Abstract
Study Objectives:
Insomnia is a common disorder, yet its proposed behavioral phenotypes are seldom differentiated. Two consecutive studies were designed to investigate psychologic characteristics and treatment preferences of people with idiopathic insomnia (IdI) relative to psychophysiologic insomnia (PI).
Design:
Cross-sectional, two-group comparison studies.
Setting:
Specialized sleep research center.
Participants:
40 participants (29 female, mean age 46 yr) participated in study 1. An additional cohort of 61 adults (48 female, mean age 37 yr) participated in study 2. In total, samples comprised 51 participants with PI and 50 with IdI. All participants met diagnostic criteria for their respective insomnia phenotype.
Interventions:
N/A
Measurements and Results:
Study 1 investigated sensitivity to arousal conditioning and sleep effort using self-report measures. Consistent with a model of conditioned arousal, participants with PI exhibited greater behavioral inhibition, i.e., sensitivity to threat and higher levels of sleep preoccupation. Study 2 investigated illness perceptions and cognitions and coping styles using self-report scales, and explored treatment acceptability based on the evaluation of 3 therapeutic scenarios. Results lend support to the hypothesis that IdI is considered somewhat more permanent than PI. Behavioral intervention was preferred to pharmacotherapy by both groups, and an acceptance treatment was considered more favorably by IdI study participants than by those with PI.
Conclusions:
Many similarities between IdI and PI were observed across psychologic measures, and both groups exhibited a preference for behavioral treatment. However, their distinctive characteristics appear to suggest that an acceptance-based therapy may also be appropriate for some people with IdI.
Citation:
Espie CA; Barrie LM; Forgan GS. Comparative investigation of the psychophysiologic and idiopathic insomnia disorder phenotypes: psychologic characteristics, patients' perspectives, and implications for clinical management. SLEEP 2012;35(3):385-393.
Keywords: Insomnia, phenotype, psychology, cognitive behavior therapy, acceptance, cognition, arousal, treatment
INTRODUCTION
Insomnia is a prevalent disorder that is challenging to manage,1,2 partly because of its inherent heterogeneity and the limited data available on the benefits (or disadvantages) of matching treatment to clinical presentation.3,4 The International Classification of Sleep Disorders, 2nd Edition (ICSD-2) provides diagnostic criteria for several primary insomnia subtypes, including psychophysiologic insomnia (PI) and idiopathic insomnia (IdI).5 The goal of the current study was to compare the way that adults with each of these subtypes perceive their insomnia, and to consider any resultant implications for clinical management.
According to ICSD-2, PI develops in adulthood, may be linked to identifiable precipitating events and/or stressors, and comprises both psychologic and physiologic features such as conditioned arousal, sleep-incompatible behavior, sleep preoccupation, and excessive focus on and anxiety about sleep. In contrast, IdI is a lifelong complaint with a chronic course and few periods of sustained remission. It has been suggested that IdI may be resistant to treatment.6 There is a limited amount of research literature on IdI, perhaps because the condition is relatively uncommon and affects fewer than 10% of those presenting with insomnia complaints.1 Moreover, conceptualizations of insomnia development and maintenance seem to fit more closely with PI than they do with IdI.7–11
IdI is partly defined by the absence of precipitating and maintaining factors, and patients appear to exhibit only minor psychologic abnormalities. The use of denial and repression as coping strategies has been suggested, and there may be an association with neurodevelopmental disorders.12,13 Higher levels of “arousability” have been reported in patients with IdI,6 and it may be that IdI represents the extreme of an insomnia continuum, where there is less of a psychologic and more of a physiologic characteristic. Somatic hyperarousal may be present in all insomnias,14,15 but may be a particular feature of IdI. In this regard it should be taken into consideration that conditioning of arousal also may be a factor in IdI, although this possibility has not been investigated. On the other hand, some research has found no major differences between IdI and PI on either polysomnography (PSG) recordings or psychologic measures.16 This finding is consistent with the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) Work Group conclusion that, although the PI and IdI classifications offered some clinical value, there was limited empirical support to propose their distinction.17 Perhaps because of the relative paucity of additional studies on idiopathic insomnia in the period between major editions, the DSM-5 committee appears to be taking a similar view in classifying a single insomnia disorder without subtypes being specified. (For further information see: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=65).
Clearly more research is warranted to better understand why PI and IdI appear to be (at least) clinically distinctive. The purpose of this study, therefore, was to compare how people meeting diagnostic criteria for PI and IdI conceptualize their own sleep difficulties. The study objective was to investigate similarities, and potential differences, in patients' personal perspectives, that might inform how we should approach management. Here we report two comparative studies, each having a separate clinically defined IdI sample, with its own PI comparison group. We first present the methods and results of each study, followed by an integrated discussion.
STUDY 1: COMPARATIVE INVESTIGATION OF AROUSAL CONDITIONING AND SLEEP EFFORT IN PI AND IDI
Methods
Participants
Eligible participants (18-65 yr) were recruited from the University of Glasgow Sleep Centre (UGSC) outpatient clinics and through local media advertisement, with most participants (approximately 75%) located via the media source. Rigorous clinical inclusion criteria for PI and IdI applied to all potential participants were as follows:
Initial telephone screening using the UGSC screening interview.
In-person, clinical evaluation against generic DSM-IV and specific ICSD-2 (PI and IdI) insomnia criteria, including detailed sleep history review.
More specifically, PI participants had to have developed persistent insomnia during adulthood, with no prior history of sleep disturbance; IdI participants had to have persistent and unremitting sleep problems since childhood.
