Abstract
(See the editorial commentary by Overton, on pages 697–9.)
Background. The immunogenicity of a high hemagglutinin (HA) dose or a second dose of influenza vaccine in human immunodeficiency virus (HIV)–infected individuals has not been fully explored.
Methods. One hundered ninety-two HIV-infected individuals aged 18–64 years were stratified by CD4 cell count (<200 cells/mL or ≥200 cells/mL) and randomized to receive 2 doses of 15 μg or 30 μg HA 2009 H1N1 vaccine 21 days apart. Hemagglutination inhibition (HAI) and microneutralization (MN) antibodies were measured on days 0, 10, 21, 31, 42, and 201.
Results. Recipients of 30 μg HA had significantly higher HAI geometric mean titers (GMTs), compared with recipients of 15 μg HA on days 10 (139.0 vs 51.9; P = .01), 21 (106.7 vs 51.9; P = .001), and 31 (130.0 vs 73.7; P = .03) but not on days 42 (91.8 vs 61.6; P = .11) and 201 (43.0 vs 27.0; P = .08). When analyzed by CD4 cell count stratum, HAI GMTs were significantly higher among 30 μg HA recipients than among 15 μg HA in the CD4 cell count <200 cells/mL stratum on days 21 and 31 and the MN GMTs on days 10, 21, 31, and 42 (P < .05). In the CD4 cell count ≥200 cells/mL stratum, MN GMTs were significantly higher among recipients of 30 μg HA than among recipients of 15 μg HA on day 10 (P = .03).
Conclusion. Increasing the HA dose of the 2009 H1N1 vaccine improves the vaccine’s immunogenicity in HIV-infected individuals.
Clinical Trials Registration. NCT00992433.
The number of individuals with human immunodeficiency virus (HIV) infection or AIDS in the United States was >1 million in 2006 [1]. Before the widespread use of highly active antiretroviral treatment (HAART), studies suggested that HIV-infected individuals were at increased risk of complications from influenza [2, 3]. The introduction of HAART has resulted in a reduction in the rates of cardiopulmonary-related hospitalization during the influenza season, but the rates remain comparable to those in other high-risk groups [4]. These and other case series suggesting that patients with AIDS have prolonged illness with influenza have provided the rationale for the recommendation to vaccinate HIV-infected individuals against influenza annually [5–7].
The data on whether HIV-infected individuals have worse outcomes than does the general population when infected with 2009 H1N1 influenza virus are inconclusive [8, 9]. The immunologic responses to the inactivated 2009 H1N1 influenza vaccine, as measured by seroprotection rates, seroconversion rates, and postvaccination geometric mean antibody titers (GMTs), were generally lower among HIV-infected patients, compared with the general population [10–13]. A single dose of AS03-adjuvanted 2009 H1N1 vaccine also resulted in lower seroprotection and seroconversion rates among HIV-infected individuals than among the general population, and 2 doses of the adjuvanted vaccine were needed to increase the response level to that seen in uninfected individuals [14–16]. This difference in the immunologic response to vaccine between HIV-infected and HIV-uninfected individuals is more striking, because most of the HIV-infected patients in the aforementioned studies were receiving antiretroviral treatment, with good CD4 cell count and virologic responses. One exception is a study by Manuel et al [17], in which HIV-uninfected individuals had unexpectedly lower seroconversion rates than did HIV-infected individuals after a single dose of AS03-adjuvanted 2009 H1N1 vaccine (67% vs 77%, respectively; P = .12).
It is unclear whether a higher hemagglutinin (HA) antigen dose in the inactivated 2009 H1N1 vaccine or a second dose of the same vaccine would improve the immunologic response in HIV-infected patients. We evaluated the immunogenicity and safety of 1 and 2 doses of the 2009 H1N1 vaccine at concentrations of 15 μg or 30 μg HA per dose in HIV-infected individuals, stratified by CD4 cell count (<200 cells/mL or ≥200 cells/mL) at enrollment.
METHODS
Participants
HIV-infected men and nonpregnant women aged 18–64 years were eligible to enroll. All participants were medically stable and had received seasonal influenza vaccine (2009–2010) at least 2 weeks before enrollment. Participants treated for opportunistic infections had to have been receiving treatment with stable symptoms for at least 2 weeks before enrollment.
