Abstract
Study Objective:
Objective physiologic data on sleep and nocturnal breathing at initial exposure and during acclimatization to high altitude are scant. We tested the hypothesis that acute exposure to high altitude induces quantitative and qualitative changes in sleep and that these changes are partially reversed with acclimatization.
Design:
Prospective observation.
Setting:
One night in a sleep laboratory at 490 meters, the first and the third night in a mountain hut at 4559 meters.
Participants:
Sixteen healthy mountaineers.
Intervention:
Altitude exposure.
Measurements:
Polysomnography, questionnaire evaluation of sleep and acute mountain sickness.
Results:
Compared to 490 m, median nocturnal oxygen saturation decreased during the 1st night at 4559 m from 96% to 67%, minute ventilation increased from 4.4 to 6.3 L/min, and the apnea-hypopnea index increased from 0.1 to 60.9/h; correspondingly, sleep efficiency decreased from 93% to 69%, and slow wave sleep from 18% to 6% (P < 0.05, all instances). During the 3rd night at 4559 m, oxygen saturation was 71%, slow wave sleep 11% (P < 0.05 vs. 1st night, both instances) and the apnea/hypopnea index was 86.5/h (P = NS vs. 1st night). Symptoms of AMS and of disturbed sleep were significantly reduced in the morning after the 3rd vs. the 1st night at 4559 m.
Conclusions:
In healthy mountaineers ascending rapidly to high altitude, sleep quality is initially impaired but improves with acclimatization in association with improved oxygen saturation, while periodic breathing persists. Therefore, high altitude sleep disturbances seem to be related predominantly to hypoxemia rather than to periodic breathing.
Citation:
Nussbaumer-Ochsner Y; Ursprung J; Siebenmann C; Maggiorini M; Bloch KE. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. SLEEP 2012;35(3):419-423.
Keywords: Altitude, hypoxia, sleep, apnea, ventilation
INTRODUCTION
Sleep at altitude is often perceived as disturbed and unrefreshing.1,2 Moreover, staying and sleeping at altitude is associated with the risk of developing high altitude illnesses3 including acute mountain sickness (AMS), which is typically associated with insomnia and high altitude pulmonary edema (HAPE) causing cough and breathlessness. Data from several studies suggest that acute exposure to hypoxia alters sleep architecture by frequent awakenings and a reduction of deep sleep.4–9 The conclusions from these data are hampered by the small sample size and variations in the study setting (i.e., hypobaric chamber vs. field studies), ascent rate, and sleeping altitude. Whether changes in sleep persist during a stay at altitude over a few days remained uncertain.
Periodic breathing, an oscillatory pattern of waxing and waning of ventilation with periods of hyperventilation alternating with central apnea or hypopnea is often observed during sleep at altitude.10–12 Whether it is the main cause of arousals and fragmented sleep at altitude is controversial, since arousals without a clear temporal relation to periodic breathing cycles have been described.13
With the limitations discussed above, only a few studies using different ascent protocols and either real or simulated altitude in small numbers of subjects have evaluated the effects of a prolonged stay at altitude on sleep and breathing with partially inconsistent results.14–16 Therefore, the independent effects of altitude and of acclimatization could not be conclusively assessed. Considering the increasing popularity of mountain tourism and the requirements for professional personnel to work at altitude, more scientific data on the effects of altitude on sleep is desirable. To address this point, we tested the hypothesis that acute exposure to high altitude induces quantitative and qualitative changes in sleep and that these changes are partially reversed with acclimatization over a few days.
MATERIALS AND METHODS
Participants
Healthy, physically fit subjects were invited to participate by newspaper advertisements. Subjects who had spent > 5 nights above 2500 m within the last 30 days before the beginning of the study and those with chronic or acute medical conditions requiring medical treatment were excluded. Participants gave written informed consent, and the study was approved by the institutional ethics committee.