Evidence of a current complaint of insomnia, verified by review of a minimum of 1 wk of sleep diary records; scores > 5 on the Pittsburgh Sleep Quality Index (PSQI)18 and > 8 on the Insomnia Severity Index (ISI).19
Exclusion criteria were as follows:
Anyone developing insomnia between the ages of 12 and 17 yr (to reduce overlap and increase the specificity and homogeneity of the samples).
Any symptomatic evidence of narcolepsy, sleep apnea, restless legs syndrome/periodic limb movement disorder, circadian rhythm sleep disorder, or parasomnia using the UGSC sleep diagnostic interview.
Any history or present-state diagnosis of psychopathologic disorder; somatic disorder related to the onset and/or course of insomnia; evidence of substance abuse; taking medications known to influence sleep; or unstable medical condition. The Beck inventories (the Beck Depression Inventory (BDI-II)20 and the Beck Anxiety Inventory (BAI)21) were used to assist evaluation of mental state and to describe and compare the final samples.
All assessment procedures were carried out by final-year, doctoral-level clinical psychology interns who were trained and supervised in behavioral sleep medicine by the first author (CAE). Consistent with current clinical assessment practice for such patients, PSG was not conducted.
Design
Cross-sectional, two-group comparison of PI and IdI.
Hypotheses
Consistent with a model of arousal conditioning, people with PI will exhibit greater sensitivity to threat (in general), and greater attention to sleep and sleep effort specifically, than will their IdI counterparts.
Measures
Behavioral Inhibition/Behavioral Activation Scale:
Based on Gray's seminal work, the Behavioral Inhibition/Behavioral Activation Scales (BIS/BAS) were developed to assess a person's dispositional sensitivity to threat (behavioral inhibition: avoidance of negative outcomes) and to reward (behavioral activation: pursuit of positive outcomes). 22–24 BIS sensitivity (also known as threat sensitivity) underlies the experience of anxiety.25,26 Consequently, we were interested in the possibility that, based on their differing historical experiences with sleep/insomnia, PI and IdI might express behavioral sensitivity in different ways.
The BIS/BAS comprises 24 items across four subscales: BIS Sensitivity (7 items: e.g., “criticism or scolding hurts me quite a bit”; “if I think something unpleasant is going to happen I usually get pretty “worked up”), and BAS Sensitivity, namely BAS Drive (4 items: e.g., “I go out of my way to get things I want”; “if I see a chance to get something I want I move on it right away”), BAS Fun Seeking (4 items: e.g., “I will often do things for no other reason than that they might be fun”; “I often act on the spur of the moment”) and BAS Reward Responsiveness (5 items: e.g., “When I'm doing well at something I love to keep at it”; “it would excite me to win a contest”). Responses are on a 4-point scale, ranging from 1 (“very true for me”) to 4 (“very false for me”), with higher scores indicating greater sensitivity. The scales have good psychometric properties in other populations. Internal consistencies in our own sample were also acceptable (Cronbach α = 0.77, 0.76, 0.78, and 0.70, respectively).
Glasgow Sleep Effort Scale:
According to ICSD-2,5 IdI “is not associated with specific precipitating or perpetuating factors” (p. 12), whereas the related notions of sleep preoccupation and striving for sleep are regarded as “essential features” of the acquisition and maintenance of PI, i.e., “Learned associations are marked by overconcern with the inability to sleep. A cycle develops in which the more one strives to sleep, the more agitated one becomes, and the less able one is to fall asleep….Concerns about sleep grow progressively over months or years as sleep gradually deteriorates until the desire to obtain good sleep becomes the person's major concern” (p. 6). Indeed, such selective attention to sleep is now recognized as important in several contemporary models of insomnia.8–11 Consequently, we wanted to compare PI and IdI on this dimension.
The Glasgow Sleep Effort Scale (GSES) is a short (7-item) scale specifically designed to assess effortful preoccupation with sleep.27 The GSES can be used to support a diagnosis of primary insomnia, particularly psychophysiologic insomnia,27 and it also differentiates people with insomnia associated with mental disorder from normal sleepers.28 A cutoff score ≥ 3 correctly identifies 93% of insomnia patients and excludes 87% of normal sleepers, representing a likelihood ratio of +7.3 (4.39–12.20) for insomnia patients relative to −0.08 (0.04-0.17) for normal sleepers. The GSES has a single factor structure with acceptable internal consistency (Cronbach α = 0.77) and respondents score each item on a 3-point Likert scale ranging from 2 (“very much”) to 0 (“not at all”).27
Results
Sample demographics and sleep characteristics
Forty eligible participants (29 female) with a mean age of 46.1 yr (standard deviation (SD) 14.5) were recruited (Table 1). There were no differences between groups on descriptive demographic, sleep, or mental health dimensions. PSQI and ISI mean scores were indicative of clinical insomnia of moderate severity, whereas BAI and BDI scores suggest mild anxiety and minimal depressive symptoms. These results confirm that the groups were well matched
Table 1.
Demographic, mental health, and sleep characteristics of Study 1 samples; and between-group comparisons on behavioral sensitivity scales
| Psychophysiologic insomnia (n = 20) | Idiopathic insomnia (n = 20) | |
|---|---|---|
| Demographic | ||
| Age (yr) | 49.95 (12.7) | 42.15 (15.4) |
| Sex [n (%)] | ||
| Female | 15 (75) | 14 (70) |
| Male | 5 (25) | 6 (30) |
| Mental health | ||
| Beck Depression Inventory II | 9.6 (6.64) | 5.65 (7.24) |
| Beck Anxiety Inventory | 12.75 (11.12) | 8.2 (7.46) |
| Sleep | ||
| Age of onset (yr) | 34.33 (13.8) | 4.7 (4.1) |
| Insomnia duration (yr) | 16.33 (11.0) | 37.15 (14.2) |
| Pittsburgh Sleep Quality Index | 13.11 (2.95) | 12.85 (3.88) |
| Insomnia Severity Index | 17.39 (4.67) | 15.25 (5.77) |
| Behavioral sensitivity | ||
| Inhibition sensitivity | 22.85 (2.71) | 18.65 (3.77) |
| Activation sensitivity - drive | 10.25 (2.93) | 9.5 (2.52) |
| Activation sensitivity - fun | 10.60 (2.74) | 11.85 (2.83) |
| Activation sensitivity - reward | 15.95 (2.01) | 16.0 (3.03) |
All data represent mean (SD), unless otherwise stated.
