Abstract
Semi-preparative high-speed counter-current chromatography (HSCCC) was successfully performed for isolation and purification of caffeic acid 4 bioactive flavonoids from Eupatorium adenophorum Spreng using stepwise elution with a pair of two-phase solvent systems composed of ethyl acetate–methanol–water at the volume ratios of 10: 1: 10 and 5: 1: 5 (v/v). From 378.5 mg of crude extract 24.1 mg of caffeic acid, 6.7 mg of 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoylglucopyranoside), 6.5 mg of quercetagetin 7-O-(6”-O-acetyl-β-D-glucopyranoside), 31.8 mg of eupalitin 3-O-β-D-galactopyranoside and 36.7 mg of eupalitin were obtained with the purities of 96.0%, 91.2%, 82.3%, 95.1% and 85.6%, respectively. The structures of the separated compounds were identified by EI-MS, 1HNMR and 13CNMR.
Keywords: Stepwise high-speed counter-current chromatography, 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoylglucopyranoside), Eupalitin, Quercetagetin 7-O-(6”-O-acetyl-β-D-glucopyranoside), Eupalitin 3-O-β-D-galactopyranoside, Eupatorium adenophorum Spreng
INTRODUCTION
Eupatorium adenophorum Spreng native in Central America, mainly in Mexico is a toxic weed which causes damage of many farmlands, pasture fields and forests due to its strong allelopathy which inhibits growth of other plants with a strong ability to adapt to different environmental conditions [1]. Since the 1950s, outbreak of this Crofton weed mainly in the southwest of China has become serious, producing the damage of native ecosystems and causing great economic losses. Therefore, the government has tried to eradicate this weed using several methods including manual, chemical and biological methods without significant progress [2, 3].
However, utilization of the weed gives advantages over simple control processes. As a natural plant, E. adenophorum Spreng has some bioactive components. Anti-oxidant properties and anti-bacterial bioactivities of flavonoids 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside), quercetagetin 7-O-(6”-O-acetyl-β-D-glucopy-ranoside) and eupalitin 3-O-β-D-galactopyranoside have been reported [4]. Previously bioassays have shown that caffeic acid has considerable anti-oxidative properties with both radical-scavenging and metal-chelating abilities [5]. Eupalitin is a kind of flavonoids, which are effective components of some medicines [6].
High-speed counter-current chromatography (HSCCC), being a support-free liquid-liquid partition method [7], eliminates irreversible adsorption of sample onto the solid support [8], and it has been widely used in preparative separation of active compounds from traditional Chinese herbs and other natural products [9]. In our previous work [10] caffeic acid was separated from E. adenophorum Spreng and applied in the synthesis of caffeic acid-intercalated layered double hydroxide. In this study only one pure component (Caffeic acid) was obtained using an isocratic elution by HSCCC. However, the use of HSCCC for the isolation and purification of the bioactive components from E. adenophorum Spreng was improved in the present paper in such a way that five bioactive components could be purified by stepwise elution HSCCC by one step operation.
One often confronts with some target components that have large partition coefficient to retain in the column for a long period of time. In this case chromatographic condition can be modified to facilitate elution of these compounds without loss of peak resolution: One is called stepwise elution which is effectively used in the purification of natural products to improve the separation and purification as previously reported [11]. The other method is called elution-extrusion countercurrent chromatography that has been found extremely suitable for high-throughput separation [12]. The present paper describes the successful preparative separation and purification of caffeic acid and four bioactive components including 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoylglucopyranoside), quercetagetin 7-O-(6”-O-acetyl-β-D-glucopyranoside), eupalitin 3-O-β-D-gal-actopyranoside and eupalitin from E. adenophorum Spreng by stepwise elution HSCCC.
