Abstract
Recently, methicillin-resistant Staphylococcus aureus (MRSA) has surpassed HIV as the most deadly pathogen in the United States, accounting for over 100,000 deaths per year. In orthopedics, MRSA osteomyelitis has become the greatest concern in patient care, despite the fact that improvements in surgical technique and aggressive antibiotic prophylaxis have decreased the infection rate for most procedures to less than 5%. This great concern is largely due to the very poor outcomes associated with MRSA osteomyelitis, which includes 30–50% failure rates for revision surgery. Thus, there is a need to develop additional therapeutic interventions such as passive immunization, particularly for immunocompromised patients and the elderly who are typically poor responders to active vaccines. Using a novel murine model of implant-associated osteomyelitis in which a stainless steel pin is coated with bioluminescent S. aureus and implanted transcortically through the tibial metaphysis, we discovered that mice protect themselves from this infection by mounting a specific IgG2b response against the peptidoglycan hydrolase, glucosaminidase (Gmd), an enzyme involved in cell wall digestion during binary fission. Since this subunit of S. aureus autolysin is essential for bacterial growth, and no genetic variation has been identified among clinical strains, we propose that monoclonal antibodies against this enzyme would have multiple mechanisms of action, including promotion of opsonophagocytosis and direct inhibition of enzyme function. Here we review the field of MRSA osteomyelitis and our research to date on the development of an anti-Gmd passive immunotherapy.
Keywords: Osteomyelitis, Methicillin-Resistant Staphylococcus aureus (MRSA), Autolysin, Glucosaminidase, Passive immunization
Introduction
Osteomyelitis is a bacterial infection of bone that is characterized by progressive inflammatory bone destruction (osteolysis) coupled with reactive bone formation, and can involve either a small portion or several regions of any bone. The initial infection that causes osteomyelitis can occur either from hematogenous seeding of the pathogen from another site in the body, or from direct inoculation via a traumatic or surgical wound (1). This results in an acute infection that usually lasts several days or weeks, and may require antibiotic and/or surgical intervention. When the causative microorganism persists for more than 10 days and causes further destruction of bone, the infection is then considered chronic osteomyelitis (2). Staphylococcus aureus (S. aureus) is the single leading cause of both acute and chronic osteomyelitis in children and adults, accounting for approximately 80% of these infections (3). Other microorganisms such as coagulase-negative staphylococci, Streptococcus spp, Enterococcus spp, and Mycobacterium tuberculosis may also cause osteomyelitis, but S. aureus is by far the most prevalent bacteria found due to virulence factors that help it evade a number of host defenses (1).
Osteomyelitis is generally classified using either of two systems: the Waldvogel (4–7) and the Cierny-Mader (8;9) systems. The Waldvogel classification system is based on the extent, duration, and mechanism of bone infection, whereas the Cierny-Mader classification system also considers the immune state and risk factors of the host, thus offering comprehensive treatment options that best fit the patient’s needs. Because of this, the Cierny-Mader classification system is considered more clinically relevant and is therefore more widely used (10). Regardless of the model of classification used, antibiotic and surgical treatments are designed on a patient-to-patient basis according to the distinct type of osteomyelitis presented, and not all patients will respond to either strategy (1;3;11).
The number of bone infections has increased over the last few decades, due to an increase in the number of prosthetic and fracture-fixation devices being placed by orthopedic surgeons (12). Although improvements in surgical technique and aggressive antibiotic prophylaxis have decreased the infection rate following orthopedic implant surgery to less than 5%, osteomyelitis remains a serious problem (13;14). The gravity of these infections is amplified by the fact that approximately 50% of clinical isolates are drug-resistant strains of S. aureus, such as methicillin-resistant S. aureus (MRSA), that are commonly acquired in both hospital and community settings (15). Additionally, MRSA has surpassed HIV as the most deadly pathogen in North America and continues to make the management of chronic osteomyelitis more difficult. Current estimates of two-stage revision surgery for MRSA periprosthetic infection suggest that reinfection rates are approximately 15–25% (16–18). However, these numbers may actually be higher because they do not account for the fraction of patients who cannot undergo revision surgery due to persistent infection that does not permit device reimplantation. This indicates that there is a major need for alternative interventional strategies, particularly for immunocompromised individuals (i.e., patients with diabetes or who are HIV-infected), those taking immunosuppressive medications, and the elderly who collectively comprise the majority of patients undergoing total joint replacement (TJR) surgery.