As expected, IdI and PI group allocation was supported by the IdI group reporting significantly younger mean age of insomnia onset (younger than age 5 yr compared with age 34 yr: t (38) = 9.1, P < 0.0001) and longer insomnia duration (37 yr compared with 16 yr: t (38) = 5.01, P < 0.001).
Behavioral sensitivity
Table 1 also summarizes between-group comparisons on the BIS/BAS scales. As can be seen, the PI group scored higher than the IdI group on the BIS scale (t (38) = 4.04, P < 0.001) indicating higher levels of threat sensitivity in PI. Given that historical differences are crucial to testing of the group factor (PI versus IdI), we repeated this analysis, conservatively correcting for age as a covariate, because it had been found to correlate with some of the dependent variables. The model remained significant when age was in the equation (F(2,37) = 7.98, P = 0.001). There were, however, no significant differences between PI and IdI on any of the three BAS dimensions.
Sleep effort
An omnibus, multivariate test (again with age as a covariate) was conducted across the 7 items of the GSES. This test revealed a significant between-group multivariate effect (F(7, 31) = 3.40, P = 0.008). Subsequent univariate comparisons revealed that this effect was accounted for by 4 GSES items differing between the PI and IdI groups (Table 2).
Table 2.
Between-group comparison of Study 1 samples on items from the Glasgow Sleep Effort Scalea
| Psychophysiologic insomnia (n = 20) | Idiopathic insomnia (n = 20) | |
|---|---|---|
| Glasgow sleep effort scale | ||
| 1. I put too much effort into sleeping when it should come naturally | 1.10 (0.79) | 0.70 (0.73) |
| 2. I feel I should be able to control my sleep | 1.15 (0.59) | 0.90 (0.64) |
| 3. I put off going to bed at night for fear of not being able to sleep | 0.50 (0.61) | 0.75 (0.72) |
| 4. I worry about not sleeping if I cannot sleep | 1.35 (0.59) | 1.05 (0.76) |
| 5. I am no good at sleeping | 1.20 (0.69) | 1.70 (0.57) |
| 6. I get anxious about sleeping before I go to bed | 0.80 (0.69) | 0.30 (0.57) |
| 7. I worry about the consequences of not sleeping | 1.40 (0.68) | 0.90 (0.64) |
All data represent mean (SD), unless otherwise stated.
Specifically, PI endorsed “putting too much effort into sleeping” (F(2) = 4.41, P = 0.019), being “anxious about sleeping before going to bed” (F(2) = 3.87, P = 0.030) and “worrying about the consequences of not sleeping” (F(2) = 3.34, P = 0.046) more strongly than did IdI. Conversely, there was a nonsignificant trend for the IdI participants to endorse being “no good at sleeping” more strongly than PI (F(2) = 3.01, P = 0.062).
The results from Study 1, therefore, broadly confirm the hypothesis that people with PI exhibit greater levels of threat sensitivity in general and a more pronounced sleep-related focus than people with IdI.
STUDY 2: COMPARATIVE INVESTIGATION OF ILLNESS PERCEPTION, COPING STYLE, AND TREATMENT ACCEPTABILITY IN PI AND IDI
Methods
Participants
Eligibility criteria and recruitment procedures were identical to those used in Study 1 with 2 exceptions. First, the conservative exclusion zone for age of insomnia onset was set slightly larger, between the ages of 10 and 18 yr. No one whose insomnia started within this age range was accepted into the study. Second, anyone with previous or current experience of psychologic treatment for insomnia was excluded, and none of the participants was on pharmacotherapy for insomnia at the time of the study. Also in this study, the Dysfunctional Beliefs and Attitudes About Sleep Scale (DBAS-16)29 was added to give a further descriptive profile of the samples.
Design
Cross-sectional, two-group comparison of PI and IdI.
Hypotheses
People with IdI will regard their insomnia as more permanent and will be more accepting of it than will people with PI. Both groups will rate a behavioral treatment as preferable to pharmacotherapy, but the IdI group will also consider an “acceptance treatment” in preference to pharmacotherapy.
Measures
Illness Perception Questionnaire-Revised:
Acknowledgment that IdI (in particular) is an unrelenting condition suggests that it may be worthwhile considering patients' perceptions from a “chronic symptom” perspective. Pain and fatigue come to mind as parallel, poorly understood psychophysiologic conditions often displaying poor treatment response,31,32 and being associated with clinician frustration and helplessness (“compassion fatigue).33 The Illness Perception Questionnaire-Revised30 (IPQ-R: 21 items) has proven valuable in such disorders to investigate patient perspectives, so it was selected as a suitable measure to explore illness perceptions of IdI relative to PI. Moreover, the IPQ-R is validated to permit the word illness to be replaced, in this instance with insomnia, to make it a disease-specific scale.