EXPERIMENTAL
Apparatus
Semi-preparative HSCCC was performed using a Model GS10A multilayer coil planet centrifuge (Beijing Institute of New Technology Application, Beijing, China) equipped with a polytetrafluoroethylene multilayer coil of 110 m × 1.6 mm I.D. with a total capacity of 230 ml. The β value of the preparative column ranged from 0.5 to 0.8. The solvent was pumped into the column with a Model NS-1007 constant-flow pump (Beijing Institute of New Technology Application, Beijing, China). Continuous monitoring of the effluent was achieved with a Model 8823A-UV Monitor (Beijing Institute of New Technology Application, Beijing, China) at 254 nm. A manual sample injection valve with a 10 ml loop (for the preparative HSCCC) was used to introduce the sample into the column. A portable recorder Yokogawa Model 3057 (Sichuan Instrument Factory, Chongqing, China) was used to plot the chromatogram. A rotary evaporator was also used.
The high-performance liquid chromatography (HPLC) equipment used was a Shimadzu LC-20A system including two LC-20A solvent delivery units, an SPD-M20A UV-VIS photodiode array detector (DAD), a Model 7725 injection valve with a 20 μl loop, an SCL-20A system controller, and a Class-VP-LC workstation (Shimadzu, Kyoto, Japan).
Reagents
All organic solvents used for HSCCC were of analytical grades and purchased from Beijing Chemical Factory (Beijing, China). Methanol used for HPLC analysis was of chromatographic grade and purchased from Tianjin Huaxi Special Reagent Factory (Tianjin, China).
E. adenophorum Spreng was obtained from Institute of Environment and Sustainable Development in Agriculture, The Chinese Academy of Agricultural Sciences, and identified by Dr. Guoliang Zhang. Caffeic acid and other standards were purchased from National Institute for the Control of Pharmaceutical & Biological Products (Beijing, China).
Preparation of Crude Extract of E. Adenophorum Spreng
About 20 g of dried powders of E. adenophorum Spreng was extracted (refluxed) for 1.5 h once with 300 ml of 95% aqueous ethanol, and the extract was filtered by a six-layer pledget. The filtrate was concentrated to dryness under reduced pressure yielding 1.10 g of a crude sample, which was subsequently used for HSCCC isolation.
Preparation of Two-phase Solvent System and Sample Solutions
The solvent system utilized in the present study was prepared by thoroughly equilibrating ethyl acetate–methanol–water (100: 1: 100, 50: 1: 50, 10: 1: 10 or 5: 1: 5, v/v) in a separatory funnel at room temperature and the two phases separated shortly before use.
The sample solutions were prepared by dissolving the crude extract in the lower phase at suitable concentrations according to the preparative purpose.
Measurement of Partition Coefficients (K)
The K values were determined as follows: each solvent mixture was thoroughly equilibrated in a test tube and the two phases were separated, 2 ml of each pre-equilibrated phase was delivered into another test tube to which about 2 mg of the sample standard was added. The test tube was shaken vigorously to equilibrate the contents. Then 1 ml of each phase was evaporated to dryness and the residues dissolved in 1 ml of methanol-water (45: 55, v/v) were analyzed by HPLC. The K value was expressed as the peak areas in the upper phase divided by that in the lower phase. The measurement was carried out at 35°C.
Separation Procedure
Semi-preparative HSCCC was performed with a Model GS10A HSCCC instrument as follows: the multilayer coiled column was first entirely filled with the stationary upper phase of the first solvent system followed by elution of the lower phase through the head end of the column at a flow-rate of 2 ml/min, while the apparatus was rotated at 800 rpm. After hydrodynamic equilibrium was reached, as indicated by a clear mobile phase eluting at the tail outlet, the sample solution (378.5 mg in 10 ml of lower phase of the first solvent system) was injected through the injection valve. After an appropriate time of elution, the mobile phase was switched to the lower phase of the second solvent system. The effluent from the outlet of the column was continuously monitored with a UV detector at 254 nm. Each peak fractions was collected according to the chromatogram.
