Abstract
The morphogenesis of Drosophila sensory bristles is dependent on the function of their actin and microtubule cytoskeleton. Actin filaments are important for bristle shape and elongation, while microtubules are thought to mediate protein and membrane trafficking to promote growth. We have identified an essential role for the bristle cuticle in the maintenance of bristle structure and shape at late stages of bristle development. We show that the small GTPase Rab11 mediates the organized deposition of chitin, a major cuticle component in bristles, and disrupting Rab11 function leads to phenotypes that result from bristle collapse rather than a failure to elongate. We further establish that Rab11 is required for the plasma membrane localization of the ZP domain-containing Dusky-like (Dyl) protein and that Dyl is also required for cuticle formation in bristles. Our data argue that Dyl functions as a Rab11 effector for mediating the attachment of the bristle cell membrane to chitin to establish a stable cuticle. Our studies also implicate the exocyst as a Rab11 effector in this process and that Rab11 trafficking along the bristle shaft is mediated by microtubules.
Keywords: Drosophila, Rab11, Dyl, Microtubules, Bristle, Chitin, Cuticle, Growth, Transport, p150glued
INTRODUCTION
Polarized extensions of epidermal cells, such as the sensory bristles, arista laterals and wing hairs of Drosophila melanogaster, are good model cell types for studying the processes that govern cytoskeleton-mediated morphogenesis (Fei et al., 2002; Guild et al., 2005; Tilney et al., 2004; Tilney et al., 1995; Tilney and DeRosier, 2005; Turner and Adler, 1998). However, little is known about the mechanisms that mediate membrane and protein transport during the growth of these structures. Here we report that decreased function in components of the intracellular trafficking and secretion machinery, such as the Rab11 GTPase, produces a dramatic mutant phenotype by a novel mechanism, namely bristle collapse, and that this is associated with a defect in cuticle deposition.
Time-lapse studies have shown that, except for the initial stages of bristle development when growth is primarily isotropic, bristle growth is highly polarized in the axial direction (Fei et al., 2002). A variety of genetic and inhibitor studies argue that actin and microtubules play different roles in bristle growth (Cant et al., 1994; Fei et al., 2002; He and Adler, 2001; Petersen et al., 1994; Tilney et al., 2000a; Turner and Adler, 1998). Large bundles of F-actin help to maintain the shape of the bristle, which is long and tapered with a small but reproducible curve, and the polymerization of actin at the tip has been thought to drive extension (Tilney et al., 1996; Tilney et al., 2000b). The microtubule cytoskeleton has been suggested to function in the transport of material along the proximal distal axis of the bristle (Fei et al., 2002; Guild et al., 2002; Tilney et al., 2000a). Once the bristle has reached its full length the actin filament bundles disappear and bristle shape is thought to be maintained by the chitinous exoskeleton (Tilney et al., 1996).
Rab11 plays an important role in polarized transport in epithelial cells (Wang et al., 2000), in protein recycling from endosomes to the plasma membrane and in the trans-Golgi network (Band et al., 2002; Hales et al., 2001; Schlierf et al., 2000; Ullrich et al., 1996; Volpicelli et al., 2002; Wilcke et al., 2000). Drosophila Rab11 mediates the transport of rhodopsin to the apical plasma membrane domain of growing rhabdomeres (Satoh et al., 2005), is required for membrane recycling during cellularization (Pelissier et al., 2003), organizes microtubule polarity in oocytes (Dollar et al., 2002) and is required for the asymmetric cell divisions that give rise to the cells of the bristle sensory organ (Emery et al., 2005). A role in bristle morphogenesis is implied by the bristle morphology phenotypes of hypomorphic Rab11 genotypes (Jankovics et al., 2001).
We have examined the role of Rab11 in bristle morphogenesis, making use of the controlled expression of Rab11 double-stranded (ds) RNA to knock down Rab11 function after the formation of the sense organ lineage. This treatment led to the macrochaetae being reduced to short stubs. Surprisingly, in vivo imaging revealed that this extreme phenotype did not result from a failure in bristle elongation but was due to a failure in stability, as bristles expressing Rab11 dsRNA grew and then collapsed. This was correlated with defects in the organization of chitin bands in the bristle cuticle, suggesting an important role for Rab11 in the secretion and patterned deposition of chitin. Our studies further implicate Dusky-like (Dyl), a ZP domain-containing protein, as a Rab11 effector for chitin deposition. Previous studies found that dyl functions in the formation of embryonic denticles, likely by mediating the connection between the apical plasma membrane and the forming cuticle (Fernandes et al., 2010). We found that dyl was required for the organized deposition of chitin in bristles and further that Rab11 function was required for the plasma membrane localization of Dyl. The exocyst is known to be a Rab11 effector in many contexts (Emery et al., 2005; He and Guo, 2009; Langevin et al., 2005). We found that knocking down the expression of exocyst components resulted in a similar stub bristle phenotype, suggesting that is also involved in chitin deposition. Our data also establish that the intracellular transport of Rab11 in growing bristles is dependent on microtubules.
MATERIALS AND METHODS
Fly culture and strains
All flies were grown on standard media. Oregon R was used as a wild-type control. The kkv, Rab11 and dyl mutant and deficiency lines were obtained from the Bloomington Drosophila Stock Center (BDSC) at Indiana University. Most of the stocks used for generating flp/FRT somatic clones, UAS-GFP-Rab11, UAS-GFP-Rab11, neur-Gal4, ap-Gal4 and ptc-Gal4 were also obtained from the BDSC. The UAS-Rab11dsRNA.pWIZ stock was provided by D. Ready (Purdue University); Rab1193Bi/TM6, Rab11ex1/TM6, Rab11ΔFRT/TM6, and y w hs-flp; FRT5377 hrp-GFP/TM3 by R. Cohen (University of Kansas); UAS-Klp10A by T. Uemera (Kyoto University); and UAS-nod-βgal and UAS-kinesin-βgal by Y. N. Jan (University of California, San Francisco). The UAS-dyl stocks and the anti-Dyl antibodies were kindly provided by F. Payre (Université Paul Sabatier, Toulouse, France). RNAi stocks for exocyst/Rab11 and cuticle genes were obtained from the Vienna Stock Center (V lines) and from the BDSC (T lines from the Harvard collection). Key lines for RNAi experiments included V102166 (dyl), V22198 (Rab11), T27314 (sec6), T27483 (sec10), T27499 (sec15), T28041 (exo70), T28712 (exo84) and T28874 (sec5). Additional RNAi-inducing transgene lines were used for confirming experiments.
Note that previous studies using the GFP-Rab11 fusion protein refer to it as Rab11-GFP; however, an examination of how the transgene was constructed indicates that Rab11 is fused to the C-terminus of GFP (Emery et al., 2005).
Temperature-controlled gene expression
The GAL4/UAS system was used to direct transgene expression. neur-Gal4 was used to drive gene expression in bristles. Temporal control of expression utilized a temperature-sensitive Gal80 protein (McGuire et al., 2004). Animals were grown at 21°C, white prepupae collected and pupae shifted to 29°C at the desired stages of development.
Generation of kkv and Rab11 clones
kkv mutant clones were generated by crossing w hs-flp; FRT82 ubi-gfp/TM6 females to FRT82 kkv1/TM6 males. Vials were heat shocked at 37°C for 1 hour to induce flp and clone formation. Rab11ex1 clones were induced in an analogous experiment. Rab11ΔFRT clones were induced as described (Bogard et al., 2007).