The IPQ-R includes a timeline: acute/chronic subscale (6 items), which measures perceptions of permanency (high subscale score indicates permanence: e.g., “my insomnia is likely to be permanent rather than temporary”; “I expect to have this insomnia for the rest of my life”) and a timeline: cyclical subscale (4 items), which measures if an individual perceives his or her illness to be variable, with either a cyclical nature or being constant and unrelenting (high subscale score indicates variability: e.g., “I go through cycles in which my insomnia gets better and worse”; “my insomnia is very unpredictable”). The two other IPQ-R subscales are also of interest. The personal control subscale (6 items) and treatability subscale (5 items) elicit individuals' beliefs in relation to having personal control over their illness (high subscale score indicates perceived lack of control: e.g., “I have the power to influence my insomnia”) or if they believe a treatment might be effective in curing their illness (high subscale score indicates low treatment expectations: e.g., “There is nothing that can help my condition”).
Participants responded to each item using a 5-point Likert scale ranging from 0 (strongly disagree) to 4 (strongly agree). Several previous adaptations to particular health populations have yielded good internal consistency (Cronbach α = 0.70-0.90α = 0.70-0.90).30 We obtained similar values across the subscales in the current study (Cronbach α = 0.68-0.89).
Illness Cognition Questionnaire:
Similar to the IPQ-R, the Illness Cognition Questionnaire (ICQ) was developed for use in chronic diseases,34 and can be worded specifically to the “illness” in question. We included the ICQ because it evaluates two important components of mental state, in our case in relation to insomnia. One scale measures the cognitive construct of “acceptance” (6 items: e.g., “I think I can handle the problems related to my insomnia, even if the insomnia gets worse” and “I can cope effectively with my insomnia”). A high score is indicative of low acceptance. The “helplessness” scale then provides an additional measure of impact/sense of control (6 items: e.g., “my insomnia controls my life” and “my insomnia limits me in everything that is important to me”).A high score indicates less helplessness/more control.
Items are rated ranging from 1 (agree) to 4 (disagree) (subscale score range = 4-24) The ICQ is reported as a reliable and valid assessment of perceptions of patients with a chronic disease (Cronbach α = 0.84-0.91).34 In our study, subscale internal reliabilities were Cronbach α = 0.81 (acceptance) and 0.77 (helplessness).
Coping Style (Brief Cope):
We also wanted to compare how people with PI and IdI perceive that they typically respond when confronted with difficult or stressful events. The 28-item Brief Cope profiles 14 coping styles: active coping, planning, positive refraining, acceptance, humor, religion, using emotional support, using instrumental support, self-distraction, denial, venting, substance use, behavioral disengagement, and self-blame.35 Responses are scored ranging from 1 (“I usually don't do this at all”) to 4 (“I usually do this a lot”). Some examples of items include “trying to see it in a different light, to make it seem more positive” (positive reframing), “turning to work or other activities to take my mind off things” (self-distraction), and “making fun of the situation” (humor). The Brief Cope is said to have psychometric properties consistent with its original 60-item version (Cronbach α ≤ 0.90).35 We did not check the internal consistency of this short-form version because each subscale comprised only 2 items.
Treatment Acceptability Scale:
Finally, we included a scenario-based assessment of patients” perceptions by adapting the treatment acceptability/preferences paradigm by Morin et al.44 to include a novel treatment descriptor on acceptance treatment. In this regard it should be noted that there is growing evidence that acceptance-based strategies may be associated with better emotional adjustment across a range chronic health conditions.58,59 The traditional Treatment Acceptability Scale (TAS) incorporates a behavioral treatment and a pharmacologic treatment scenario, each of which participants rate for treatment acceptance (2 items), willingness to comply, suitability for sleep onset, and for sleep maintenance problems, perceived effectiveness (2 items), and side effects.36 Each dimension is rated ranging from 1 to 6, giving a possible range of 8-48 per scale. We added an Acceptance Therapy scenario to explore this additional perspective to the way adults with PI and IdI might conceptualize insomnia treatment. The treatments were described in three ways.
Behavioral treatment is a nondrug treatment method aimed at teaching individuals a set of skills to help overcome their sleep problem. It provides specific guidelines for changing poor sleep habits and for regulating sleep schedules. Education about sleep hygiene factors (e.g., bedroom environment) is also provided. Pharmacologic treatment consists of taking a prescribed pill at a specified time. The prescribed medication is a naturally-occurring hormone that is essential for sleep. The specific dosage would be based on the nature and severity of the patient's sleep problem. Acceptance treatment is a nondrug treatment method aimed at encouraging acceptance of insomnia. It is designed to develop strategies for overcoming the effect of insomnia on a patient's life (e.g., engaging in increased activity or reducing distress caused by insomnia-associated thinking).
We modeled the pharmacologic treatment on a melatonin receptor agonist (mRA) for three reasons. First, previous studies have already indicated that a conventional sleeping pill is not particularly favored by people with insomnia, with behavioral treatment receiving higher ratings36,37; second, we wanted to “match” the novelty aspect of acceptance therapy, as a new(er) approach; and third, how people might respond to an mRA proposition was in itself interesting. In our study, all three TAS achieved satisfactory reliability (behavioral: Cronbach α = 0.88; pharmacologic: Cronbach α = 0.82; acceptance: Cronbach α = 0.92).
Results
Sample demographics and sleep characteristics
A total of 61 adults with insomnia (48 females; mean age 36.6 (15.1) yr) participated. There were no differences between PI and IdI groups on sex or age, or on educational or relationship status (Table 3). Likewise, the groups were well matched on health status (Short Form-36)39 and alcohol consumption, and had at most minor anxiety and/or depressive symptoms. Both PI and IdI groups reported sleep disturbance, in the moderate range on the PSQI and ISI, with no significant between-group differences. Average duration of insomnia was 7 years for the PI group and 26 years for the IdI group, yet dysfunctional thinking about sleep did not differ between groups, either in terms of Dysfunctional Beliefs and Attitudes About Sleep (DBAS-16) total score (Table 3) or on its subscales. None of the participants had received previous psychologic treatment. Some in both groups reported occasional previous use of prescribed and nonprescribed medication for insomnia, although none was taking prescribed medication at the time of assessment.