HPLC Analyses and Identification of HSCCC Peak Fractions
The crude extract of E. adenophorum Spreng and HSCCC peak fractions were all analyzed by HPLC. The analyses were performed with a Shimadzu ODS column (150 mm × 4.6 mm I.D.). The mobile phase composed of methanol-0.05% phosphoric acid water (50: 50, v/v) was isocratically eluted at a flow-rate of 0.5 ml/min and the effluent was monitored at 254 nm by a DAD detector.
Identification of the target compounds was based on MS, 1H-NMR and 13C-NMR spectra.
RESULTS AND DISCUSSION
HPLC Analyses
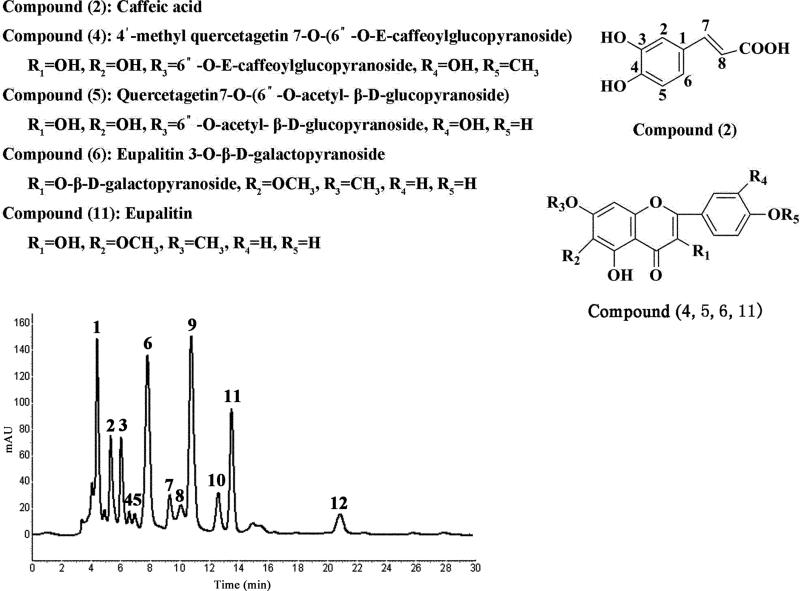
As shown in Fig. 1, HPLC analysis of the crude extract of E. adenophorum Spreng mainly produced over 12 peaks, among which the peaks 2, 4, 5, 6 and 11 correspond to caffeic acid, 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside), quercetagetin 7-O-(6”-O- acetyl -β-D-glucopyranoside), eupalitin 3-O-β-D-galactopyranoside and eupalitin with the contents of 6.7%, 1.8%, 1.8%, 17.2% and 9.7%, respectively, based on HPLC analysis compared with the external standard.
Fig. 1.
HPLC analyses of the crude extract of E. adenophorum Spreng. HPLC conditions: a Shimadzu ODS column (150 mm × 4.6 mm I.D.). Mobile phase: methanol-0.05% phosphoric acid water (50: 50, v/v), flow-rate: 0.5 ml/min, monitored at 254nm by a DAD detector. Peak 2: caffeic acid; Peak 4: 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside); Peak 5: quercetagetin 7-O-(6”-O-acetyl-β-D- glucopyranoside); Peak 6: eupalitin 3-O-β-D-galactopyranoside; Peak 11: eupalitin.
Partition Coefficients and HSCCC Purification
In order to achieve an efficient resolution of target compounds, a two-phase solvent system composed of ethyl acetate–methanol–water was examined using semi-preparative HSCCC by varying the mutual volume ratio, since this solvent system has been successfully applied to separate various samples with a moderate degree of polarity [13–16]. In the present study, the K values of the five compounds were determined for selecting suitable solvent systems (Table 1).
Table 1.