Immunostaining
Immunostaining was performed by standard protocols on paraformaldehyde fixed material (see He et al., 2005). Primary antibodies used were: rabbit anti-GFP (1:2000, Molecular Probes), mouse anti-acetylated tubulin (1:1000, Sigma), rabbit anti-β-galactosidase (1:10,000, Cappel) and anti-Dyl (1:200, F. Payre). Alexa 488- and Alexa 568-conjugated secondary antibodies (1:250) were from Molecular Probes. For F-actin staining, we used Alexa Fluor phalloidin (488 or 568, Molecular Probes).
Chitin staining using CBD-Rhodamine probe
To visualize chitin in bristles, fixed thoraces were incubated in a 1:100 dilution of Rhodamine-conjugated chitin-binding probe (New England Biolabs) (Gangishetti et al., 2009) in PBS containing 0.3% Triton X-100 for 4 hours at room temperature, rinsed in PBS and mounted. Similar results were obtained using labeled wheat germ agglutinin, although with this reagent it was better not to add detergent to permeabilize the cells.
Inhibition of cytoskeleton using drugs and genetics
Thoraces from pupae at 40-42 hours after puparium formation (APF) (25°C) were cultured in Schneider’s medium (1×) in the presence of drugs prior to fixation and staining. We also used the timed expression of UAS-Klp10A to disassemble microtubules. Klp10A is a member of the kinesin-13 family that binds to the minus ends of microtubules and induces their depolymerization (Ems-McClung and Walczak, 2010; Heald, 2004) and it has been found to be an effective mediator of microtubule breakdown in several tissues/developmental stages in Drosophila and other systems (Ems-McClung and Walczak, 2010; Goodwin and Vale, 2010; Goshima and Vale, 2005; Heald, 2004; Schimizzi et al., 2010; Sharp et al., 2005; Shimada et al., 2006). We found that Klp10A reduced the density of bristle microtubules, as detected by transmission electron microscopy, to ∼40% of wild-type levels. This might be an underestimate as microtubule density decreases as bristles elongate (Tilney et al., 2000a) and elongation is severely inhibited by Klp10A expression.
Disruption of chitin synthases using nikkomycin
Nikkomycin (5 mg/ml in water; Sigma) was injected into pupal heads between 28 and 32 hours APF (25°C) using a microinjection apparatus. The drug-treated pupae were compared with water-injected controls.
Live imaging studies
Animals were placed on double-sided sticky tape and part of the pupal case was removed to provide a window that exposed the head and thorax. A coverslip coated with a small volume of halocarbon oil (Sigma) was placed onto the pupa with both sides supported by a piece of rubber. Pupae were imaged every 3-4 hours at 20× and 60× while being incubated at 29°C between imaging sessions. Confocal observations were made on a Nikon Eclipse TE200 microscope equipped with a CARV spinning disc confocal attachment (ATTO). The FRAP and in vivo imaging experiments were performed on a BioRad Radiance 2100 confocal and a Zeiss 510 Meta confocal microscope at the Keck Center for Cellular Imaging at the University of Virginia.
Image analysis
Confocal images were obtained using a CARV spinning disc unit on a Nikon Eclipse TE200 microscope or on a Zeiss Meta confocal microscope. Cuticle images were obtained using a SPOT digital camera (National Diagnostics) on a Zeiss Axioskop 2 microscope. Images were analyzed and scale bars added using ImageJ and processed using Adobe Photoshop.
RESULTS
Rab11 and bristle development
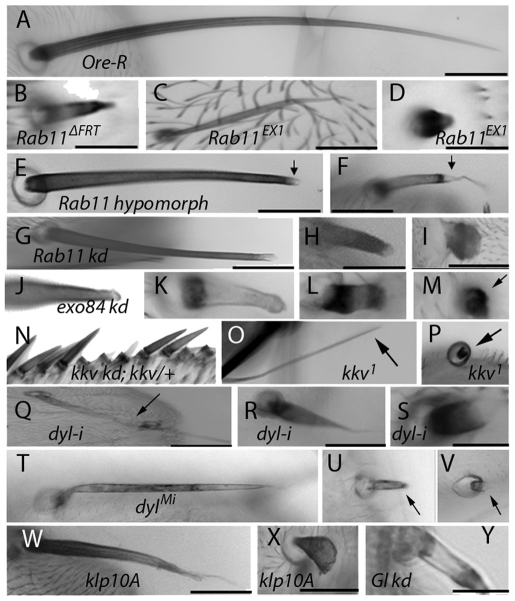
We induced unmarked clones of two different Rab11 null alleles – Rab11ΔFRT and Rab11ex1 (Bogard et al., 2007; Dollar et al., 2002) – and observed missing bristles. This was expected because Rab11 is required for the formation of the bristle sense organ lineage (Emery et al., 2005). We also observed occasional abnormal bristles on these flies that were shortened, malformed (Fig. 1B,C) or reduced to stubs (Fig. 1D). We suggest that perdurance of the Rab11 gene product allowed these bristles to bypass the Rab11 requirement for bristle determination. These phenotypes partly overlapped those seen in Rab11 hypomorphs (Rab1193Bi/Rab11ex1). The hypomorphs showed a weaker bristle phenotype that was mostly obvious in macrochaetae, where the bristles were shorter than wild type and had tips that were either blunt or very thin and wavy (Fig. 1E,F). Interestingly, the distal parts of the Rab11 hypomorph bristles often lacked pigmentation and were almost transparent (Fig. 1E,F, arrows).
Fig. 1.
Bristle phenotypes. (A) Scutellar bristle from wild-type (Ore-R) adult. (B) Thoracic bristle from putative Rab11ΔFRT null clone. (C) Thoracic bristle from putative Rab11ex1 null clone. (D) Abdominal bristle from putative Rab11ex1 null clone. (E,F) Thoracic bristles from Rab11 hypomorph (Rab1193Bi/Rab11ex1) adults. Arrows point to pigmentation defect at the tip. (G-I) Rab11 knockdown (kd) thoracic bristles. Knockdown starting 19 hours APF (G), 12 hours APF (H), or at white prepupal stage (I). (J-M) exo84 kd bristles (arrow points to bristle in M). (N) Wing bristles from flies with disrupted kkv function (kkv RNAi; kkv/+). (O,P) Scutellar bristles from putative kkv1 clones (arrows point to bristles). (Q-S) Thoracic bristles from dyl kd (w; dyl RNAi/+; neur-Gal4/+). Arrow points to affected bristle in Q. (T-V) Bristles from a dylML02088 escaper fly (arrows point to bristles). (W,X) Thoracic bristles from flies expressing Klp10A. Expression starting 15 hours APF (W) or at white prepupal stage (X). (Y) Thoracic bristle from fly with p150glued kd from white prepupal stage to adult. All images were taken at 10× magnification. Scale bars: 100 μm.
We found that expressing Rab11 dsRNA after the determinative cell divisions was able to produce a much stronger bristle phenotype than the hypomorph. The strength of the mutant phenotype was proportional to the duration of RNAi induction and the particular RNAi-inducing transgene used (Fig. 1G-I). The strongest phenotype resulted in bristles that were reduced to short stubs (Fig. 1I). Such phenotypes were seen with close to complete cellular penetrance for the scutellar macrochaetae. It was possible to obtain very strong phenotypes in all bristles with the appropriate choice of transgenes and temperature shifts (supplementary material Fig. S1).