Vaccine
The vaccine used in this study was the licensed inactivated 2009 H1N1 vaccine (Novartis). The vaccine was provided as 0.5-mL prefilled syringes, each containing 15 μg of the A/California/7/2009 influenza virus HA for intramuscular administration. For participants randomized to receive 30 μg HA, 2 injections of 15 μg HA were given, 1 in each deltoid region.
Study Design
This was a multisite, open-label study with the primary objective of assessing the antibody response after 1 and 2 doses of vaccine at the 15-μg or 30-μg dose levels in HIV-1–seropositive adults stratified by CD4 cell count. A CD4 cell count obtained within 3 months of enrollment was used for stratification purposes. Randomization was stratified by CD4 cell count (<200 cells/mL or ≥200 cells/mL), and participants were assigned to receive vaccine at 15 μg HA or 30 μg HA. The planned sample size of 60 individuals per dose level in each CD4 cell count stratum was based on logistical considerations. Assuming that participants who received vaccine with 15 μg HA have a response rate of 50%, the study has 80% power to detect an increase of ≥25% in the response rate of participants who received vaccine with 30 μg HA in a specific CD4 cell count stratum. The study only accrued a total of 71 participants in the CD4 cell count <200 cells/mL stratum, reducing the power based on the same assumptions to 60% in that stratum.
Study Procedures and Definitions
Written informed consent was obtained from the participants, and if eligible, they were randomized to 1 of 2 groups: 15 μg HA or 30 μg HA. Participants were vaccinated on days 0 and 21. Blood samples for antibody assays were collected at baseline and on days 10, 21 (before dose 2), 31, 42, and 201. CD4 cell count and HIV RNA levels (VL) were measured at baseline and on day 31. Participants were assessed for 20 minutes after each injection and were asked to record solicited adverse events for 7 days thereafter. Ten days after each injection, an in-clinic evaluation of symptoms was done. Unsolicited adverse events were collected for 21 days after each injection. Information on chronic medical conditions and serious adverse events was collected at 2, 4, and 6 months (day 201) after the second dose. A serious adverse event was defined as Guillain-Barré syndrome or as resulting in death, life-threatening, requiring inpatient hospitalization, or prolongation of existing hospitalization, resulting in congenital anomaly, resulting in a significant disability, or any other medical event that may jeopardize the participant and require intervention to prevent one of the aforementioned outcomes. Adverse events were defined as mild (grade 1) if the symptoms caused discomfort, moderate (grade 2) if the symptoms caused interference with regular activities, and severe (grade 3) if the symptoms interrupted daily activities.
Laboratory Assays
CD4 cell count and VL measurements were performed at Clinical Laboratory Improvements Amendments-certified–certified laboratories. Hemagglutination inhibition (HAI) and microneutralization (MN) antibody assays were performed at Southern Research Institute (Birmingham, Alabama). A genetically modified reassortant A/California/07/2009 virus (Centers for Disease Control and Prevention, 2009712112) was used in the assays. The starting dilution for the assay was defined as 1:10. Samples with negative results were assigned a titer of 5, and a titer of ≥10 was defined as a detectable response. The GMT of duplicate results for each specified time point was used for all immunogenicity calculations. Details of the serologic tests have been described elsewhere [18]. Seroconversion was defined as a 4-fold increase in antibody titer if the baseline titer was ≥10 or as achieving a titer of 40 after vaccination if the baseline titer was 5. Seroprotection was defined as a titer (HAI or MN) ≥40.
Statistical Methods
Safety analyses were based on an intent-to-treat population; Fisher exact test was used to compare reactogenicity rates between dose groups. Immunogenicity analyses were based on a modified intent-to-treat population. GMTs of antibody responses were computed after transforming the results on a logarithmic scale, assuming that asymptotic normality conditions were satisfied on this scale, and converting back to the original scale. With use of results from days 21 and 42, a general estimating equation model was used to estimate the effect of the second vaccination on immune response, adjusting for HA dose and CD4 cell count stratum.