Partition coefficient (K) of the compounds in different solvent systems
| Ethyl acetate–methanol–water (v/v) | Partition coefficient (K) |
||||
|---|---|---|---|---|---|
| Compound 2 | Compound 4 | Compound 5 | Compound 6 | Compound 11 | |
| 5:1:5 | 0.777 | 0.936 | 1.367 | 2.998 | 7.703 |
| 10:1:10 | 0.808 | 1.043 | 1.623 | 15.82 | 29.51 |
| 50:1:50 | 0.745 | 0.891 | 0.949 | 30.58 | 63.91 |
| 100:1:100 | 0.351 | 0.370 | 0.445 | 9.384 | 11.92 |
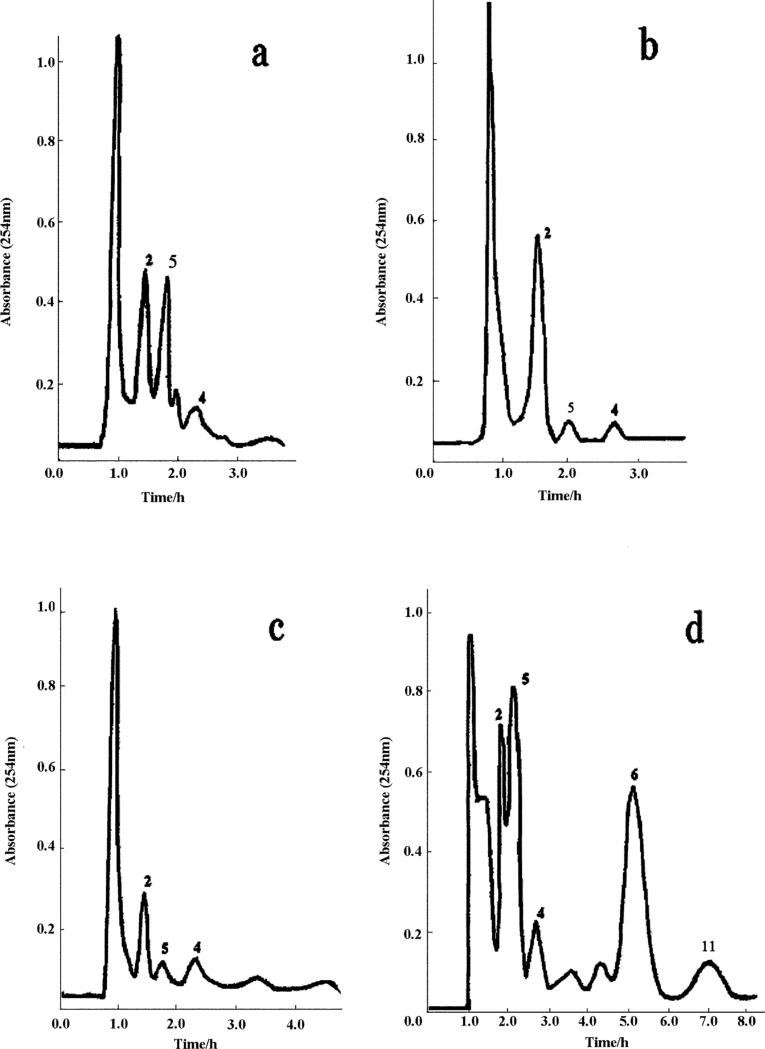
After trying all of the above solvent systems, a set of the two-phase solvent systems composed of ethyl acetate–methanol–water (10: 1: 10, v/v) and (5: 1: 5, v/v) was finally chosen for stepwise elution. The results were illustrated in Fig. 2a, 2b, 2c and 2d. Although Fig. 2a and Fig. 2b showed that the two-phase solvent system composed of ethyl acetate–methanol–water could separate and purify 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside) (peak 4) and quercetagetin 7-O-(6”-O-acetyl-β-D- glucopyranoside) (peak 5) at the volume ratio (100: 1: 100, v/v) or (50: 1: 50, v/v), the compound caffeic acid (peak 2) was mixed with unknown compounds as indicated by the HPLC analysis. As seen in Fig. 2c, using two-phase solvent system composed of ethyl acetate–methanol–water (10:1:10, v/v), caffeic acid (peak 2), 4'-methyl quercetagetin 7-O-(6”-O-E- caffeoyl glucopyranoside) (peak 4) and quercetagetin 7-O-(6”-O- acetyl-β-D-glucopyranoside) (peak 5) were purified from polar impurities while eupalitin 3-O-β-D-galactopyranoside (peak 6) and eupalitin (peak 11) were long retained in the column. On the other hand, using the solvent system composed of ethyl acetate– methanol–water (5: 1: 5, v/v) five target compounds were separated in a short elution time as shown in Fig. 2d, while caffeic acid (peak 2) was contaminated with unknown impurities as seen in the HPLC analysis. These results suggested that the combined use of these two solvent systems in stepwise elution could provide an excellent purification of these five target compounds. This strategy was successfully demonstrated in Fig. 3 where the crude extract was first eluted with the solvent system with a volume ratio at 10: 1: 10 (v/v) until all polar impurities were eluted out followed by the elution with the second solvent system with a volume ratio at 5: 1: 5 (v/v). In this way these five components were purified within a short period of time.