As a test of the functionality of a GFP-Rab11 fusion protein, we constructed flies that were UAS-GFP-Rab11/+; Rab1193Bi neur-Gal4/Rab11ex1. The hypomorphic bristle phenotype in such flies was completely rescued both in length (supplementary material Fig. S2) and morphology, confirming that the GFP-Rab11 protein was functional. To test whether GFP-Rab11 was intact we analyzed extracts of wing discs from UAS-gfp-Rab11/ptc-Gal4 larvae by western blotting. A single band of the expected size was detected (supplementary material Fig. S3). We concluded that GFP-Rab11 was intact, functional and an accurate reporter for Rab11.
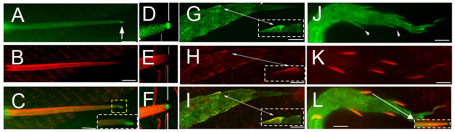
The accumulation of an organizing/marking protein at a particular region of the plasma membrane is often a crucial step in the growth of polarized cell structures (Pruyne et al., 2004; Tang, 2001). Since Rab11 mutants showed a strong bristle phenotype with altered tip morphology, we examined the localization of GFP-Rab11 in developing bristles by immunostaining and by direct in vivo imaging. It was most abundant in the cell body and punctate localization was observed in the shaft (Fig. 2A-F, Fig. 3A,B). Interestingly, the tagged Rab11 was enriched at the distal tip of growing bristles throughout the period of bristle elongation. This was also reported by Hayashi and colleagues (Otani et al., 2011). The GFP-Rab11 at the tip was distal to the large bundles of F-actin (Fig. 2D-F), suggesting that it could have an organizing function, similar to that which Rab11 is thought to have in the oocyte for microtubules (Dollar et al., 2002). Recent FRAP experiments by Otani et al. showed Rab11 movement and localization to be very dynamic in growing bristles (Otani et al., 2011), a result we independently obtained.
Fig. 2.
GFP-Rab11 localization in bristles and laterals. GFP-Rab11 (green) localization was visualized by immunostaining with anti-GFP antibody. F-actin (red) was visualized by phalloidin staining. (A) GFP-Rab11 is found in the bristle cell body and shaft. It is enriched at the tip (arrow). (B) F-actin for the bristle in A. (C) Merge of A and B. The tip is magnified in the inset. (D-F) Localization of GFP-Rab11 with respect to actin filaments. (D) GFP-Rab11 is enriched at the tip (arrow). (E) F-actin for bristle shown in D. (F) Merge of D and E. Note that Rab11 at the tip is distal to the F-actin. (G-I) Early stages of lateral outgrowth (28 hours APF, 25°C). (G) GFP-Rab11. (H) F-actin. (I) Merged image. Note the very early tip enrichment of GFP-Rab11. Insets are magnifications of the indicated regions (arrowed). (J-L) Middle stage of lateral outgrowth (32 hours APF, 25°C). (J) GFP-Rab11. Arrowheads point to Rab11 at tip. (K) F-actin. (L) Merged image. All images were taken at 60× magnification. Scale bars: 10 μm.
Fig. 3.
Rab11 intracellular transport is microtubule dependent. Time-lapse imaging of bristles expressing GFP-Rab11 and Klp10A under neur-Gal4 as compared with control bristles expressing only GFP-Rab11. (A-C) GFP-Rab11 localization in a wild-type bristle cell during its growth phase. Note the tip enrichment of Rab11 (arrows). Note the cell body GFP-Rab11 (arrowheads). (D) GFP-Rab11 localization in a wild-type bristle cell near the end of its growth phase. Note the decreased tip enrichment. (A′-D′) Magnified images of the bristle tip from A-D, respectively. (E-G) GFP-Rab11 localization in a bristle expressing Klp10A during its growth phase (starting ∼24 hours APF). (H) GFP-Rab11 localization in a bristle expressing Klp10A after its growth phase. (E′-H′) Magnified images of the bristle tip from E-H, respectively. (I-L) Highly enhanced versions of images E-H that allow the bristle shaft structure (arrows) to be seen. Images are of a single bristle tracked in each set. Images were taken at 60× magnification and represent a projection of optical sections. In control images C and D, the entire length of the bristle cell is not shown. Scale bars: 10 μm.
If Rab11 accumulation at the tip is required for polarized growth, we predicted that it should initiate at very early stages in bristle development. When bristle outgrowth begins, the shaft cell is located below the socket cell, making it difficult to reliably analyze protein localization. We therefore examined the lateral branches of the arista for GFP-Rab11 localization. The cytoskeleton of elongating laterals is similar to that of bristles, but they differ in that the initial site of outgrowth is not covered (He and Adler, 2001). At the time of initiation of outgrowth (28 hours APF, 25°C) a crescent of GFP-Rab11, often with a small blob-like protrusion, formed on the plasma membrane at the site of outgrowth (Fig. 2G-I). F-actin also accumulated at this site (Fig. 2H,I) but GFP-Rab11 appeared to be more focused at the tip than the actin (Fig. 2I). Four hours later, when extension had started, the tip localization of GFP-Rab11 was retained and the shaft also showed localization (Fig. 2J-L). As observed in growing bristles, the tip localization of Rab11 was distal to the actin bundles.
Studies in mammalian cells and Drosophila have asserted the importance of both actin filaments and microtubules in the Rab11-mediated transport of proteins (Casanova et al., 1999; Dollar et al., 2002; Hales et al., 2001; Hales et al., 2002; Lapierre et al., 2001; Leung et al., 2000; Satoh et al., 2005). It has been suggested that the microtubules play a key role in transporting proteins required for bristle growth (Fei et al., 2002; Tilney et al., 2000a), although experimental evidence for this is lacking.
We first tested the role of the cytoskeleton in GFP-Rab11 localization by culturing pupal thoraces in the presence of drugs that are known to disrupt actin or microtubules. Disruption of the microtubule cytoskeleton had a greater effect on GFP-Rab11 distribution than disruption of the actin cytoskeleton (supplementary material Fig. S4), but these experiments were not definitive. Therefore, as an alternative method for inhibiting microtubule function, we used the GAL4/UAS/Gal80ts system to express Kinesin-like protein (KLP) 10A in growing bristles (see Materials and methods). Klp10A induces microtubule depolymerization (Goshima and Vale, 2005; Heald, 2004; Sharp et al., 2005; Shimada et al., 2006).
Using time-lapse imaging, we tracked bristle development in pupae expressing GFP-Rab11 and Klp10A as compared with those expressing only GFP-Rab11. As observed previously, GFP-Rab11 localized to the bristle tip as early as 28 hours APF in the control bristles (Fig. 3A). The localization at the tip and in the shaft increased over the next 8 hours (Fig. 3B-D). Interestingly, the tip localization decreased after the elongation phase, although GFP-Rab11 remained abundant throughout the shaft (Fig. 3D). By comparison, GFP-Rab11 localization in the Klp10A-expressing bristles was dramatically reduced, both in the shaft and the bristle tip (Fig. 3E-H), but not in the cell body. We concluded that Rab11 moves from the cell body to the growing shaft in a microtubule-dependent manner.