Protocol
Within one month after baseline evaluation in Zurich (490 m), participants travelled to Alagna (1170 m), Italy, ascended to Passo Salati (2971 m) by cable car and walked to the Gnifetti hut (3610 m) within 2-3 h to spend the night there. The following morning, subjects climbed to the Regina Margherita hut (4559 m) within 4-6 h and stayed there for 4 nights. Polysomnographies were performed during one night in Zurich and during the first and the third night at 4559 m from approximately 22:00 to 06:00. Subjects were not allowed to take any prophylactic medication, but were treated with dexamethasone tablets 4 mg twice daily if they suffered from severe AMS (Lake Louise score > 4).
Measurements
Polysomnography
Polysomnography was performed with a portable, battery powered device (LifeShirt, VivoMetrics, Ventura, CA, USA).10,17,18 Derivations included a central electroencephalogram (EEG) (C3A2), electrooculogram (EOG) and submental electromyogram (EMG) leads, pulse oximetry, calibrated respiratory inductive plethysmography, and capnography of expired air. Relative gains of rib cage and abdominal inductance signals were determined in the evening by the qualitative diagnostic calibration procedure,19 and their sum was calibrated in absolute units (L) by rebreathing into a bag of known volume (0.8 L) during several breaths.10 Volume calibration was validated in the morning at the end of sleep studies and revealed that tidal volume estimates were within 20% of calibrated values.
Sleep stages and arousals were scored according to standard criteria.20 Apneas/hypopneas were defined as previously described.10 Briefly, a reduction of the inductive plethysmographic sum volume signal to < 50% of the preceding 2 min baseline during ≥ 10 s or during 5-10 s was scored as apnea/hypopnea if it occurred as part of a periodic breathing pattern. Central apneas/hypopneas were distinguished from obstructive apneas/hypopneas by the absence of rib cage-abdominal asynchrony or paradoxical motion. The apnea/hypopnea index (AHI) was computed as the mean number of events per hour of sleep. The oxygen saturation measured by pulse oximetry was quantified in terms of the mean value during sleep (SpO2), and the oxygen desaturation index (> 3% dips) expressed as events per hour of time in bed, since periodic breathing occurs during sleep as well as during wakefulness and to allow comparison to previous studies not using EEG.
Questionnaire and clinical examination
Subjective sleep quality was evaluated by visual analogue scales according to the Leeds questionnaire.21,22 For each question listed below, a vertical mark had to be placed on a line 100 mm in length. If no change was experienced compared to the usual sleep, the mark had to be placed in the center (at 50 mm). Perceived changes were represented by the distance of the mark from the center, with values < 50 mm (left of center) indicating worse and > 50 mm (right of center) indicating better. The following statements were rated: “Sleep was more restlessness than usual/more restful than usual.” “There were more periods of wakefulness than usual/fewer periods of wakefulness than usual.” “How did you feel on waking (tired/alert)”.
Symptoms of acute mountain sickness were assessed by the Lake Louise protocol self-report and clinical assessment.23 A Lake Louise total score > 4 was considered to indicate acute mountain sickness. The question on insomnia within the Lake Louise questionnaire is reported separately.
Data Analysis
Data are summarized as medians and quartiles. The effects of altitude and of acclimatization were evaluated by Wilcoxon matched pairs tests. A probability of P < 0.05 was considered significant.
RESULTS
Subjects
Twenty subjects were recruited. Three subjects withdrew after the baseline examination at Zurich, and one could not participate in polysomnography at 4559 m because of severe AMS. Therefore, data from 16 subjects (13 men, 3 women) were available for analysis. Median age was 45 (quartile range 33 to 50) years, the body mass index was 23.8 (23.1 to 25.2) kg/m.2 Five subjects suffered from severe AMS on the second day at 4559 m and received dexamethasone, 4 mg twice daily.
Sleep Studies
A total of 47 polysomnographic recordings could be analyzed; one recording was lost because of technical failure.