Fig. 2.
HSCCC chromatograms for optimization suitable solvent systems for separation of the targets from the crude extract of E. adenophorum Spreng. Solvent system: a. ethyl acetate–methanol–water at volume ratio of (100: 1: 100, v/v); b. ethyl acetate–methanol–water at volume ratio of (50: 1: 50, v/v); c. ethyl acetate–methanol–water at volume ratio of (10: 1: 10, v/v); d. ethyl acetate–methanol–water at volume ratio of (5: 1: 5, v/v). Stationary phase: organic phase; mobile phase: lower aqueous phase. Flow-rate: 2.0 ml/min; revolution speed: 800 rpm; sample: 378.5 mg dissolved in 10 ml lower phase. Retentions of the stationary phase of these four solvent systems were 69.6%, 69.6%, 65.2% and 56.5%, respectively.
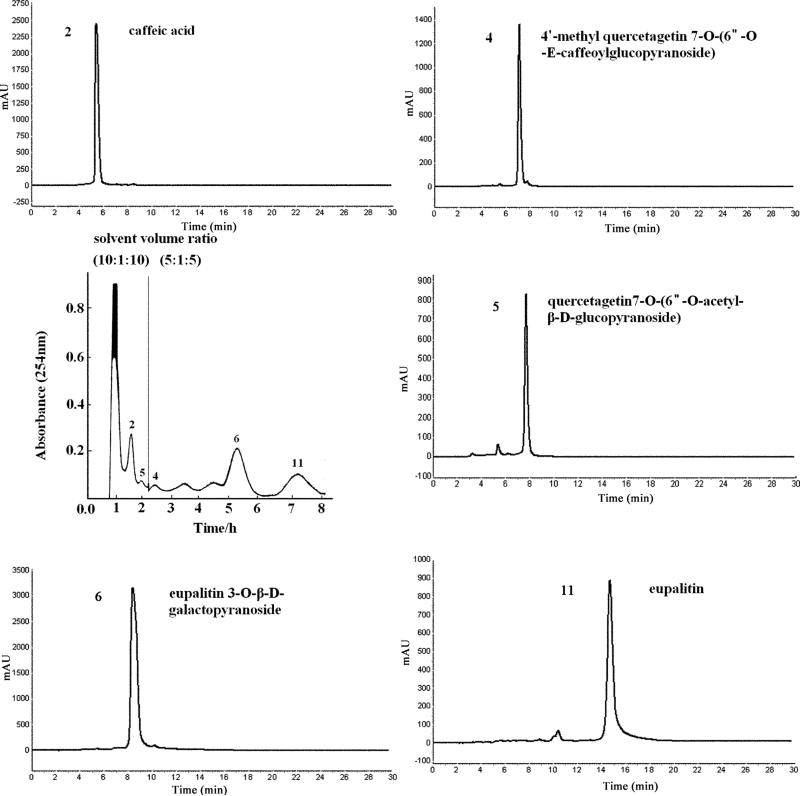
Fig. 3.