In the Drosophila oocyte the posterior accumulation of Rab11 is required for polarizing microtubules so that plus ends are preferentially located in the posterior (Dollar et al., 2002). We speculated that Rab11 could be performing a similar role in bristles to mediate the polarized transport of protein(s) and membrane to the tip. To test this hypothesis, we analyzed microtubule polarity in bristles by expressing reporter proteins. Both Kin-βgal and GFP-Nod (Cui et al., 2005; McDonald et al., 1990) are plus-end reporters and they localized to the bristle cell body (supplementary material Fig. S5A,B). By contrast, the Nod-βgal fusion protein is a minus-end reporter (Clark et al., 1997; Cui et al., 2005) and it localized to the distal tip (supplementary material Fig. S5C). These observations indicated that microtubules are polarized in bristles with their minus ends toward the distal tip and plus ends toward the cell body. Similar results on the polarity of microtubules were reported by Bitan et al. (Bitan et al., 2010). Surprisingly, the relationship between Rab11 and microtubule ends is reversed in bristles as compared with oocytes (Clark et al., 1997; Dollar et al., 2002). We found the Nod-βgal and Kin-βgal reporters to be very toxic. Even a short period of expression of Nod-βgal led to bloated bristle tips (supplementary material Fig. S5A). In adult bristles this treatment led to bristles with a variety of tip abnormalities, including reduced pigmentation, thin wispy shafts and swollen tips (supplementary material Fig. S5E). By contrast, expression of Kin-βgal led to bristles that were frail and thinned (supplementary material Fig. S5F,G). We suggest that these polarity reporters act as dominant-negative motors and block transport along microtubules, which leads to their accumulating in a polarized fashion. The toxic nature of the microtubule polarity reporters made it difficult for us to use them to reliably analyze a possible role for Rab11 in bristle microtubule organization.
Rab11 is important for chitin deposition and bristle stability
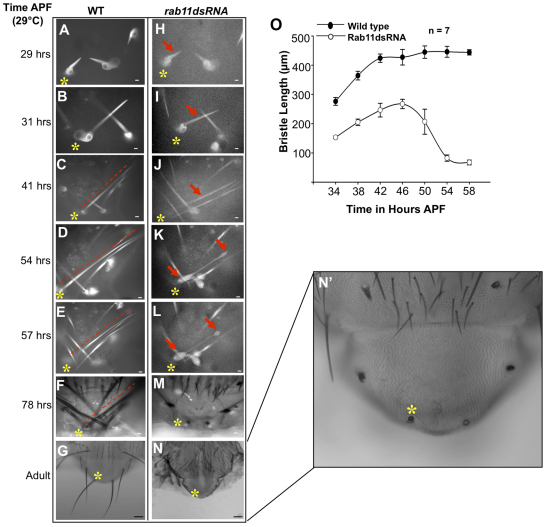
We hypothesized that the phenotypes displayed by Rab11 mutants were due to a defect in bristle elongation. To determine whether this was the case, we performed live imaging studies in pupae expressing Rab11 dsRNA as well as wild-type controls (Fig. 4). neur-Gal4>Tubulin-GFP was used for imaging developing bristles. Rab11 dsRNA-expressing bristles were shorter and grew more slowly than control bristles in the growth phase (29-42 hours APF) (Fig. 4O). Nevertheless, they did elongate and by 46 hours APF reached 260 μm in length, which was more than half the length of controls (Fig. 4I,J). In addition to growing more slowly, bristles from Rab11 dsRNA-expressing pupae also started displaying deformities/bulges at later stages (54-57 hours APF; Fig. 4K,L). These deformities worsened over time and led to the collapse of the bristle (Fig. 4M,O).
Fig. 4.
Rab11 is essential for bristle stability. (A-N′) Time-lapse imaging of wild-type and Rab11 dsRNA-expressing bristles. All images except G and N (2.5×) were taken at 20× (oil immersion) magnification. Images are shown of the same pupa tracked from 29 hours APF to adult. Asterisks and arrows mark the bristle being tracked; dashed line is parallel to the bristle. Images represent single optical sections. (A-G) Wild-type pupal thoracic bristles (control). (H-N′) Rab11 kd bristles (kd starting at white prepupal stage). Arrows in K and L indicate regions of bristle that showed deformities. (N′) Enlargement of N. Scale bars: 10 μm in A-F,H-M; 100 μm in G,N. (O) The growth of wild-type and Rab11 dsRNA-expressing bristles over time as determined by measuring the length of bristles (μm) at different times of development (hours APF at 29°C). Mean ± s.d.
We concluded that the dramatic stub phenotype was not due to a failure of bristle outgrowth but rather represented what was left after bristle collapse. Examining numerous bristles we observed that the details of the deformities and collapse were highly variable. That is, the regions where the bulges first arose and the path of changes that preceded the collapse were not consistent. However, we first observed the deformities at ∼48 hours APF regardless of their initial location along the shaft (Fig. 4K,L). We also examined bristles from pupae expressing Rab11 dsRNA for a shorter period of time (supplementary material Fig. S6). The adult bristles from such flies displayed weaker bristle phenotypes (supplementary material Fig. S6A-G). In such cases, we found that bristles elongated to a greater extent and then snapped, broke or collapsed, leaving behind a shorter bristle with an abnormal tip (supplementary material Fig. S6E-G).
The exocyst is known to be a Rab11 effector (Emery et al., 2005; He and Gou, 2009; Langevin et al., 2005) and Sec5 colocalizes with Rab11 in growing bristles (Otani et al., 2011). We tested seven of the eight exocyst components (sec5, sec6, sec8, sec10, sec15, exo70 and exo84) for a function in bristle morphogenesis using the same timed RNAi knockdown (kd) we used for Rab11. For all seven we observed bristle abnormalities that spanned the range seen with Rab11, including the stub phenotype (Fig. 1J-M). The frequency of exocyst kd-induced stub phenotypes was, however, routinely lower than that seen for Rab11, and UAS-Dicer-2 was needed as an RNAi enhancer (Dietzl et al., 2007). In our best experiments, ∼75% of the scutellar bristles displayed the stub phenotype. We did not carry out time-lapse in vivo imaging experiments on exocyst kd bristles, but observations of fixed 42-hour pupae indicated that the adult stub bristle phenotype was due to collapse. Consistent with the exocyst functioning in Rab11-independent processes, lethality was routinely more extensive with exocyst kd. Our results suggest that the exocyst is a Rab11 effector during bristle development.
Our experiments showed that bristle collapse occurred late in development (Fig. 4O), around the time when actin filament breakdown is initiated (Guild et al., 2002). We therefore examined actin filaments in bristles before and at the time of collapse to determine whether actin breakdown was affected in the Rab11 kd bristles. We did not detect any change in the timing of actin filament breakdown due to Rab11 kd (supplementary material Fig. S7). We concluded that the collapse was not mediated by an effect on F-actin.