The great need for novel interventions is reinforced by the fact that approximately 112,000 orthopedic device-related infections occur each year in the United States, at an approximate cost of $15,000–70,000 per incident (3). While the infection rates for joint prosthesis and fracture-fixation devices have been only 0.3–11% and 5–15% of cases, respectively, over the last decade (1;19), these infections may lead to amputation or even death. Additionally, although unproven, the popularization of “minimally invasive surgery” for elective TJR, in which a very small incision can lead to complications from the prosthesis contacting skin during implantation, has been associated with a marked increase in the incidence of osteomyelitis (20). These infections require a very expensive two-stage revision surgery, and recent reports suggest that success rates could be as low as 63% (16–18).
At present, the only prophylactic treatments available for preventing MRSA infection in patients undergoing TJR surgery are preoperative antibiotics, such as vancomycin. However, the overuse of these “last resort” antibiotics is resulting in the emergence of strains with resistance to even our most potent antibiotics (21). Therefore, it is imperative that immunocompromised and elderly patients, who collectively account for most of the 1.5 million TJRs performed annually in the United States, have access to alternative interventional strategies. Thus, a vaccine that would decrease the MRSA incidence by 50–80% would reduce the number one complication of joint replacement and open fracture repair procedures, and it would also cut the healthcare burden by a similar amount (22).
Microbial Pathogenesis of Osteomyelitis and Targets for Immunotherapy
Based on over 150 years of research, a clear paradigm to explain microbial pathogenesis has emerged. This model also applies to osteomyelitis. The initial step of infection occurs when a single bacterium invades the body. At this point the microbe must respond to environmental changes and express virulence genes that will help it defeat innate immunity and provide it with adhesin receptors to attach to the host. The microbe is also dependent on the presence of host structures from necrotic tissue or a foreign body such as an implant. Successful completion of these steps leads to an exponential growth phase, which ceases at the point of nutrient exhaustion and/or the development of adaptive immunity. Following the exponential growth phase, the bacteria are forced to persist under dormant growth conditions within a complex extracellular matrix referred to as a biofilm. At this point the infection is now chronic and cannot be eradicated by drugs or host immunity. Because of the critical importance of initial attachment, the focus in this field has been on the cell surface adhesins that specifically interact with extracellular matrix components known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (23). In fact, the majority of anti-S. aureus vaccines that have been developed to date have been directed against MSCRAMMs that are important to host tissue colonization and invasion (24). The goal of these vaccines was to generate antibodies that block the attachment to host tissues by binding to the adhesins. Unfortunately, S. aureus has many adhesins, such that inhibition of one may not be sufficient to prevent bacterial attachment.
S. aureus Autolysin as a Primary Target
In gram-positive and gram-negative bacteria, autolysins play an important role in cell separation and cell wall remodeling during normal binary fission. The 138-kDa S. aureus autolysin is proteolytically processed on the cell surface to produce two active enzymes, N-acetylmuramyl-L-alanine amidase (amidase, 62-kDa) and endo-β-N-acetylglucosaminidase (glucosaminidase, 51-kDa), that remain non-covalently attached to the cell surface (25–28).