HSCCC chromatogram of the crude extract of E. adenophorum Spreng and the HPLC chromatograms of the separated compounds. HSCCC stepwise elution with solvent systems 1 and 2 was used. Solvent system 1: ethyl acetate–methanol–water (10: 1: 10, v/v), solvent system 2: ethyl acetate– methanol–water (5: 1: 5, v/v); stationary phase: upper organic phase of solvent system 1; mobile phase: 330 ml of lower aqueous phase of solvent system 1 and 750 ml of lower aqueous phase of solvent system 2; flow-rate: 2.0 ml/min; revolution speed: 800 rpm, sample: 378.5 mg dissolved in 10 ml of the lower phase of solvent system 1.
This stepwise elution was applied for the preparative separation of 378.5 mg of the crude extract of E. adenophorum Spreng. As shown in Fig. 3, the separation was started with the first solvent system composed of ethyl acetate–methanol–water (10: 1: 10, v/v) and, after caffeic acid (peak 2) and quercetagetin 7-O-(6”-O-acetyl-β-D- glucopyranoside) (peak 5) were eluted (2 hours and 45 minutes), the mobile phase was switched to the second solvent system composed of ethyl acetate–methanol–water (5: 1: 5, v/v). Then, 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside) (peak 4), eupalitin 3-O-β-D-galactopyranoside (peak 6) and eupalitin (peak 11) were eluted out in this order with well resolved peaks.
Purity Determination and Chemical Structure Identification of HSCCC Fractions
The HSCCC fractions were collected and evaporated to dryness under reduced pressure, yielding 24.1 mg of fraction 2, 6.7 mg of fraction 4, 6.5 mg of fraction 5, 31.8 mg of fraction 6 and 36.7 mg of fraction 11 with the purities of 96.0%, 91.2%, 82.3%, 95.1% and 85.6%, respectively, based on HPLC analysis (Fig. 3).
The structural identification of these five fractions was carried out by MS, 1H-NMR and 13C-NMR spectra as follows:
Fraction 2: EI-MS m/z: 180, 163, 136, 89, 77. 13C-NMR (DMSO-d6) δ: 168.9 (C-9), 148.8 (C-4), 146.2 (C-3), 145.5 (C-7), 126.7 (C-1),121.9 (C-6), 116.6 (C-5), 115.9 (C-8), 115.5 (C-2). 1H-NMR (DMSO-d6) δ:12.132 (1H, COOH), 7.703 (1H, H-8), 7.519 (1H, H-7), 6.897 (1H, H-6), 6.883 (1H, H-5), 6.916 (1H, H-2), 5.012 (1H, 3-OH), 4.999 (1H, 4-OH). Compared with the data given in [17], this compound was identified as caffeic acid.
Fraction 4: FAB-MS m/z: 695, 679, 347, 333 and 163. 1H-NMR (CD3OD): 7.681 (1H, H-2'), 7.651 (1H, H-6'), 7.450 (1H, H-3”'), 6.861 (1H, H-5”'), 6.561 (1H, H-9”'), 6.492 (1H, H- 8”'), 6.171 (1H, H-2”'), 5.061 (1H, H-1” ), 4.301 and 4.243 (1H each, H2-6”), 3.862 (3H, 4'-OCH3).
Fraction 5: FAB-MS m/z: 523, 319. 1H-NMR (CD3OD): 7.722 (1H, H-2'), 7.631 (1H, H-6'), 6.871 (1H, H-5'), 6.862 (1H, H-8), 5.012 (1H, H-1”), 4.491 and 4.201 (1H each, H2-6”), 2.030 (3H, 6”-OCOCH3). Compared with the data given in [18], the two components were identified as 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoyl glucopyranoside) and quercetagetin 7-O-(6”-O-acetyl-β-D- glucopyranoside) respectively.