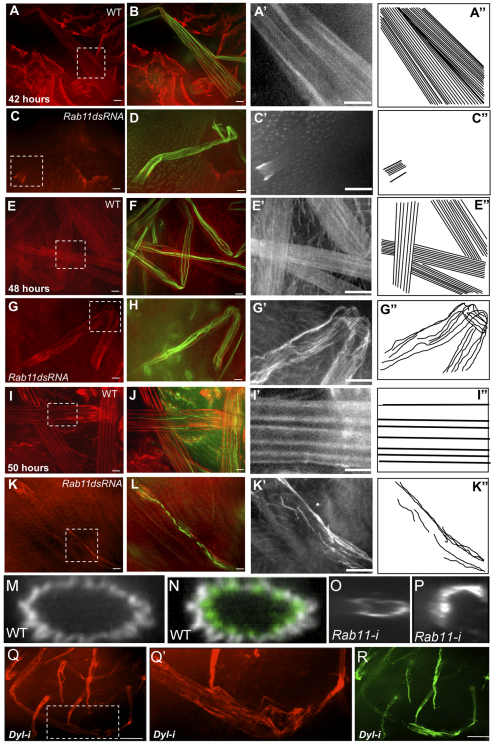
The correlation between the timing of actin filament breakdown and the collapse of Rab11 kd bristles suggested that collapse was associated with a failure in a component that provided structural integrity after actin bundle breakdown. The cuticular exoskeleton was a likely candidate; therefore, we examined the deposition and accumulation of chitin in wild-type and Rab11 dsRNA-expressing bristles. Chitin is a major component of cuticle but the timing and arrangement of chitin deposition in Drosophila bristles has not been reported. We first detected chitin at ∼42 hours APF. It was first seen proximally and shortly thereafter all along the shaft bristle (Fig. 5A,A′). As development proceeded, chitin was found in increasingly distinct bands that ran parallel to the long axis of the bristle (Fig. 5E,E′,I,I′). When we analyzed z projections we observed that chitin was external to the bundles of F-actin and covered the entire bristle circumference (Fig. 5M,N). The banded pattern seen in optical sections and maximal projections is likely to be a consequence of the fluted shape of the bristles and variation in chitin thickness. As expected for an extracellular component (Moussian et al., 2005), we were able to stain bristle chitin in the absence of permeabilization with detergent (supplementary material Fig. S8A).
Fig. 5.
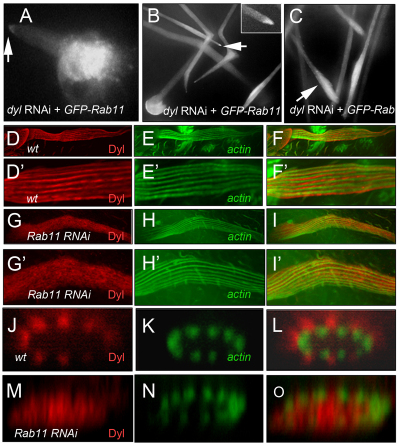
Chitin organization in bristles. (A-L) Chitin (red) and actin (green) in wild-type bristles and Rab11 kd bristles at 42, 48 and 50 hours APF. Magnifications of chitin staining (boxed regions) are shown in grayscale in A′,C′,E′,G′,I′,K′, and interpretive drawings of these in A″,C″,E″,G″ I″,K″. (M,N) Projection of z-stacks from images of wild-type bristles stained for chitin (grayscale) and F-actin (green) localization. (O,P) Projection of z-stacks of Rab11 kd bristles showing uneven chitin deposition along the bristle circumference. (Q-R) Chitin (red) and F-actin (green) in dyl kd bristles. (Q′) Enlargement with enhanced contrast of boxed region from Q. Scale bars: 10 μm.
Rab11 kd bristles displayed dramatically altered chitin banding. The abnormalities ranged from delayed deposition (Fig. 5C), bands that did not run parallel to the long axis of the bristle, to regions with a complete loss of chitin (Fig. 5G′,K′). These abnormalities were usually more severe in older bristles, suggesting that there was not only a defect in the deposition of chitin but also in its stability. In z projections, large chitin-free regions were often seen (Fig. 5O,P). In addition to the disorganization there was also a quantitative decline in the amount of chitin staining and this also became more severe at later stages (supplementary material Fig. S9).
In Drosophila and other insects there are two chitin synthase genes. CS-1 is encoded by krotzkopf verkehrt (kkv) and mediates cuticle formation in the epidermis and trachea in embryos, larvae and adults (Coutinho et al., 2003; Merzendorfer and Zimoch, 2003; Ostrowski et al., 2002; Ren et al., 2005; Roncero, 2002; Zimoch and Merzendorfer, 2002). The second chitin synthase (CS-2) is thought to only function in the synthesis of the peritrophic membrane. As null alleles of kkv are embryonic lethal, to examine its function in bristle development we generated somatic clones of kkv1 [a null allele (Ostrowski et al., 2002)]. Flies carrying large or numerous clones often died as pharate adults due to an apparent defect in the integrity of the cuticle barrier. Flies with few or smaller clones often eclosed. In the abdomen of such flies, clones were often associated with ‘blobs of exudates’ (supplementary material Fig. S10C). The clone cuticle had reduced pigment and trichomes were faint as expected (supplementary material Fig. S10A) (Ren et al., 2005). Bristle abnormalities ranged from rare very short bristles to frequent moderately short bristles with abnormal pigmentation (Fig. 1N-P). Although there was overlap between the kkv1 and Rab11 kd phenotypes, the kkv1 mutant bristles generally showed a substantially weaker phenotype, suggesting that Rab11 function is likely to be required for the deposition of multiple cuticular components. A similar range of phenotypes was seen in experiments in which we injected the chitin synthase inhibitor nikkomycin into pupae (supplementary material Fig. S10D-L) (R. Nagaraj, PhD Thesis, University of Virginia, 2010).
Dusky-like functions as a Rab11 effector
The formation and maintenance of chitin is a complex process that requires the function of chitin synthases, chitin-binding proteins and chitinases (Merzendorfer and Zimoch, 2003). We identified dusky-like (dyl) as a compelling candidate for functioning in bristle cuticle formation. Dyl is a ZP domain-containing transmembrane protein that is expressed only in cuticle-secreting epithelia such as the epidermis, trachea and foregut (Fernandes et al., 2010; Hillman and Lesnick, 1970; Roch et al., 2003). Knocking down dyl function in bristles resulted in a range of bristle phenotypes (Fig. 1Q-S) that were similar to those seen with Rab11. Most notable were the stub phenotype and pigmentation abnormalities (Fig. 1Q-S). We found that heterozygosity for a deficiency that removed dyl strongly enhanced the weak scutellar bristle phenotype that resulted from dyl kd using either the ptc-Gal4 or sca-Gal4 drivers (supplementary material Fig. S11F). Recently, a stock carrying a Minos insertion into dyl became available. This mutation is recessive lethal, with most homozygotes dying as prepupae or young pupae. Rare escapers showed bristle phenotypes that were similar to those seen in the kd experiments (Fig. 1T-V). Hence, we concluded that the dyl RNAi phenotype was specific. As with Rab11, we observed alterations in chitin band deposition in dyl kd bristles (Fig. 5Q).
Similarities between bristle phenotypes suggested that Dyl might regulate Rab11 function (or vice versa) to mediate chitin deposition in bristles. To determine whether dyl is required for Rab11 localization we examined GFP-Rab11 by live imaging in dyl kd bristles. These experiments showed that dyl kd bristles grew and then collapsed like the Rab11 mutant bristles (Fig. 6A-C). Disrupting dyl function did not affect the tip localization of GFP-Rab11 (Fig. 6A,B, arrow). Normal Rab11 distribution was still evident when morphological abnormalities first appeared (Fig. 6). As the deformities worsened, GFP-Rab11 accumulated in the ‘bulges’ of collapsing bristles (Fig. 6C). We suggest that the late alteration in GFP-Rab11 localization was due to the changes in bristle morphology and was not a direct consequence of disrupting dyl function.