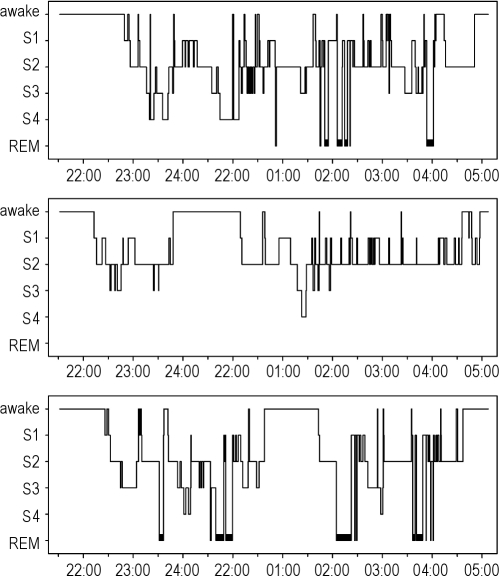
Baseline studies at 490 m revealed normal sleep, breathing patterns, and oxygen saturation (Table 1). On the first night at 4559 m, oxygen saturation was decreased, and a periodic breathing pattern with frequent central apneas/hypopneas was observed, predominantly in NREM sleep (Table 1, Figure 1). Minute ventilation had increased to almost one and a half of the value at 490 m, due to an increase in both tidal volume and breath rate. End-expiratory PCO2, a surrogate of arterial PCO2, was decreased (Table 1). Sleep analysis during the first night at 4559 m revealed major changes in the hypnograms (Figure 1). There were reductions in total sleep time, sleep efficiency, and slow wave sleep, while the total arousal index increased, although not in proportion to the number of apneas/hypopneas (Table 1, Figure 2).
Table 1.
Sleep study results
| Zurich 490 m | Margherita hut 1st night 4559 m | Margherita hut 3rd night 4559 m | |
|---|---|---|---|
| Time in bed (min) | 464 (456,484) | 485 (462,497) | 496*# (486,509) |
| Total sleep time (min) | 417 (387,439) | 320* (280,391) | 360* (282,392) |
| NREM stages 1 and 2 (%) | 73 (69,75) | 91* (88,93) | 78*# (70,90) |
| NREM stages 3 and 4 (%) | 18 (16,23) | 6* (4,7) | 11# (6,20) |
| REM (%) | 8 (6,12) | 3* (0,5) | 4# (1,12) |
| Sleep efficiency (%) | 93 (90,94) | 69* (64,80) | 75* (55,84) |
| Arousal index (/h) | 5.4 (3.5,7.3) | 17.9* (5.9,28.5) | 5.7# (2.7,15.1) |
| AHI TST (/h) | 0.1 (0,0.1) | 60.9* (21.4,79.2) | 86.5* (19.1,95.4) |
| AHI NREM (/h) | 3.1¶ (1.2,5.8) | 58.7* (20.1,81.4) | 91.3*¶ (20,102.2) |
| AHI REM (/h) | 5.5 (2.3,11.1) | 27.8 (0,103.9) | 2.1 (0,19.8) |
| Oxygen desaturation index (> 3% dips/h of time in bed) | 0.1 (0.4,2) | 31.8* (13.3,75.9) | 28.8* (13.7,51) |
| SpO2 (%) | 96 (95,96) | 67* (64,69) | 71*# (69,78) |
| End-tidal PCO2 (mmHg) | 41 (40,44) | 29* (23,31) | 29* (27,30) |
| Minute ventilation (L/min) | 4.4 (3.1,5.3) | 6.3* (5.3,8.6) | 5.1*# (4.8,6.7) |
| Tidal volume (mL) | 293 (209,300) | 335* (262,399) | 258 (225,334) |
| Breath rate (/min) | 16 (14,17) | 20* (19,22) | 20* (19,22) |
| Heart rate (/min) | 56 (50,61) | 81* (74,92) | 84* (75,89) |
N = 16. AHI TST, AHI NREM, AHI REM = apnea-hypopnea index during total sleep time, NREM, and REM sleep, respectively, SpO2 = mean oxygen saturation during sleep.