Fraction 6: FAB-MS m/z: 331. 13C-NMR (pyridine-d5) δ: 60.9 (6-OCH3), 57.3 (7-OCH3), 157.7 (C-2), 135 (C-3), 179.1 (C-4), 153.0 (C-5), 132.8 (C-6), 159.4 (C-7), 92.1 (C-8), 121.9 (C-1'), 131.9 (C-2', C-6'), 116.1(C-3', C-5'), 161.7 (C-4'), 104.3 (C-1”), 73.7 (C-2”), 75.3 (C-3”), 69.8 (C-4”), 77.5 (C-5”), 61.8 (C-6). 1H-NMR (DMSO-d6) δ: 12.560 (1H, 5-OH), 8.111 (2H, H-2' and H-6'), 6.880 (2H, H-3' and H-5'), 6.601 (1H, H-8), 3.751 (3H, 6-OCH3), 3.930 (3H, 7-OCH3, 5.411 (1H, H-1”), 4.141-6.701 (sugar protons).
Fraction 11: FAB-MS m/z: 493. 13C-NMR (pyridine-d5) δ: 60.9 (6-OCH3), 57.3 (7-OCH3), 157.7 (C-2), 135.1 (C-3) , 179.0 (C-4), 153.0 (C-5), 132.7 (C-6), 159.3 (C-7), 92.1 (C-8), 121.9 (C-1'), 131.9 (C-2', C-6'), 116.1(C-3', C-5'), 161.7 (C-4'), 61.8 (C-6). 1H-NMR (DMSO-d6) δ: 12.451 (1H, 5-OH), 8.251 (2H, H-2' and H-6'), 7.082 (2H, H-3' and H-5'), 6.971 (1H, H-8), 4.061 (3H, 7-OCH3), 3.902 (3H, 6-OCH3). Compared with the data given in [19], the two chemicals were identified to be eupalitin 3-O-β-D-galactopyranoside and eupalitin.
CONCLUSION
In order to get a satisfactory separation of the five bioactive components by HSCCC, stepwise elution was successfully performed with a pair of two-phase solvent systems composed of ethyl acetate–methanol–water at volume ratios of 10: 1: 10 and 5: 1: 5 (v/v). Using a semi- preparative unit of the HSCCC centrifuge, about a 378.5 mg amount of the crude extract was separated to yield 24.1 mg of caffeic acid, 6.7 mg of 4'-methyl quercetagetin 7-O-(6”-O-E-caffeoylglucopyranoside), 6.5 mg of quercetagetin 7-O-(6”-O-acetyl-β-D-glucopyranoside), 31.8 mg of eupalitin 3-O-β-D-galactopyranoside and 36.7 mg of eupalitin. The present study demonstrates that the stepwise elution mode can be successfully used in HSCCC to improve the purification of natural products.
ACKNOWLEDGMENTS
Financial support from Ministry of Agriculture of the people's republic of China (Project 200803022) and State Key Laboratory of Chemical Resource Engineering (Project CRE-B-2008104) are gratefully acknowledged.