Fig. 6.
Dyl and Rab11 localization in bristles. (A-C) GFP-Rab11 localization in bristle expressing dyl RNAi at (A) 29 hours, (B) 40 hours and (C) 56 hours APF at 29°C. Arrows in A and B indicate tip accumulation of GFP-Rab11 (magnified in inset in B). Arrow in C shows the accumulation of GFP-Rab11 in a blebbing region of a bristle. (D-O) Dyl localization in wild-type and Rab11 mutant bristles visualized by staining with anti-Dyl antibody. Localization of (D) Dyl and (E) F-actin in wild-type bristles. (F) Merge of D and E. (D′-F′) Magnifications of D-F, respectively. (G,H) Localization of Dyl (G) and F-actin (H) in Rab11 kd bristles. (I) Merge of G and H. (G′-I′) Magnifications of G-I, respectively. (J-O) z projections showing Dyl (J,M) and F-actin (K,N) localization around the circumference of wild-type (J,K; merge in L) and Rab11 kd (M,N; merge in O) bristles.
To determine whether Rab11 functions upstream of dyl we examined Dyl localization by immunostaining (Fernandes et al., 2010) in both wild-type and Rab11 kd bristles. In wild-type bristles, Dyl was localized in parallel stripes along the length of the shaft (Fig. 6D′) that were off set from the bundles of F-actin. In z sections, Dyl was at the periphery and external to the actin bundles. (Fig. 6J-L). Since Dyl is a transmembrane protein, we concluded that Dyl was localized to the plasma membrane of bristles. In Rab11 kd bristles, Dyl was not found in stripes and in z sections it was spread throughout the cytoplasm and not restricted to the cell periphery. Thus, Rab11 function is essential for localizing Dyl to the bristle plasma membrane.
Microtubule function is essential for bristle elongation
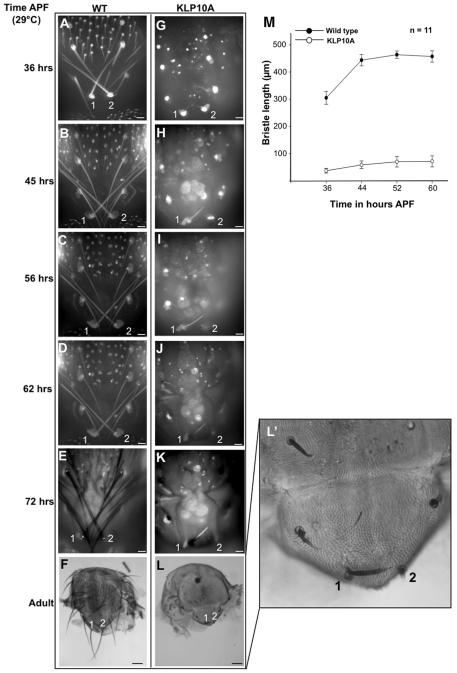
Expressing Klp10A resulted in mutant phenotypes that were similar to those of Rab11 mutants. Bristles similar to those seen in Rab11 hypomorphs (Fig. 1W) were seen when Klp10A was induced at 15 hours APF. When Klp10A was induced from the white prepupal stage onwards, we observed bristles that resembled the strong Rab11 kd stub phenotype (Fig. 1X). Previous studies with microtubule antagonists such as vinblastine produced similar phenotypes (Fei et al., 2002).
We carried out in vivo imaging to determine the basis for the Klp10A stub phenotype. In contrast to the results seen for Rab11, the expression of Klp10A resulted in a failure of bristle extension (Fig. 7). Such bristles were shorter than those of the wild type at all stages and no collapse was detected. These observations showed that, although the adult bristle phenotypes of Rab11 mutants resemble those from flies in which Klp10A was expressed, the mechanisms that gave rise to the phenotypes were very different.
Fig. 7.
Microtubules are essential for bristle growth. (A-L′) Time-lapse imaging of wild-type and Klp10A-expressing bristles. Images of the pupal bristles and adult bristles were taken at 20× and 2.5× magnification, respectively. (A-F) Wild-type bristles 1 and 2 tracked over 40 hours of development from pupa to adult stage. (G-L) Klp10A-expressing bristles 1 and 2 tracked over 40 hours of development. (L′) Magnification of L. Scale bars: 10 μm in A-E,G-K; 100 μm in F,L. (M) Growth of wild-type and Klp10A-expressing bristles over time. Mean ± s.d. Images are projections of single optical sections.
The Klp10A phenotype could be a direct consequence of the reduced number or size of microtubules or, alternatively, it could be due to an indirect effect on one or more microtubule-dependent processes such as long-distance intracellular transport. To distinguish between these possibilities, we examined bristles in which p150glued (Glued – FlyBase) function was compromised. p150glued is the largest subunit of the dynactin complex and serves as an adaptor to facilitate the binding of cytoplasmic dynein to its cargo and to meditate the interaction between dynein and microtubules (Gill et al., 1991).
Glued function was knocked down in bristle-forming cells in pupae. The strongest phenotypes we observed resembled the Rab11 hypomorphic phenotype (Fig. 1E,F). The Glued kd bristles were shorter than normal, had almost transparent tips and showed excess pigment just proximal to the tip (Fig. 1Y). We concluded that dynactin-mediated intracellular transport on microtubules is important for bristle extension and pigmentation and that a disruption of intracellular transport is likely to be the basis for the Klp10A phenotype.
DISCUSSION
Dyl functions as a Rab11 effector
We found that knocking down Rab11 function in bristles led to short stubs. Very similar bristle stub phenotypes were observed when any of seven different exocyst components or Dyl function was knocked down. In vivo imaging showed that the bristle stub phenotype was not due to a primary failure in bristle elongation. Rather, the phenotype resulted from the collapse of bristles at the time when cuticle takes over the structural support role from F-actin bundles. We documented highly abnormal chitin deposition in both the Rab11 and dyl kd, consistent with a failure of cuticle formation being the cause of the collapse.
An analogous role for Rab11 has been observed previously in S. cerevisiae, where the yeast homologs Ypt31 and Ypt32 spatially and temporally regulate the delivery of Chs3p (one of the yeast chitin synthases) to the plasma membrane to mediate chitin deposition (Ortiz and Novick, 2006). This suggests that Rab11 regulates chitin formation in bristles by mediating the transport and localization of chitin-synthesizing enzymes to sites of cuticle deposition in bristles. If this were true and it was the only function of Rab11 in bristle morphogenesis then disrupting the function of chitin synthases in bristles should cause similar phenotypes to those of Rab11 mutants. However, bristles homozygous for a null allele of kkv, which encodes the relevant chitin synthase, had, on average, weaker bristle phenotypes than Rab11 mutants, suggesting that there are cuticle targets other than chitin synthase that require Rab11 for localization, secretion or organization in bristles. Candidate targets include cuticle proteins and proteins required for cross-linking and pigmenting cuticular structures.