P < 0.05 vs. 490 m.
P < 0.05 vs. 4559 m 1st night.
P < 0.05 NREM vs. REM.
Figure 1.
The hypnogram obtained in a subject during a night at 490 m (top panel) shows a normal distribution of sleep stages and several NREM/REM sleep cycles. In contrast, the hypnogram recorded during the 1st night at 4559 m (middle panel) reveals predominantly superficial sleep stages with frequent awakenings, very rare deep sleep stages 3 and 4, and no REM sleep. The hypnogram from the 3rd night at 4559 m (bottom panel) reveals a partial restoration of normal sleep architecture.
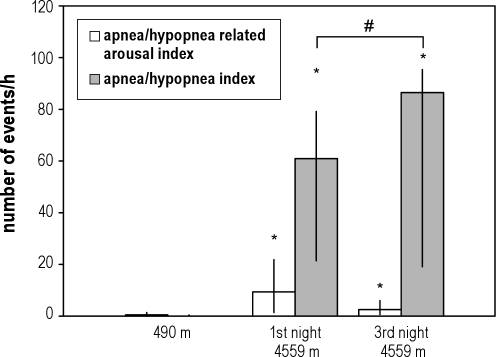
Figure 2.
Medians and quartile ranges (bars, vertical lines) of the apnea/hypopnea index (AHI) and the apnea/hypopnea related arousal index at 490 m and the 1st and the 3rd night at 4559 m. Only 11% and 4% of the apneas/hypopneas were followed by an arousal during the 1st and the 3rd nights, respectively. *P < 0.05 vs. 490 m, #P < 0.05 vs. the 1st night at 4559 m.
Repeated recordings obtained in the third night at altitude revealed a partial improvement of mean oxygen saturation (SpO2), associated with an increase in total sleep time, slow wave sleep, and REM sleep, and a reduction in the arousal index. However, the AHI increased further compared to the first night. Multiple regression analysis with percent slow wave sleep as the dependent variable and SpO2 and the AHI as the independent variables revealed a significant positive effect of SpO2 on slow wave sleep (β 0.38, P = 0.01) even after controlling for the AHI, while the negative association of the AHI and slow wave sleep did not quite reach statistical significance (β −0.26, P = 0.09). A separate analysis of data from the 5 subjects treated with dexamethasone on day 2 at 4559 m due to severe AMS revealed a major increase in oxygen saturation of 10% (quartile range 8,10) from 68% (67,69) in night 1 to 78% (76,81) in night 3 at 4559 m (P < 0.05). This increase significantly exceeded the corresponding increase of 3% (1,7) from 67% (64,70) in night 1 to 70% (68,71) in night 3 in the 11 subjects not receiving dexamethasone (P < 0.05). No change from night 1 to night 3 in any other variable listed in Tables 1 and 2 significantly differed between the 5 subjects receiving dexamethasone and the 11 subjects not receiving dexamethasone (data not shown).
Table 2.
Questionnaire evaluation
| Zurich 490 m | Margherita hut 1st night 4559 m | Margherita hut 3rd night 4559 m | |
|---|---|---|---|
| Lake Louise total score | 1 (0,1) | 6* (3,9) | 4*# (3,5) |
| Lake Louise question on insomnia | 1 (1,2) | 2* (2,3) | 2* (1,2) |
| SEQ “restlessness” | 15 (5,21) | 2* (1,6) | 18# (6,35) |
| SEQ “nocturnal wakefulness” | 14 (4,19) | 2* (1,5) | 20# (4,30) |
| SEQ “tired/alert” | 36 (28,51) | 31 (7,40) | 45# (19,80) |
N = 16. SEQ = Sleep Evaluation Questionnaire (mm).
P < 0.05 vs. 490 m.
P < 0.05 vs. 1st night at 4559 m.