REFERENCES
- 1.Zhao XB, Zhang LH, Liu DH. Comparative study on chemical pretreatment methods for improving enzymatic digestibility of crofton weed stem. Bioresource Technol. 2008;99(9):3729–3736. doi: 10.1016/j.biortech.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Gu WB, Sang WG, Ling HB, Axmacher JC. Effects of Crofton weed Ageratina adenophora on assemblages of Carabidae (Coleoptera) in the Yunnan Province, South China. Agric. Ecosyst. Environ. 2008;124(3-4):173–178. [Google Scholar]
- 3.Wang L, Qin R.H. Research Advances Related to the Invasive Herb Eupatorium adenophorum. J. Southwest For. Coll. 2004;24(3):72–75. [Google Scholar]
- 4.Liang D, Zhang BD. The new extraction and isolation progress of flavonoids. J. Zhoukou Norm. Univ. 2007;24(5):87–89. [Google Scholar]
- 5.Horn AF, Nielsen NS, Jacobsen C. Additions of caffeic acid, ascorbyl palmitate or c-tocopherol to fish oil-enriched energy bars affect lipid oxidation differently. Food Chem. 2009;112(2):412–420. [Google Scholar]
- 6.Gao DJ, Tian Y, Liang FH, Bi SY, Li TC, Chen YH, Zhang HQ, Yu AM. Investigation on hyperin–cetyltrimethylammonium bromide–fibronectin system by resonance light-scattering technique. Spectrochim. Acta, Part A. 2007;66(1):52–57. doi: 10.1016/j.saa.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Gutzeit D, Winterhalter P, Jerz G. Application of preparative high-speed counter-current chromatography/electrospray ionization mass spectrometry for a fast screening and fractionation of polyphenols. J. Chromatogr. A. 2007;1172(1):40–46. doi: 10.1016/j.chroma.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y. Recent advances in counter-current chromatography. J. Chromatogr. 1991;538(1):3–25. doi: 10.1016/s0021-9673(01)91617-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhou TT, Fan GR. Application of high--speed counter--current chromatography in the preparation of active components in natural products. J. Chin. Pharm. Univ. 2007;38(5):391–395. [Google Scholar]
- 10.Wei Y, Gao YL, Zhang K, Ito Y. Isolation of caffeic acid from eupatorium adenophorum spreng by high-speed countercurrent chromatography and synthesis of caffeic acid-intercalated layered double hydroxide. J. Liq. Chromatogr. R. T. 2010;33(6):837–845. doi: 10.1080/10826071003684471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Zhang TY, Ito Y. Preparative isolation of osthol and xanthotoxol from Common Cnidium Fruit (Chinese traditional herb) using stepwise elution by high-speed counter-current chromatography. J. Chromatogr. A. 2004;1033(2):373–377. doi: 10.1016/j.chroma.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 12.Lu YB, Ma WY, Hu RL, Berthod A, Pan YJ. Rapid and preparative separation of traditional Chinese medicine Evodia rutaecarpa employing elution-extrusion and back-extrusion counter-current chromatography: Comparative study. J. Chromatogr. A. 2009;1216(19):4140–4146. doi: 10.1016/j.chroma.2008.10.095. [DOI] [PubMed] [Google Scholar]
- 13.Wu HK, Su Z, Yang Y, Ba H, Akber, Aisa HJ. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss. et Huet. by high-speed counter-current chromatography. J. Chromatogr. A. 2007;1176(1-2):217–222. doi: 10.1016/j.chroma.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Xie QQ, Wei Y, Zhang GL. Separation of flavonol glycosides from Flaveria bidentis (L.) Kuntze by high-speed counter-current chromatography. Sep. Purif. Technol. 2010;72(2):229–233. [Google Scholar]
- 15.Zhao MB, Ito Y, Tu PF. Isolation of a novel flavanone 6-glucoside from the flowers of Carthamus tinctorium (Honghua) by high-speed counter-current chromatography. J. Chromatogr. A. 2005;1090(1-2):193–196. doi: 10.1016/j.chroma.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Wei Y, Xie QQ, Dong WT, Ito Y. Separation of epigallocatechin and flavonoids from Hypericum perforatum L. by high-speed counter-current chromatography and preparative high-performance liquid chromatography. J. Chromatogr. A. 2009;1216(19):4313–4318. doi: 10.1016/j.chroma.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YM, Wang TZ, Wang ZX. Studies on Chemical Constituents in Dried Buds of Lonicera similis Hemsl. China. J. Chi. Mater. Medica. 2001;26(1):45–47. [PubMed] [Google Scholar]
- 18.Ramachandran Nair, A.G., Gunasegaran R, Krishnan S, Bayet C, Voirin B. Flavonol glycosides from leaves of Eupatorium Glandulosum. Phytochem. 1995;40(1):283–285. [Google Scholar]
- 19.Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S. Int. Immunopharmacol. 2005;5(3):541–553. doi: 10.1016/j.intimp.2004.11.001. [DOI] [PubMed] [Google Scholar]