We identified Dyl as such a target. We established that Rab11 is required for the plasma membrane localization of Dyl and that chitin is similarly disorganized in dyl and Rab11 mutants. It is therefore likely that Dyl functions as a Rab11 effector in mediating patterned chitin deposition in bristles. Dyl, like other ZP (zona pellucida) domain-containing proteins is membrane anchored and shares homology with several vertebrate and invertebrate apical matrix components. Genes encoding sixteen ZP domain proteins have been identified in Drosophila melanogaster, eight of which have been shown to have highly specific roles in the shaping of embryonic epidermal denticles, where they mediate interactions between the apical extracellular matrix and the epidermal cell membrane (Fernandes et al., 2010). Dyl could therefore function to mediate interactions between chitin and/or other cuticle components and the bristle cell membrane to ensure the proper addition and organization of the cuticle. Interestingly, Dyl was the only ZP domain protein among the four that we examined to affect bristle development: dusky, miniature and dumpy did not show bristle phenotypes. This suggests that ZP domain proteins have cell type-specific functions. This strengthens the possibility that Dyl functions as a cuticle-specific effector of Rab11 function in bristle cells. Rab11 presumably has dyl-independent functions earlier in bristle development, as Rab11 is required for the asymmetric cell divisions that give rise to the sense organ (Emery et al., 2005) but we found no evidence for a similar requirement for dyl.
Does the exocyst function as a Rab11 effector in bristle cuticle formation?
Based on what is known from other systems, it is expected that any gene(s) encoding a product that is required for the trafficking of Dyl to the plasma membrane or for the secretion of chitin (or chitin synthase) and cuticle proteins would share the Rab11/dyl bristle stub phenotype. We observed such phenotypes upon kd of each of the seven exocyst-encoding genes examined, providing strong support for the proposal that the exocyst functions as a Rab11 effector for bristle cuticle deposition. It is interesting that a genome-wide screen failed to identify a role for exocyst component genes such as sec3, sec5, sec6, sec8 and sec15 in bristle morphogenesis (Mummery-Widmer et al., 2009). This is due to organismal lethality in the kd, and uncovering the exocyst role in bristle morphogenesis required a temporally limited kd that would be very laborious in a genome-wide screen.
ZP domain proteins and the generation of cuticle diversity
In considering the role of Rab11 and Dyl in bristle morphogenesis it is worth noting that the defect was not simply a quantitative problem with chitin deposition but rather there appeared to be a profound disruption in chitin organization in the mutants. This suggests that Rab11 and Dyl not only function in the secretion of chitin but also serve to pattern the extracellular space for chitin deposition and cuticle formation. Different insect cuticles have different physical properties consistent with their different physiological functions. ZP domain proteins seem likely to play a role in generating this specificity. This appears to be the case in the embryo, where different ZP domain proteins localize to different parts of developing embryonic denticles and mutations in the relevant genes produce distinct phenotypes (Fernandes et al., 2010). In addition, based on their mutant phenotypes it appears that the dusky and miniature genes, which also encode ZP domain proteins, have an important function in the formation of wing blade cuticle but not in bristle or hair cuticle (Roch et al., 2003). Further studies on this family of genes could provide unique insights into the evolution of cuticle structure and function, which has facilitated the extraordinary diversity of insect morphology.
The distal tip localization of Rab11
We found that GFP-Rab11 preferentially accumulated at the tip of growing bristles. This was also seen by Hayashi and co-workers (Otani et al., 2011), who found that the IKKε kinase was required for the tip localization of Rab11. IKKε is also localized to bristle tips, as is the interacting SpnF protein (Bitan et al., 2010; Otani et al., 2011). Interfering with the function of these genes leads to bristle morphological abnormalities that are nonetheless not as severe as the stub phenotypes we describe here (Abdu et al., 2006; Bitan et al., 2010; Otani et al., 2011; Shapiro and Anderson, 2006). Thus, Rab11 mislocalization appears to create a less serious problem for bristle morphogenesis than a lack of Rab11. These observations suggest that Rab11 function is required all along the growing bristle for Dyl insertion into the membrane and chitin deposition.
Supplementary Material
Acknowledgments
We thank the many members of the fly community who provided reagents, particularly F. Payre and R. Cohen.
Footnotes
Funding
This work was supported by a grant from the National Institutes of Health [GM-37136 to P.N.A.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.074252/-/DC1
References
- Abdu U., Bar D., Schupbach T. S. (2006). spn-F encodes a novel protein that affects oocyte patterning and bristle morphology in Drosophila. Development 133, 1477–1484 [DOI] [PubMed] [Google Scholar]
- Band A. M., Ali H., Vartiainen M. K., Welti S., Lappalainen P., Olkkonen V. M., Kuismanen E. (2002). Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 531, 513–519 [DOI] [PubMed] [Google Scholar]
- Bitan A., Guild G. M., Bar D. D., Abdu U. (2010). Asymmetric microtubule function is an essential requirement for polarized organization of the Drosophila bristle. Mol. Cell. Biol. 30, 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard N., Lan L., Xu J., Cohen R. S. (2007). Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134, 3413–3418 [DOI] [PubMed] [Google Scholar]
- Cant K., Knowles B. A., Mooseker M. S., Cooley L. (1994). Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J. Cell Biol. 125, 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J., Wang X., Kumar R., Bhartur S. G., Navarre J., Woodrum J. E., Altschuler Y., Ray G. S., Goldenring J. R. (1999). Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. E., Jan L. Y., Jan Y. N. (1997). Reciprocal localization of Nod and Kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development 124, 461–470 [DOI] [PubMed] [Google Scholar]
- Coutinho P. M., Deleury E., Davies G. J., Henrissat B. (2003). An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328, 307–317 [DOI] [PubMed] [Google Scholar]
- Cui W., Sproul L. R., Gustafson S. M., Matthies H. J., Gilbert S. P., Hawley R. S. (2005). Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Mol. Biol. Cell 16, 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–157 [DOI] [PubMed] [Google Scholar]
- Dollar G., Struckhoff E., Michaud J., Cohen R. S. (2002). Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development 129, 517–526 [DOI] [PubMed] [Google Scholar]
- Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M., Gonzalez K., Juergen A. (2005). Asymmetric Rab11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763–773 [DOI] [PubMed] [Google Scholar]
- Ems-McClung S. C., Walczak C. E. (2010). Kinesin-13s in mitosis: key players in the spatial and temporal organization of spindle microtubules. Semin. Cell Dev. Biol. 21, 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X., He B., Adler P. N. (2002). The growth of Drosophila bristles and laterals is not restricted to the tip or base. J. Cell Sci. 115, 3797–3806 [DOI] [PubMed] [Google Scholar]
- Fernandes I., Chanut-Delalande H., Ferrer P., Latapie Y., Waltzer L., Affolter M., Payre F., Plaza S. (2010). Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev. Cell 18, 64–76 [DOI] [PubMed] [Google Scholar]
- Gangishetti U., Breitenbach S., Zander M., Saheb S. K., Müller U., Schwarz H., Moussian B. (2009). Effects of benzoylphenylurea on chitin synthesis and orientation in the cuticle of the Drosophila larva. Eur. J. Cell Biol. 88, 167–180 [DOI] [PubMed] [Google Scholar]