There are many features that make the S. aureus autolysin a very attractive target for anti-S. aureus vaccine investigation. First, it is highly conserved among staphylococci. For example, glucosaminidase (Gmd) is greater than 95% conserved across all strains of S. aureus in the public database, and approximately 85% conserved among other staphylococci (29). Second, it is essential for complete separation of daughter cells following binary fission. S. aureus bacteria deficient in autolysin still divide, but daughter cells fail to separate, leading to the generation of large clusters that fall out of suspension (30). Third, Gmd is located on the extracellular surface of the bacterium, potentially focusing the immune response on a vulnerable part of the cell. In support of this, scanning electron micrographs of anti-Gmd immune complexes on the surface of S. aureus demonstrated that the antibody binds in immediate proximity to digested cell wall (28). Fourth, autolysins may have a potential role in biofilm formation (31;32). For example, previous studies demonstrated that autolysins are involved in the initial attachment of Staphylococcus bacteria to a polymer surface (33), and that murein hydrolases are regulated by effector genes that control bacterial death and lysis in the case of biofilm formation (31;32). Finally, elevated levels of anti-Gmd antibodies were detected in serum from mice that survived a challenge with S. aureus (34). Collectively, this information provides a strong rationale for anti-Gmd therapy for osteomyelitis, as the antibodies have multiple potential mechanisms to achieve cytostatic and cytolytic activity, and the extremely high evolutionary conservation of the enzyme suggests that compensatory mutations to achieve antigenic variation and immune evasion may not be possible.
Glucosaminidase as a Protective Antigen
Recently, Li et al. identified Gmd as an important component of immune protection in mice (34;35). A time course study was performed in which a stainless steel pin coated with 1 × 106 UAMS-1 S. aureus was inserted transcortically through the tibia of each of five mice. At sacrifice, DNA was extracted from the infected tibia and analyzed by quantitative real time PCR to determine the number of copies of the nuc gene per infected tibia. Because each S. aureus bacterium contains a single copy of the nuc gene in its genome, we can directly quantify the in vivo bacterial load as a measure of infection (36).
In order to identify potentially protective antigens, pre-immune and convalescent sera were used as the primary antibody in Western blots of total S. aureus protein extract. Bands with significantly increased intensity were observed in the convalescent serum at 26-, 34-, 38-, and 56-kDa, suggesting that these antigens are somehow involved in the protective immune response.
The molecular identity of these potentially protective antigens was then determined. Convalescent serum was used to probe total S. aureus extract that was separated by two-dimensional polyacrylamide gel electrophoresis (35). A polypeptide with strong reactivity in the convalescent serum was detected that was not detected in the pre-immune serum. This protein was isolated from preparative Coomassie blue-stained gels, digested with trypsin, and then analyzed by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), which resolved 70 individual peptide peaks. The amino acid sequence from every peptide was a 100% match with the active Gmd subunit of S. aureus autolysin.
Preliminary Experiments Involving Anti-glucosaminidase Antibodies
We hypothesized that inhibiting the function of Gmd would disrupt critical steps in the growth cycle of S. aureus. Thirty-six mouse IgG1 monoclonal antibodies were generated against the Gmd enzyme, and a series of experiments was designed to test the effects of these antibodies on enzyme activity. Five were chosen as candidate anti-Gmd antibodies for the first round of screening based on their high-affinity binding to both native and recombinant His-tagged Gmd.
We predicted that S. aureus cultures grown in the presence of anti-Gmd antibodies would be inhibited in their growth. Initial experiments focused on the change in light scattering detected at A490 as cultures of S. aureus grew in the presence or absence of anti-Gmd antibodies. Using Xen29 S. aureus as a model organism, we set up cultures and measured light scattering at various time points using a plate reader. We found that antibodies against Gmd reduced the growth-related light scattering of S. aureus in our in vitro growth assays. This led us to believe that we were indeed altering the enzymatic activity of Gmd, but we needed additional evidence to understand the mechanism by which we were altering S. aureus growth.
To try and understand how our anti-Gmd antibodies were working, we set up several cultures of Xen29 S. aureus similar to those used in the growth assays, only larger in volume. If we were indeed inhibiting Gmd enzymatic activity, we predicted that we should see an increased number of cell clusters and sedimentation since Gmd is intimately involved in cell separation. Gross observation of cultures treated with anti-Gmd antibodies showed sedimentation of cells at the bottom of the vial in comparison to Xen29 grown in the absence of these antibodies (Fig. 1), leading us to believe that failed binary fission may be causing the bacteria to fall out of suspension. This was consistent with similar observations made in two separate reports using autolysin mutant S. aureus strains that could not produce glucosaminidase and amidase (30;37).
Fig. 1.