Symptoms and Clinical Examination
On the morning after the first night at altitude, the Lake Louise Score indicated that subjects suffered from symptoms of AMS and impaired sleep quality, with a greater degree of perceived nocturnal wakefulness and restlessness (Table 2). These symptoms were partially relieved in the morning after the third night at 4559 m; the changes did not differ between the 5 subjects receiving dexamethasone and the remaining 11 subjects (data not shown).
DISCUSSION
Our study is the first to provide comprehensive physiologic data including neurophysiologic and cardiorespiratory variables along with subjectively perceived sleep quality and symptoms of AMS during short-term acclimatization to high altitude. Altitude induced hypoxemia during the first night at 4559 m which was associated with a reduction in total sleep time, slow wave sleep, and REM sleep, and an increased number of arousals. Breathing patterns were characterized by increased minute ventilation and frequent central apneas/hypopneas. Three days of acclimatization resulted in partial improvements of oxygen saturation and of alterations in sleep architecture in the third night at 4559 m despite a further increase in apneas/hypopneas, suggesting that periodic breathing was not the predominant cause of sleep disturbances at altitude. Symptoms of AMS and subjective sleep disturbances were prominent after arrival at 4559 m but improved significantly with acclimatization.
The few previous studies that assessed the effect of acute hypobaric hypoxia on sleep and breathing disturbances are difficult to compare because of different study settings (field versus hypobaric chamber studies), differences in ascent rates, modes of transportation (cable car versus climbing), and sleeping altitudes.5,6,8,9,24 Our field study performed in a relatively large number of subjects provides novel insights since it was performed in a realistic setting, i.e., study participants carried their own equipment, and the climbing rate and route were similar to that during recreational mountaineering in the Alps. Our results extend findings from some earlier hypobaric chamber studies suggesting a greater amount of superficial and fragmented sleep at a simulated altitude of 4000 m compared to sea level.7,8 In 12 soldiers pre-acclimatized to 1000 m, polysomnography performed in a mountain facility in the night after arrival at 3613 m revealed reductions in slow wave sleep and sleep efficiency but no change in REM sleep, and an increase in arousals compared to baseline studies at 1000 m.6 In 14 trekkers ascending from 1400 to 5000 m in Nepal over the course of 12 days, slow wave sleep and sleep efficiency were reduced at some but not all altitudes.9 Since this study comprised a prolonged exposure to increasing altitudes the effects of hypoxia and of acclimatization could not be separately assessed and compared to data from the current and the other cited studies.
The effects of altitude acclimatization on sleep have not been conclusively studied. Goldenberg et al.14 performed sleep studies in 12 subjects on day 4 and 24 at 4800 m. Six subjects were randomized to loprazolam and 6 to placebo. In the placebo group, acclimatization resulted in an increase of total sleep time and the percentage of NREM stage 3 and REM sleep. No data immediately after arrival was recorded. Zielinski et al.15 found no change in sleep variables in 9 subjects studied at 760 m and in the 1st and 6th night after arrival and stay at 3200 m. Correspondingly, Salvaggio et al.16 did not find any changes in sleep variables with acclimatization from day 8 to day 28 at 5050 m in 5 subjects.
In the current study, short-term acclimatization over 3 days at 4559 m resulted in a partial recovery of sleep structure with increases in slow wave sleep, REM sleep and a reduction in the arousal index. During the same period, the amount of periodic breathing persisted. These finding are consistent with our previous observations in climbers acclimatizing at 4433 m and 5530 m.10 Multiple regression analysis of data from the current study further revealed a positive effect of oxygen saturation on slow wave sleep at altitude after controlling for the AHI which is consistent with the hypothesis that acclimatization improved sleep quality via an independent effect on oxygen saturation despite a further increase in the AHI.The opposite trends of changes in the (increasing) apnea/hypopnea index and in the (decreasing) arousal index during acclimatization suggest that periodic breathing was not the predominant cause of sleep fragmentation at high altitude. Correspondingly, only a minor fraction of the apneas/hypopneas that occurred as part of high altitude periodic breathing was followed by an arousal (Figure 2), which is in line with previous observations.9,13
Consistent with a hypoxic stimulation of ventilation, we found an increase in minute ventilation and a decrease in PetCO2 in the first night at 4559 m. A high sensitivity to CO2 and hypoxia along with a reduced CO2 reserve are thought to be major determinants of periodic breathing. Thus, the increase in the apnea/hypopnea index in night 3 compared to night 1 at 4559 m despite a lesser degree of hypoxemia might be related to an increase in ventilatory drive induced by acclimatization.