- Gill S. R., Schroer T. A., Szilak I., Steuer E. R., Sheetz M. P., Cleveland D. W. (1991). Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115, 1639–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S. S., Vale R. D. (2010). Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Vale R. D. (2005). Cell Cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells. Mol. Biol. Cell 16, 3896–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild G. M., Connelly P. S., Vranich K. S., Michael K., Tilney L. G. (2002). Actin filament turnover removes bundles from Drosophila bristle cells. J. Cell Sci. 115, 641–653 [DOI] [PubMed] [Google Scholar]
- Guild G. M., Connelly P. S., Ruggiero L. V., Kelly A., Tilney L. G. (2005). Actin filament bundles in Drosophila wing hairs: Hairs and bristles use different strategies for assembly. Mol. Biol. Cell 16, 3620–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. M., Griner R., Hobdy H. K., Dorn M. C., Hardy D., Kumar R., Navarre J., Chan K. L., Lapierre L. A., Goldenring J. R. (2001). Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 276, 39067–39075 [DOI] [PubMed] [Google Scholar]
- Hales C. M., Vaerman J. P., Goldenring J. R. (2002). Rab11 Family Interacting Protein 2 associates with myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 277, 50415–50421 [DOI] [PubMed] [Google Scholar]
- He B., Adler P. N. (2001). Cellular mechanisms in the development of the Drosophila arista. Mech. Dev. 104, 69–78 [DOI] [PubMed] [Google Scholar]
- He B., Guo W. (2009). The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Fang X., Emoto K., Jan Y. N., Adler P. N. (2005). The Tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with Furry during Drosophila wing hair development. Mol. Biol. Cell 16, 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R. (2004). Cell division: burning the spindle at both ends. Nature 427, 300–301 [DOI] [PubMed] [Google Scholar]
- Hillman R., Lesnick L. (1970). Cuticle formation in the embryo of Drosophila melanogaster. J. Morph. 131, 383–396 [Google Scholar]
- Jankovics F., Sinka R., Erdelyi M. (2001). An interaction type of genetic screen reveals a role of the rab11 gene in oskar mRNA localization in the developing Drosophila melanogaster oocyte. Genetics 158, 1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Sibarita J. B., Aresta S., Murthy M., Schwarz T., Camonis J., Bellaiche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Curr. Opin. Cell Biol. 9, 355–376 [DOI] [PubMed] [Google Scholar]
- Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W., Mercer J. A., Bahler M., Goldenring J. R. (2001). Myosin Vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S. M., Ruiz W. G., Apodaca G. (2000). Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 11, 2131–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. B., Stewart R. J., Goldstein S. B. (1990). The kinesin-like Ncd protein of Drosophila is a minus end-directed microtubule motor. Cell 63, 1159–1165 [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Mao Z., Davis R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, 16–20 [DOI] [PubMed] [Google Scholar]
- Merzendorfer H., Zimoch L. (2003). Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 206, 4393–4412 [DOI] [PubMed] [Google Scholar]
- Moussian B., Erika T., Tonning A., Helms S., Schwarz H., Nüsslein-Volhard C., Anne E. U. (2005). Drosophila Knickkopf and retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133, 163–171 [DOI] [PubMed] [Google Scholar]
- Mummery-Widmer J. L., Yamazaki M., Stoeger T., Novatchkova M., Bhalerao S., Chen D., Dietzl G., Dickson B. J., Knoblich J. A. (2009). Genome-wide analysis of Notch signaling in Drosophila by transgenic RNAi. Nature 458, 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D., Novick P. J. (2006). Ypt32 regulates the translocation of Chs3p from an internal pool to the plasma membrane. Eur. J. Cell Biol. 85, 107–116 [DOI] [PubMed] [Google Scholar]
- Ostrowski S., Dierick H. A., Bejsovec A. (2002). Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics 161, 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Oshima K., Onishi S., Takeda M., Shinmyozu K., Yonemura S., Hayashi S. (2011). IKK regulates cell elongation through recycling endosome shuttling. Dev. Cell 20, 219–232 [DOI] [PubMed] [Google Scholar]
- Pelissier A., Chauvin J. P., Lecuit T. (2003). Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr. Biol. 13, 1848–1857 [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Lankenau D. H., Mitchell H. K., Young P., Corces V. G. (1994). Forked proteins are components of fiber bundles present in developing bristles of Drosophila melanogaster. Genetics 136, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse M. A., Gao L., Dong Y., Bretscher A. (2004). Mechanisms of polarized growth and organelle segregation in yeast. Ann. Rev. Cell Dev. Biol. 20, 559–591 [DOI] [PubMed] [Google Scholar]
- Ren N., Zhu C., Lee H., Adler P. N. (2005). Gene expression during Drosophila wing morphogenesis and differentiation. Genetics 171, 625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch F., Alonso C. R., Akam M. (2003). Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J. Cell Sci. 116, 1199–1207 [DOI] [PubMed] [Google Scholar]
- Roncero C. (2002). The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41, 367–378 [DOI] [PubMed] [Google Scholar]
- Satoh A. K., O’Tousa J. E., Ozaki K., Ready D. F. (2005). Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132, 1487–1497 [DOI] [PubMed] [Google Scholar]
- Schimizzi G. V., Currie J. D., Rogers S. L. (2010). Expression levels of a kinesin-13 microtubule depolymerase modulates the effectiveness of anti-microtubule agents. PLoS ONE 5, e11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf B., Fey G. H., Hauber J., Hocke G. M., Rosorious O. (2000). Rab11b is essential for recycling of transferrin to the plasma membrane. Exp. Cell Res. 259, 257–265 [DOI] [PubMed] [Google Scholar]
- Shapiro R. S., Anderson K. (2006). Drosophila Ik2, a member of the IκB kinase family, is required for mRNA localization during oogenesis. Development 133, 1467–1475 [DOI] [PubMed] [Google Scholar]
- Sharp D. J., Mennella V., Buster D. W. (2005). Klp10A and KLP59C: the dynamic duo of microtubule depolymerization. Cell Cycle 4, 1482–1485 [DOI] [PubMed] [Google Scholar]
- Shimada Y., Yonemura S., Ohkura H., Strutt D., Uemuera T. (2006). Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10, 209–222 [DOI] [PubMed] [Google Scholar]
- Tang B. (2001). Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J. Neurochem. 79, 923–930 [DOI] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J. (2005). How to make a curved Drosophila bristle using straight actin bundles. Proc. Natl. Acad. Sci. USA 102, 18785–18792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S., Guild G. M. (1995). F actin bundles in Drosophila bristles. Two filament cross-links are involved in bundling. J. Cell Biol. 130, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Guild G. M. (1996). F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J. Cell Biol. 135, 1291–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Vranich K. A., Shaw M. K., Guild G. M. (2000a). Actin filaments and microtubules play different roles during bristle elongation in Drosophila. J. Cell Sci. 113, 1255–1265 [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Vranich K. A., Shaw M. K., Guild G. M. (2000b). Regulation of actin filament cross-linking and bundle shape in Drosophila bristles. J. Cell Biol. 148, 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Guild G. M. (2004). Microvilli appear to represent the first step in actin bundle formation in Drosophila bristles. J. Cell Sci. 117, 3531–3538 [DOI] [PubMed] [Google Scholar]
- Turner C. M., Adler P. (1998). Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech. Dev. 70, 181–192 [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli L. A., Lah J. J., Fang G., Goldenring J. R., Levey A. I. (2002). Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 22, 9776–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kumar R., Navarre J., Casanova J. E., Goldenring J. R. (2000). Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 275, 29138–29146 [DOI] [PubMed] [Google Scholar]
- Wilcke M., Johannes L., Galli T., Mayau V., Goud B., Salamero J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 151, 1207–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimoch L., Merzendorfer H. (2002). Immunolocalization of chitin synthase in the tobacco hornworm. Cell Tissue Res. 308, 287–297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.