Table 3.
Demographic, health, and sleep characteristics of study 2 samplesa
| Psychophysiologic insomnia (n = 31) | Idiopathic insomnia (n = 30) | |
|---|---|---|
| Demographic | ||
| Sex [n (%)] | ||
| Female | 24 (77.4) | 24 (80) |
| Male | 7 (22.6) | 6 (20) |
| Age (yr) | 38.52 (14.5) | 34.63 (15.7) |
| Education [n (%)] | ||
| Secondary completed | 14 (45.2) | 10 (33.3) |
| Tertiary completed | 17 (54.8) | 20 (66.7) |
| Relationship status [n (%)] | ||
| With partner | 13 (41.9) | 14 (46.7) |
| Living alone | 18 (58.1) | 16 (53.3) |
| Health | ||
| Alcohol (units/wk) | 6.67 (5.85) | 6.8 (6.66) |
| BDI II | 12.39 (6.67) | 10.41 (5.88) |
| BAI | 7.74 (3.98) | 8.35 (5.8) |
| SF-36 | ||
| Physical | 76.37 (15.34) | 82.73 (12.51) |
| Mental | 63.18 (18.37) | 66.37 (17.92) |
| Sleep | ||
| PSQI | 10.25 (3.76) | 10.90 (3.38) |
| ISI | 14.74 (5.29) | 16.23 (4.7) |
| DBAS-16 | 76.28 (25.76) | 74.73 (29.34) |
All data represent mean (SD), unless otherwise stated. BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; DBAS-16, Dysfunctional Beliefs and Attitudes About Sleep Scale; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; SF-36, Short Form-36.
Illness perception
Both groups perceived their sleep difficulties to be relatively chronic, scoring above the mid-point on the IPQ-R permanency subscale (mean item scores: Table 4). However, the IdI group perceived their insomnia to have greater permanency than did the PI group (t (59) = 2.18, P = 0.033). There were no significance differences between groups on the variability, personal control, or perceived treatability subscales. It should be noted, however, that mean scores suggest that both groups tended to view their insomnia as variable and outside of their control, and had doubts about its treatability.
Table 4.
Between-group comparison of study 2 samples on illness perception, illness cognition, coping style, and treatment acceptability scalesa
| Psychophysiologic insomnia (n = 31) | Idiopathic insomnia (n = 30) | |
|---|---|---|
| Illness perception questionnaire | ||
| Permanency | 3.44 (0.68) | 3.84 (0.69) |
| Variability | 2.99 (0.97) | 2.78 (1.11) |
| Personal control | 3.04 (0.65) | 2.83 (0.72) |
| Treatability | 3.24 (0.53) | 3.25 (0.57) |
| Illness cognition questionnaire | ||
| Acceptance | 14.4 (4.67) | 15.7 (4.21) |
| Helplessness | 16.5 (4.42) | 17.2 (4.50) |
| Coping style (Brief Cope) | ||
| Active coping | 5.33 (1.45) | 5.85 (1.35) |
| Planning | 5.27 (1.41) | 5.69 (1.54) |
| Positive reframing | 4.63 (1.45) | 5.38 (1.81) |
| Acceptance | 5.67 (1.37) | 5.92 (1.74) |
| Humor | 4.33 (1.94) | 5.85 (2.20) |
| Religion | 3.67 (2.14) | 2.92 (1.87) |
| Emotional support | 4.23 (2.05) | 4.69 (2.22) |
| Instrumental support | 4.70 (2.00) | 4.28 (1.65) |
| Self distraction | 4.73 (1.48) | 5.73 (1.22) |
| Denial | 2.83 (1.39) | 3.19 (1.58) |
| Venting | 4.63 (2.61) | 4.28 (1.45) |
| Substance use | 2.70 (1.29) | 2.92 (1.29) |
| Behavioral disengagement | 3.10 (1.54) | 3.23 (1.73) |
| Self blame | 4.23 (1.98) | 4.12 (1.95) |
| Treatment acceptability scale | ||
| Behavioral therapy | 41.3 (7.63) | 42.19 (8.84) |
| Pharmacotherapy | 32.9 (9.34) | 34.17 (7.23) |
| Acceptance therapy | 30.2 (9.74) | 36.20 (8.80) |
All data represent mean (SD), unless otherwise stated.
Illness cognition
Both PI and IdI groups scored in the midscale range on the ICQ acceptance subscale, indicating moderate levels of insomnia acceptance/lack of acceptance (Table 4). However, there were no significant differences between participants with PI and IdI. Likewise, there were no differences between groups on the ICQ helplessness scale (both P > 0.05).
Coping strategies
A comparison of profiles of use of the 14 different strategies measured by the Brief Cope is also presented in Table 4. Interestingly, the top three endorsed strategies (interpreting mean scores from highest to lowest) for PI were acceptance, active coping, and planning. Acceptance and active coping were also the two top-rated strategies in the IdI group, but with the use of humor scoring equal second. Between-group comparison showed that patients with IdI rated using humor to cope significantly more often than those with PI (t (59) = −2.73, P = 0.008). The only other strategy that differentiated between group was self-distraction, which also was used more often by patients with IdI than by those with PI (t (59) = −2.72, P = 0.009]. These findings fail to achieve significance after conservative adjustment for multiple comparisons (critical value: P < 0.004).