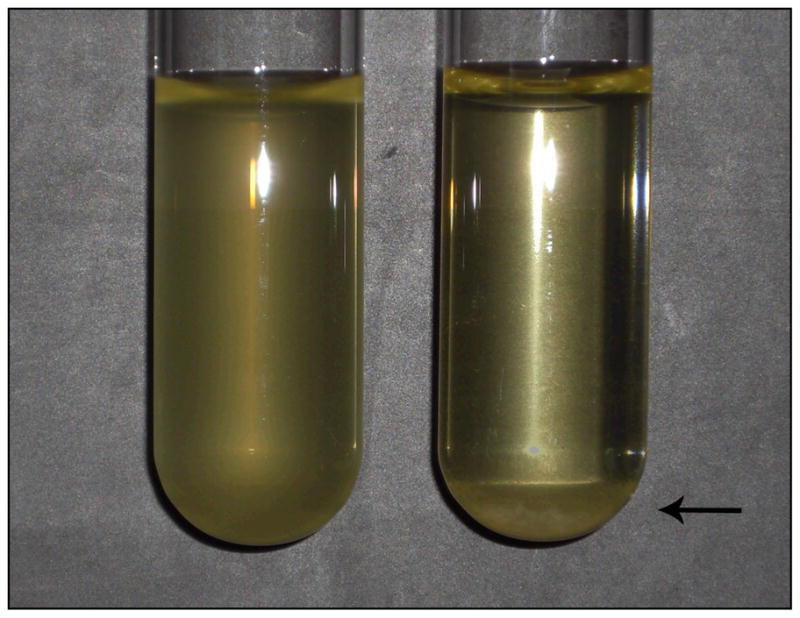
S. aureus cultures treated with an anti-glucosaminidase antibody grew as large clusters that fell out of suspension. S. aureus grown in Luria-Bertani broth under normal conditions (left), as compared to S. aureus grown in the presence of the anti-glucosaminidase monoclonal antibody 1C11 (right). Sedimentation is indicated by an arrow.
Scanning electron microscopy also revealed that S. aureus treated with anti-Gmd antibodies grew as large clusters of twenty or more bacteria, in contrast to the mostly single-cell and doublet suspension of the control culture (Fig. 2). Additionally, S. aureus treated with anti-Gmd antibodies had a rough outer appearance in comparison to cultures grown in the absence of these antibodies, which is consistent with a previous report using a Gmd-deficient strain (38).
Fig. 2.
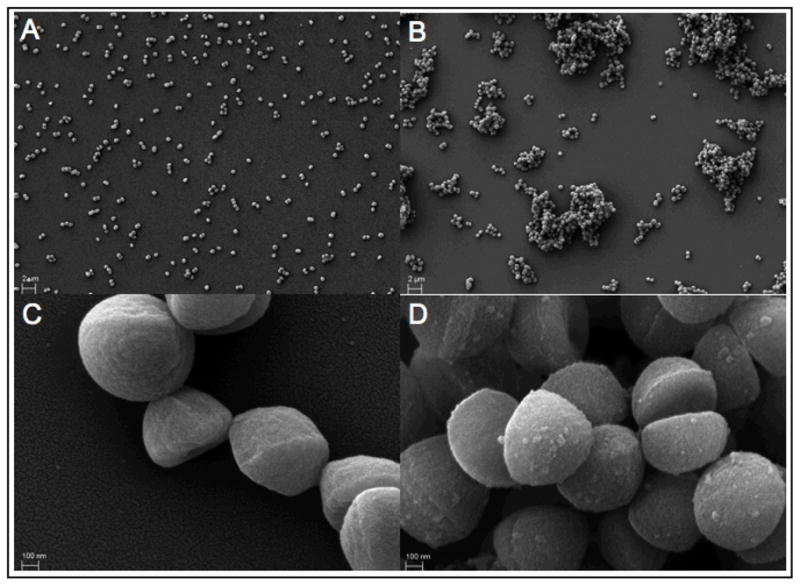
Anti-glucosaminidase antibodies alter the growth habit of S. aureus in vitro. S. aureus grew as a uniform suspension of mostly single bacteria and doublets in Luria-Bertani broth under normal growth conditions (A). In contrast, S. aureus grown in the presence of the anti-glucosaminidase monoclonal antibody 1C11 grew as large clusters (B), as revealed by scanning electron microscopy. Micrographs C and D are representative magnified views of A and B, respectively. Note the presence of a rough outer surface on S. aureus treated with the antibody, as seen in micrograph D.