Objective alterations in sleep structure and breathing instability at altitude were associated with subjective feelings of insomnia, nocturnal wakefulness, restless sleep and symptoms of AMS (Table 2). AMS may have contributed to the sleep disturbances in the first night at 4559 m and the improvements in slow wave sleep, REM sleep, sleep fragmentation and subjective sleep disturbances in parallel with reductions of the AMS score additionally supports an interaction between AMS and sleep quality. On day 2 at 4559 m, 5 of the 16 subjects were treated with dexamethasone for major symptoms of AMS. These subjects experienced a rapid relief of AMS symptoms and an increase in SpO2. Dexamethasone might therefore have influenced their sleep and breathing in night 3 to some extent either directly or by improving AMS and promoting acclimatization. The fact that similar trends of changes in objective sleep variables and improvements in subjective sleep quality and AMS were also observed in the majority of subjects not receiving dexamethasone underlines the independent effects of acclimatization.
In summary, our study in a relatively large group of healthy subjects provides new insights into the effects of high altitude acclimatization on sleep and nocturnal breathing. Our data demonstrate qualitative and quantitative alterations in sleep structure in the first night at altitude that are partially reversed after 3 days of acclimatization. Since periodic breathing persisted in the third night it is not the main determinant of high altitude sleep disturbances.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the subjects for study participation and the team of the Margherita hut for hospitality. The study was supported by a grant from the Zurich Center for Integrative Human Physiology (ZIHP), the Hartmann Müller foundation and the Fondazione Crivelli (Switzerland). Work for this study was performed at University Hospital Zurich.
REFERENCES
- 1.Jafarian S, Gorouhi F, Taghva A, Lotfi J. High-altitude sleep disturbance: results of the Groningen Sleep Quality Questionnaire survey. Sleep Med. 2008;9:446–9. doi: 10.1016/j.sleep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Anholm JD, Powles AC, Downey R, III, et al. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis. 1992;145:817–26. doi: 10.1164/ajrccm/145.4_Pt_1.817. [DOI] [PubMed] [Google Scholar]
- 3.Maggiorini M, Buhler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. BMJ. 1990;301:853–5. doi: 10.1136/bmj.301.6756.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussbaumer-Ochsner Y, Schuepfer N, Siebenmann C, Maggiorini M, Bloch KE. High altitude sleep disturbances monitored by actigraphy and polysomnography. High Alt Med Biol. 2011 doi: 10.1089/ham.2010.1073. In press. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno K, Asano K, Okudaira N. Sleep and respiration under acute hypobaric hypoxia. Jpn J Physiol. 1993;43:161–75. doi: 10.2170/jjphysiol.43.161. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont M, Batejat D, Pierard C, et al. Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude. Sleep. 2007;30:1527–33. doi: 10.1093/sleep/30.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaumont M, Batejat D, Coste O, et al. Effects of zolpidem and zaleplon on sleep, respiratory patterns and performance at a simulated altitude of 4,000 m. Neuropsychobiology. 2004;49:154–62. doi: 10.1159/000076723. [DOI] [PubMed] [Google Scholar]