Treatment acceptability
Table 4 also summarizes between-group comparisons across the three treatment scenarios on the TAS task. Mixed-model analysis of covariance (with age as a covariate) revealed significant effects of group (F(1) = 5.34, P = 0.025), treatment type (F(2) = 3.11, P = 0.049), and a near-significant group by treatment interaction (F(2) = 3.73, P = 0.059). There was no significant difference between PI and IdI in patient ratings of behavioral treatment or pharmacologic treatment. For the acceptance-based approach to insomnia, however, there was a significant effect, with the IdI group rating this intervention as acceptable relative to the PI group's lower ratings (t (61) = 2.40, P = 0.02). To consider the possibility that treatment acceptability covaried with duration of insomnia (independent of subtype), we correlated insomnia duration in the PI group with ratings for each treatment scenario. No significant effects were observed (behavioral (rho = −0.054); acceptance (rho = 0.026); pharmacologic (rho = −0.164); all P > 0.40).
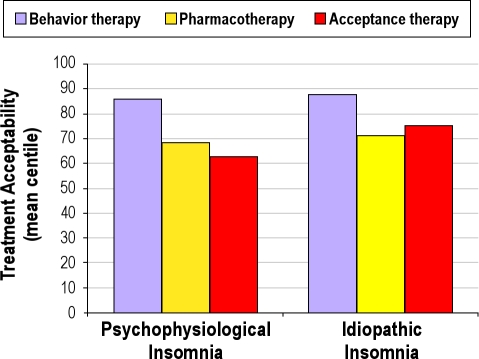
Treatment acceptability scores for the PI and IdI groups are also presented as centiles in Figure 1. Related t-tests indicate that behavioral treatment was the highest rated both within the PI group (behavioral versus pharmacologic: t(31) = 3.30; P = 0.003; behavioral versus acceptance: t (31) = 5.03; P < 0.001] and the IdI group (behavioral versus pharmacologic: t (30) = 3.45; P = 0.002; behavioral versus acceptance: t (30) = 2.79; P = 0.01). Despite the visual impression that the PI group rated pharmacologic treatment higher than acceptance treatment, and that the IdI group rated acceptance higher than pharmacologic treatment, neither effect was not supported statistically (both P > 0.30).
Figure 1.
Between group comparison of Treatment Acceptability centile scores for Study 2 samples.
The results from Study 2 lend some support to the hypothesis that IdI participants regard their insomnia as somewhat more permanent than PI. Whereas neither group regards insomnia as acceptable, an acceptance treatment was considered more favorably by IdI than by PI participants. Behavioral intervention was preferred to pharmacotherapy by both groups.
DISCUSSION
Despite some early interest in comparing and contrasting the insomnia phenotypes of IdI and PI, scientific progress has been limited. Although ICSD-2 retained the subtypes proferred in the original ICSD,38 due to limited research data they were not included in DSM-IV and they seem unlikely to be in the DSM-5 nosology (expected publication in 2013). Nonetheless, their “clinical utility” has been recognized (compassion fatigue, DSM-IV). Therefore, we took this consensus clinical framework to conduct two consecutive studies, with the purpose of comparing and contrasting the patient perspectives and insomnia treatment preferences of people with IdI and PI. We endeavored to make clinical differentiation both valid and robust; e.g., by excluding anyone whose sleep problem developed during their teenage years, and by matching the IdI and PI groups on demographic factors, insomnia severity, and associated (but minimal) psychopathology. Thus, we recruited two relatively homogeneous insomnia subgroups that differed primarily, and categorically, on their history of poor sleep experience.
In relation to results, the first thing to be said is that we observed many similarities between IdI and PI, across the psychologic measures that we applied. Both groups reported helplessness and that their insomnia felt out of control, and they expressed doubts about its treatability (ICQ and IPQ data). Also trying to accept their situation and to cope with it actively rather than passively seemed characteristic (Brief Cope). They also endorsed similar levels of dysfunctional thinking (DBAS-16) and both groups had a clear preference for a behavioral approach to insomnia intervention (TAS). Nevertheless, some hypothesized between-group differences were supported.
In Study 1, people with PI exhibited higher levels of behavioral inhibition or threat sensitivity (BIS), consistent with an etiologic model of vulnerability to conditioned arousal in PI. This vulnerability might find its expression during an insomnia acquisition phase, perhaps at a time of stress.8,10,40 Likewise, analysis of sleep effort data (GSES) appears consistent with the ICSD-2 account that people with PI characteristically strive hard to sleep, and worry excessively about the consequences of not sleeping.10,11 By way of contrast, there was a trend for people with IdI to endorse the notion that sleeping is just something that they are not any good at; perhaps not so much a learned performance failure, as a fundamental (lifelong) inability. This would be consistent with a trait hyperarousal perspective on IdI.
In Study 2, whereas both groups perceived their sleep problems to be chronic, and possibly permanent, this belief was held more strongly by people with IdI (IPQ). It is interesting, therefore, that as hypothesized, participants with IdI rated an acceptance treatment highly, an approach that seemed in comparison relatively unacceptable to people with PI (TAS). So we might speculate that there are phenotypical differences between IdI and PI in their readiness to adapt to a “living with insomnia” perspective. IdI participants were also more likely to use humor as a form of coping (Brief Cope). Humor is integral to some psychotherapeutic traditions, including Frankl's logotherapy approach,41 from which paradoxic intention therapy for insomnia was first derived,42 and also in what has become known as Acceptance and Commitment Therapy (ACT).43 Perhaps humor provides some relief from the effect of symptoms on the person with insomnia, rather than directly influencing their occurrence per se? This would be consistent also with the comparatively higher use of self-distraction as a coping strategy in IdI.
We also replicated previous TAS experimental findings that behavioral therapy is highly rated by people with insomnia (here in both PI and IdI) and that it is generally preferred to pharmacotherapy,36,37 in this case, to the scenario of an mRA compound, although we did not specifically mention melatonin in the study. However, our data also hint (nonsignificant trend) that people with PI might prefer pharmacotherapy to accepting that they have a chronic problem that they might need to live with (TAS).