Potential Future Clinical Applications
For practical reasons that pertain to the size and scope of a potential phase 3 clinical trial designed to prove the efficacy of an anti-Gmd passive immunization, we have chosen to focus our efforts towards an adjuvant immunotherapy for MRSA-infected TJR patients who are candidates for a two-stage exchange arthroplasty. The primary goal of this intervention is to prevent the seeding of residual bacteria onto the sterile implant during revision surgery and in the post-operative period. Since these bacteria must go through a planktonic growth phase to colonize the prosthesis, anti-Gmd mAbs in combination with standard chemotherapy could inhibit the re-seeding process better than antibiotics alone and significantly increase the success rates.
Ultimately, we envision a role for our passive immunotherapy in prevention of the primary infections that cause so much subsequent trauma. Specific indications include prophylaxis for all patients undergoing TJR surgery, patients receiving artificial heart valves, and children at risk for infection in neonatal intensive care units.
Conclusion
As indicated by the increase in bone infections over the last few decades, there is a great need for alternative interventional strategies for the treatment of osteomyelitis. This demand is reinforced by three situations. First, not all patients respond to antibiotic or surgical intervention. Second, approximately 50% of clinical isolates are drug-resistant strains of S. aureus, most notably MRSA. Finally, with the exception of several “last resort” antibiotics, there are no prophylactic treatments that can help high-risk patients, especially immunocompromised patients and the elderly who are the primary recipients of TJR surgery. By using a novel murine model of implant-associated osteomyelitis in which a stainless steel pin is coated with bioluminescent S. aureus and implanted transcortically through the tibial metaphysis, we discovered that mice protect themselves from this infection by mounting a specific IgG2b response that includes antibodies against Gmd, an enzyme that is intimately involved in cell separation and cell wall remodeling during S. aureus growth and binary fission. These initial experiments show that anti-Gmd antibodies alter the growth habit of S. aureus, and suggest that Gmd may be a target for direct growth inhibition and focusing immune effectors.
Acknowledgments
The authors would like to thank Tony Chen for assistance with the electron microscopy. This work was supported by Codevax, Inc., and research grants from the NIH, P50 AR054041, R43 AI085844, and T32 AR053459.
Footnotes
Conflict of Interest:
J.L. Daiss is a paid consultant for Codevax, Inc. E.M. Schwarz has received equipment and supplies from Codevax, Inc., with whom he also has a patent licensing arrangement. J.J. Varrone and D. Li have nothing to disclose.
Peer Review:
This article has been peer-reviewed.
References
- 1.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004 Jul 24–30;364(9431):369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 2.Mader JT, Norden C, Nelson JD, Calandra GB. Evaluation of new anti-infective drugs for the treatment of osteomyelitis in adults. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992 Nov;15( Suppl 1):S155–161. doi: 10.1093/clind/15.supplement_1.s155. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004 Apr 1;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 4.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970 Feb 5;282(6):316–322. doi: 10.1056/NEJM197002052820606. [DOI] [PubMed] [Google Scholar]
- 5.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects (second of three parts) N Engl J Med. 1970 Jan 29;282(5):260–266. doi: 10.1056/NEJM197001292820507. [DOI] [PubMed] [Google Scholar]
- 6.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970 Jan 22;282(4):198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 7.Waldvogel FA, Medoff G, Swartz MN. Treatment of osteomyelitis. N Engl J Med. 1970 Oct 8;283(15):822. doi: 10.1056/NEJM197010082831523. [DOI] [PubMed] [Google Scholar]
- 8.Cierny G., 3rd Chronic osteomyelitis: results of treatment. Instr Course Lect. 1990;39:495–508. [PubMed] [Google Scholar]
- 9.Cierny G, 3rd, Mader JT. Approach to adult osteomyelitis. Orthop Rev. 1987 Apr;16(4):259–270. [PubMed] [Google Scholar]
- 10.Sia IG, Berbari EF. Infection and musculoskeletal conditions: Osteomyelitis. Best Pract Res Clin Rheumatol. 2006 Dec;20(6):1065–1081. doi: 10.1016/j.berh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Jorge LS, Chueire AG, Rossit AR. Osteomyelitis: a current challenge. Braz J Infect Dis. May–Jun;14(3):310–315. [PubMed] [Google Scholar]
- 12.Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000 Sep–Oct;8(5):285–291. doi: 10.5435/00124635-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003 Jan;85-A(1):27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Global Strategy for Containment of Antimicrobial Resistance. 2001 www.who.int/emc.