To explore the association between the demographic and clinical characteristics with the immune response (HAI or MN measured at days 21, 42, and 201; transformed as k = log2[titer/10]), we fit separate linear regression models for each of the covariates and multivariate linear regression models for immune response at each time point, including the following covariates: vaccine HA dose, CD4 cell count stratum (<200 cells/mL or ≥200 cells/mL), HIV infection duration, baseline antibody titers, VL (less than or greater than the limit of detection), age, sex, body mass index, and clinical site.
RESULTS
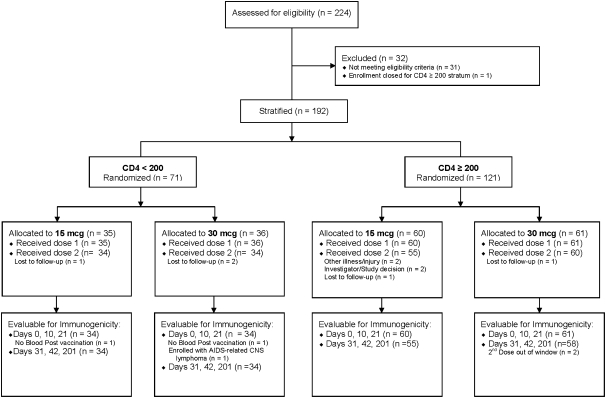
From November 2009 through April 2010, we enrolled 192 HIV-infected participants. Figure 1 displays the number of study participants screened, enrolled, and followed up through the study. Most of the study participants were male (79%), non-Hispanic (92%), and black (52%) and had a VL below the limit of detection (60.9%). The demographic and clinical characteristics of the study population by CD4 cell count stratum (<200 cells/mL vs ≥200 cells/mL) and dose group (15 μg HA vs 30 μg HA) are provided in Table 1.
Figure 1.
Flow diagram representing study participant enrollment and follow-up. The number of participants evaluable for immunogenicity includes all participants who were not evaluated because of protocol violations. Participants missing results because of a missed visit or administrative error were not excluded from evaluations at time points with nonmissing results.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| CD4 <200 cells/mL |
CD4 ≥200 cells/mL |
|||
| 15 μg HA | 30 μg HA | 15 μg HA | 30 μg HA | |
| Characteristic | (n = 35) | (n = 36) | (n = 60) | (n = 61) |
| Male sex | 28 (80%) | 26 (72%) | 48 (80%) | 49 (80%) |
| Hispanic ethnicity | 3 (9%) | 6 (17%) | 5 (8%) | 2 (3%) |
| Race | ||||
| Native American | 0 | 0 | 1 (2) | 2 (3) |
| Asian | 0 | 0 | 0 | 1 (2) |
| Black | 26 (74%) | 22 (61%) | 27 (45%) | 24 (39%) |
| White | 9 (26%) | 14 (39%) | 32 (53%) | 34 (56%) |
| Mean age (SD) | 45.7 (9.4) | 45.7 (8.3) | 45.7 (10.3) | 45.0 (9.9) |
| Mean CD4 count prior to dose 1 (SD) | 156.3 (97.3) | 147.5 (60.9) | 610.1 (269.3) | 545.1 (264.2) |
| Mean CD4 count day 31 (SD) | 157.9 (107.6) | 146.1 (52.3) | 603.4 (284.3) | 555.8 (290.2) |
| Mean BMIa (SD) | 25.9 (3.0) | 26.8 (4.8) | 26.7 (4.4) | 27.7 (6.7) |
| CD4 nadir (cells/mL) | ||||
| <99 | 27 (77.1%) | 31 (86.1%) | 14 (24.1%) | 22 (36.7%) |
| 100–199 | 8 (22.9%) | 5 (13.9%) | 10 (17.2%) | 8 (13.3%) |
| 200–299 | NA | NA | 13 (22.4%) | 13 (21.7%) |
| 300–399 | NA | NA | 8 (13.8%) | 7 (11.7%) |
| 400–499 | NA | NA | 6 (10.3%) | 5 (8.3%) |
| ≥500 | NA | NA | 7 (11.7%) | 5 (8.3%) |
| On antiretroviral therapy | 32 (91.4%) | 34 (94.4%) | 52 (86.7%) | 53 (86.9%) |