Taken together with the cognitive-behavioral literature on insomnia and its treatment, what might our results imply for research and for clinical practice?
We know that cognitive behavioral therapy (CBT) is an effective intervention for (largely undifferentiated) persistent insomnia, with approximately two thirds of patients making a sustained response.44,45–47 Therefore, there is a rationale for using CBT with both PI and IdI patients. From the data in this study, we can see that PI and IdI have common psychologic features that may form part of an insomnia-specific (e.g., dysfunctional beliefs about sleep) and a generic (e.g., sense of helplessness) focus for CBT. Moreover, both groups rated CBT highly, presumably because they thought that it would be (most) relevant to their situation.
By the same token, however, we know from the literature that one third of patients do not respond to CBT. Therefore, it seems reasonable to consider two things. First, what characterizes a nonresponder; and second, what pragmatically might be a “second line” therapy for those who fail to respond to adequately delivered CBT, as the behavioral treatment of first choice. Research has shown that it is difficult to predict, either a priori or a posteriori, who the responders and nonresponders might be48; although, importantly in relation to the current study, it seems likely that poor definition and subtyping in insomnia research studies could be one important factor.49
From our findings, we speculate that there is a rationale for considering an acceptance-based therapy as a second-line intervention for PI patients who have not responded to CBT; and potentially, as a first-line treatment for IdI. Perhaps the integration of aspects of acceptance into CBT is worth considering, although we note that the evolution of multimodal CBT over and above its components (e.g., relaxation, stimulus control, sleep restriction) has not been accompanied by greatly improved outcomes.3 On balance, therefore, we would recommend that controlled evaluation of acceptance as a stand-alone therapy against a CBT comparator would be valuable, especially if patients can be stratified by insomnia subtype so that treatment × phenotype interactions can be reported. Such research would address two important clinical caveats: if a sleep problem cannot be eliminated, then failing to reduce its effect and associated distress would amount to poor quality care; and if a sleep problem can be solved, then living with insomnia in a contented way would be a suboptimal therapeutic solution.
To encourage more research, we note that the therapeutic value of acceptance is becoming influential across a range of other chronic medical and psychologic disorders. Contemporary “third wave” techniques such as mindfulness-based stress reduction, mindfulness-relaxation, and ACT,50 raise the question of whether a person's relationship with their symptoms could change (rather than focusing on symptom reduction). In insomnia, this would translate into addressing concerns about lying awake in bed, rather than addressing directly the frequency or duration of lying awake in bed. There are several small or uncontrolled studies on acceptance and mindfulness approaches to insomnia in the literature,51–57 and this work is very much welcomed. It is worth bearing in mind, however, that people may be less likely to endorse acceptance of a problem if they are participants in a study based at a center where they are actively seeking a remedy. In this study, most participants completed measures at home. Nevertheless, the study was clearly associated with our clinical research center. Therefore, there may be methodologic advantages in also evaluating acceptance with non-treatment-seeking samples and in explicitly community settings.
In conclusion, we wish to acknowledge important limitations to our studies. We used clinical interview criteria to establish a working diagnosis. Whereas this appears valid for the purpose of the study, and PSG would be unlikely to be informative about the PI/IdI discrimination, we cannot be certain that other sleep disorders were excluded. For example, it is possible that mild obstructive sleep apnea and/or periodic limb movement disorder could be present in either or both of our insomnia phenotype samples, Also, although we reviewed diary records, actigraphy may have been useful, for example, to confirm stability of poor sleep in IdI compared with night-to-night variability in PI. We also cannot completely exclude the possibility that the differences between PI and IdI reported here relate solely to the length of time that people have had problems with insomnia. Bivariate correlations of duration of insomnia with TAS scores in the PI group suggest that this was not a factor, and we included age as a covariate in all our major analyses. However, this is a research question that should be specifically addressed in the future. We would also point out that our description of acceptance treatment was necessarily brief (40 words) to match previously published behavioral treatment descriptions,36 and possibly left the descriptor open to misinterpretation (e.g.,a coping strategy rather than a treatment). It is problematic to convey the therapeutic value (of any treatment) in so few words,and a further study should consider comparing more detailed outlines. Finally, we recognize that what people think and believe is not necessarily the best guide to what should be offered in the way of treatment, or to what will actually work for them.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Infrastructure support provided by the Dr. Mortimer and Theresa Sackler Foundation. Thanks also to Shona Currie for her assistance in recruitment and to Kerry Ross for assistance in preparing this manuscript.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Review. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Espie CA. Stepped care: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549–58. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin C, Bootzin R, Buysse D, Edinger J, Espie C, Lichstein K. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 4.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205–14. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.2nd edition. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 6.Edinger JD, Stout AL, Hoelscher T. Cluster analysis of insomniacs' MMPI profiles: relation of subtypes to sleep history and treatment outcome. Psychosom Med. 1988;50:77–87. doi: 10.1097/00006842-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 8.Perlis ML, Giles DF, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 9.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 10.Espie CA, Broomfield NM, MacMahon KMA, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 12.Hauri PJ, Olmstead EM. Childhood-onset insomnia. Sleep. 1980;3:59–65. doi: 10.1093/sleep/3.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Hauri PJ. A cluster analysis of insomnia. Sleep. 1983;6:326–38. doi: 10.1093/sleep/6.4.326. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 15.Nofzinger EA, Buysse DJ, Germain A, Price J, Miewald J, Kupfer D. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–29. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 16.Philip P, Guilleminault C. Adult psychophysiologic insomnia and positive history of childhood insomnia. Sleep. 1996;19(3):16–22. [PubMed] [Google Scholar]