- 15.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008 Oct;23(7):984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009 Jul;467(7):1706–1714. doi: 10.1007/s11999-009-0739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009 Jul;467(7):1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009 Jul;467(7):1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006 Feb;88(2):149–155. doi: 10.1302/0301-620X.88B2.17058. [DOI] [PubMed] [Google Scholar]
- 20.Graw BP, Woolson ST, Huddleston HG, Goodman SB, Huddleston JI. Minimal incision surgery as a risk factor for early failure of total hip arthroplasty. Clin Orthop Relat Res. 2010 Sep;468(9):2372–2376. doi: 10.1007/s11999-010-1300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba PS, Taneja J, Mishra B. Methicillin and Vancomycin Resistant S. aureus in Hospitalized Patients. J Glob Infect Dis. 2010 Sep;2(3):275–283. doi: 10.4103/0974-777X.68535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BY, Wiringa AE, Bailey RR, Lewis GJ, Feura J, Muder RR. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine. Mar 11;28(12):2465–2471. doi: 10.1016/j.vaccine.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsen K, Lorenz U. Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol. Aug;300(6):402–410. doi: 10.1016/j.ijmm.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Komatsuzawa H, Sugai M, Nakashima S, et al. Subcellular localization of the major autolysin, ATL and its processed proteins in Staphylococcus aureus. Microbiol Immunol. 1997;41(6):469–479. doi: 10.1111/j.1348-0421.1997.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 26.Komatsuzawa H, Sugai M, Ohta K, et al. Cloning and characterization of the fmt gene which affects the methicillin resistance level and autolysis in the presence of triton X-100 in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997 Nov;41(11):2355–2361. doi: 10.1128/aac.41.11.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S, Sugai M, Komatsuzawa H, et al. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996 Mar;178(6):1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D. Development of a Quantitative Murine Model of Implant-associated Osteomyelitis and its use to Demonstrate the Adverse Effects of Anti-resorptive Drugs and Efficacy of an Immuno-dominant Antigen Vaccine on Staphylococcus aureus Infection of Bone. Rochester, NY: Department of Biomedical Genetics, University of Rochester School of Medicine and Dentistry; 2007. [Google Scholar]
- 30.Takahashi J, Komatsuzawa H, Yamada S, et al. Molecular characterization of an atl null mutant of Staphylococcus aureus. Microbiol Immunol. 2002;46(9):601–612. doi: 10.1111/j.1348-0421.2002.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 31.Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008 Mar;72(1):85–109. doi: 10.1128/MMBR.00030-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice KC, Mann EE, Endres JL, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007 May 8;104(19):8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997 Jun;24(5):1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Gromov K, Soballe K, et al. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008 Jan;26(1):96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Gromov K, Rubery PR, O’Keefe RJ, Schwarz EM. Trans Ortho Res Soc. Vol. 31. Orthopaedic Research Society; 2006. Staphylococcus aureus autolysin is an immunodominant antigen during the establishment of implant-associated osteomyelitis. [Google Scholar]
- 36.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992 Jul;30(7):1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugai M, Komatsuzawa H, Akiyama T, et al. Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995 Mar;177(6):1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster SJ. Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J Bacteriol. 1995 Oct;177(19):5723–5725. doi: 10.1128/jb.177.19.5723-5725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