- 8.Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med. 1996;153:1864–9. doi: 10.1164/ajrccm.153.6.8665047. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PL, Edwards N, Burgess KR, Sullivan CE. Sleep architecture changes during a trek from 1400 to 5000 m in the Nepal Himalaya. J Sleep Res. 2010;19:148–56. doi: 10.1111/j.1365-2869.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Bloch KE, Latshang TD, Turk AJ, et al. Nocturnal periodic breathing during acclimatization at very high altitude at Mount Muztagh Ata (7,546 m) Am J Respir Crit Care Med. 2010;182:562–8. doi: 10.1164/rccm.200911-1694OC. [DOI] [PubMed] [Google Scholar]
- 11.Erba P, Anastasi S, Senn O, Maggiorini M, Bloch KE. Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur Respir J. 2004;24:303–8. doi: 10.1183/09031936.04.00006504. [DOI] [PubMed] [Google Scholar]
- 12.Kohler M, Kriemler S, Wilhelm EM, Brunner-Larocca H, Zehnder M, Bloch KE. Children at high altitude have less nocturnal periodic breathing than adults. Eur Respir J. 2008;32:189–97. doi: 10.1183/09031936.00119807. [DOI] [PubMed] [Google Scholar]
- 13.Khoo MC, Anholm JD, Ko SW, et al. Dynamics of periodic breathing and arousal during sleep at extreme altitude. Respir Physiol. 1996;103:33–43. doi: 10.1016/0034-5687(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg F, Richalet JP, Jouhandin M, Gisquet A, Keromes A, Larmignat P. Periodic respiration during sleep at high altitude. Effects of a hypnotic benzodiazepine, loprazolam. Presse Med. 1988;17:471–4. [PubMed] [Google Scholar]
- 15.Zielinski J, Koziej M, Mankowski M, et al. The quality of sleep and periodic breathing in healthy subjects at an altitude of 3,200 m. High Alt Med Biol. 1:331–6. doi: 10.1089/15270290050502408. [DOI] [PubMed] [Google Scholar]
- 16.Salvaggio A, Insalaco G, Marrone O, et al. Effects of high-altitude periodic breathing on sleep and arterial oxyhaemoglobin saturation. Eur Respir J. 1998;12:408–13. doi: 10.1183/09031936.98.12020408. [DOI] [PubMed] [Google Scholar]
- 17.Clarenbach CF, Senn O, Brack T, Kohler M, Bloch KE. Monitoring of ventilation during exercise by a portable respiratory inductive plethysmograph. Chest. 2005;128:1282–90. doi: 10.1378/chest.128.3.1282. [DOI] [PubMed] [Google Scholar]
- 18.Brack T, Thuer I, Clarenbach CF, et al. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest. 2007;132:1463–71. doi: 10.1378/chest.07-0121. [DOI] [PubMed] [Google Scholar]
- 19.Sackner MA, Watson H, Belsito AS, et al. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol. 1989;66:410–20. doi: 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. Washington, DC: Public Health Service, U.S. Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 21.Parrott AC, Hindmarch I. Factor analysis of a sleep evaluation questionnaire. Psychol Med. 1978;8:325–9. doi: 10.1017/s0033291700014379. [DOI] [PubMed] [Google Scholar]
- 22.Tarrasch R, Laudon M, Zisapel N. Cross-cultural validation of the Leeds sleep evaluation questionnaire (LSEQ) in insomnia patients. Hum Psychopharmacol. 2003;18:603–10. doi: 10.1002/hup.534. [DOI] [PubMed] [Google Scholar]
- 23.Maggiorini M, Muller A, Hofstetter D, Bartsch P, Oelz O. Assessment of acute mountain sickness by different score protocols in the Swiss Alps. Aviat Space Environ Med. 1998;69:1186–92. [PubMed] [Google Scholar]
- 24.Nicholson AN, Smith PA, Stone BM, Bradwell AR, Coote JH. Altitude insomnia: studies during an expedition to the Himalayas. Sleep. 1988;11:354–61. doi: 10.1093/sleep/11.4.354. [DOI] [PubMed] [Google Scholar]