- 17.Reynolds CF, Kupfer DJ, Buysse DJ, Coble P, Yeager A. Subtyping DSM- III-R primary insomnia. a literature review by the DSM IV work group on sleep disorders. Am J Psychiatry. 1991;148:432–38. doi: 10.1176/ajp.148.4.432. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. New York: The Psychological Corporation; 1996. Beck depression inventory. [Google Scholar]
- 21.Beck AT, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 23.Gray JA. Oxford: Oxford University Press; 1982. The neuropsychology of anxiety: an enquiry into the functions of the septo- hippocampal system. [Google Scholar]
- 24.Gray JA. The neuropsychology of emotion and personality. In: Stahl SM, Iversen SD, Goodman EC, editors. Cognitive Neurochemistry. Oxford: Oxford University Press; 1987. [Google Scholar]
- 25.Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatient with anxiety and mood disorders. Psychol Assess. 2004;16:244–254. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- 26.Bijttebier P, Beck I, Claes L, Vandereycken W. Gray's reinforcement sensitivity theory as a framework for research on personality-psychopathology associations. Clin Psychol Rev. 2009;29:421–30. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14:401–7. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 28.Kohn L, Espie CA. Sensitivity and specificity of measures of the insomnia experience: a comparative study of psychophysiologic insomnia, insomnia associated with mental disorder and good sleep. Sleep. 2005;28:104–12. doi: 10.1093/sleep/28.1.104. [DOI] [PubMed] [Google Scholar]
- 29.Morin CM, Vallieres A, Ivers H, Bouchard S, Bastien CH. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a briefer version (DBAS-16) Sleep. 2003;26:29. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psycholog Health. 2002;17:1–16. [Google Scholar]
- 31.Van Damme S, Crombez G, Van Houdenhove B, Mariman A, Michielsen W. Well-being in patients with chronic fatigue syndrome: the role of acceptance. J Psychosom Res. 2006;61:595. doi: 10.1016/j.jpsychores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.McCracken LM, Eccleston CA. Prospective study of acceptance of pain and patient functioning with chronic pain. Pain. 2005;118:164–9. doi: 10.1016/j.pain.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Figley CR. Compassion fatigue: psychotherapists' chronic lack of self care. J Clin Psychol. 2002;58:1433–41. doi: 10.1002/jclp.10090. [DOI] [PubMed] [Google Scholar]
- 34.Evers AWM, Kraaimaat FW, Van Lankveld W, Jongen PJH, Bijlsma JWJ. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69:1026–36. [PubMed] [Google Scholar]
- 35.Carver CS. You want to measure coping but your protocol's too long: consider the Brief COPE. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 36.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients' acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15:302–5. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 37.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24:411–17. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 38.American Sleep Disorders Association. Rochester, MN: American Sleep Disorders Association; 1990. International classification of sleep disorders. [Google Scholar]
- 39.Jenkinson C, Layte R, Wright L, Coulter A. Oxford: Health Services Research Unit, University of Oxford; 1996. The UK SF-36: an analysis and interpretation manual. [Google Scholar]
- 40.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–55. [PubMed] [Google Scholar]
- 41.Frankl VE. 2nd edition. New York: Knopf; 1965. The doctor and the soul. [Google Scholar]
- 42.Ascher LM, Turner RM. Paradoxical intention and insomnia: an experimental investigation. Behav Res Ther. 1979;17:408–11. doi: 10.1016/0005-7967(79)90015-9. [DOI] [PubMed] [Google Scholar]
- 43.Hayes SC, Strosahl K, Wilson KG. New York: Guildford Press; 1999. Acceptance and commitment therapy: an experiential approach to behavior change. [Google Scholar]
- 44.Morin CM, Bootzin RR, Buysee DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 45.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205–14. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 46.NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens Sci Statements. 2005;22:1–30. [PubMed] [Google Scholar]
- 47.Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacology. 2010;24:1577–1600. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 48.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 49.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 50.Kabat-Zin J. New York: Delta; 1990. Full catastrophe living: using the wisdom of your mind to face stress, pain and illness. [Google Scholar]
- 51.Lundh L. The role of acceptance and mindfulness in the treatment of insomnia. J Cogn Psychother. 2005;19:29–39. [Google Scholar]
- 52.Heidenreich T, Tuin I, Pflug B, Michal M, Michalak J. Mindfulness-base cognitive therapy for persistent insomnia:a pilot study. Psychother Psychosom. 2006;75:188–9. doi: 10.1159/000091778. [DOI] [PubMed] [Google Scholar]
- 53.Yook K, Lee SH, Ryu M, et al. Usefulness of mindfulness-based cognitive therapy for treating insomnia in patients with anxiety disorders: a pilot study. J Nerv Ment Dis. 2008;196:501–3. doi: 10.1097/NMD.0b013e31817762ac. [DOI] [PubMed] [Google Scholar]
- 54.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008;39:171–82. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore. 2009;5:30–6. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Britton WB, Haynes PL, Fridel KW, Bootzin RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72:539–48. doi: 10.1097/PSY.0b013e3181dc1bad. [DOI] [PubMed] [Google Scholar]
- 57.Dalrymple KL, Fiorentino L, Politi MC, Posner D. Incorporating principles from acceptance and commitment therapy into cognitive-behavioral therapy for insomnia: a case example. J Contemp Psychother. 2010;40:209–17. [Google Scholar]
- 58.Stanton AL, Revenson TA, Tennen H. Health psychology: psychological adjustment to chronic disease. Annu Rev Psychol. 2007;58:565–92. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- 59.De Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet. 2008;372:246–55. doi: 10.1016/S0140-6736(08)61078-8. [DOI] [PubMed] [Google Scholar]