| History of ≥1 opportunistic infection | 30 (85.7%) | 32 (88.9%) | 36 (60%) | 36 (59%) |
| Chronic hepatitis C coinfection | 9 (25.7%) | 9 (25%) | 14 (23.3%) | 11 (18%) |
| Chronic hepatitis B coinfection | 2 (5.7%) | 2 (5.6%) | 2 (3.3%) | 1 (1.6%) |
| Chronic hepatitis B and C coinfection | 0 | 0 | 1 (1.7%) | 0 |
| HIV RNA prior to dose 1 | ||||
| <LOD | 14 (40%) | 22 (61.1%) | 41 (68.3%) | 40 (65.6%) |
| <1000 copies/mL | 8 (22.9%) | 8 (22.2%) | 8 (13.3%) | 10 (16.4%) |
| ≥1000 copies/mL | 11 (31.4%) | 6 (16.7%) | 9 (15%) | 8 (13.1%) |
| Not tested | 2 (5.7%) | 0 | 2 (3.3%) | 3 (4.9%) |
| HIV RNA on day 31 | ||||
| <LOD | 14 (41.2%) | 21 (61.8%) | 47 (79.7%) | 45 (75%) |
| <1000 copies/mL | 16 (47.1%) | 7 (20.6%) | 3 (5.1%) | 8 (13.3%) |
| ≥1000 copies/mL | 4 (11.8%) | 6 (17.6%) | 9 (15.3%) | 7 (11.7%) |
| Receipt of 2009–2010 TIV | ||||
| 15–30 days prior to enrollment | 4 (11.1 %) | 4 (11.4%) | 8 (13.3%) | 14 (23.0%) |
| >30 days prior to enrollment | 31 (88.9%) | 32 (88.6%) | 52 (86.7%) | 47 (77.0%) |
| Mean years since HIV diagnosis (SD) | 6.3 (7.6) | 5.3 (5.0) | 9.2 (7.3) | 7.8 (7.3) |
Abbreviations: BMI, body mass index; HA, hemagglutinin; LOD, limit of detection; NA, not applicable; SD, standard deviation; TIV, trivalent inactivated influenza vaccine.
BMI measured at screening or enrollment visit. One participant was missing BMI measurement.
Vaccine Safety and Reactogenicity
Solicited systemic adverse events occurred at a frequency of 0%–31% in all study groups (Table 2). We found no increase in the frequency of solicited systemic events in participants who received the 30-μg HA dose, compared with those who received the 15-μg HA dose, or after the second vaccination, compared with the first vaccination. In the CD4 cell count ≥200 cells/mL stratum, vaccination with the 30-μg HA dose was associated with a significant increase in the frequency of solicited injection site symptoms, compared with the 15-μg HA dose (P = .04).
Table 2.
Solicited Injection Site and Systemic Adverse Events Reported by Participants Within 8 Days of Each Vaccination
| CD4 <200 cells/mL |
CD4 ≥200 cells/mL |
|||||||
| 15 μg HA |
30 μg HA |
15 μg HA |
30 μg HA |
|||||
| Adverse Event | 1st Dose | 2nd Dose | 1st Dose | 2nd Dose | 1st Dose | 2nd Dose | 1st Dose | 2nd Dose |
| Systemic symptoms | ||||||||
| ≥1 symptom | 14 (40%) | 8 (24%) | 14 (37%) | 9 (27%) | 24 (40%) | 15 (25%) | 24 (39%) | 18 (30%) |
| Fevera | 0 | 0 | 0 | 1 (3%) | 0 | 1 (2%) | 0 | 2 (3%) |
| Grade≥2 | 0 | 1 (2%) | 0 | |||||
| Feverishnessb | 4 (12%) | 3 (9%) | 3 (9%) | 3 (9%) | 7 (12%) | 5 (9%) | 3 (5%) | 7 (12%) |
| Grade≥2 | 1 (3%) | 1 (3%) | 1 (3%) | 1 (3%) | 1 (2%) | 2 (4%) | 0 | 4 (7%) |
| Malaise | 9 (26%) | 5 (15%) | 10 (28%) | 3 (9%) | 13 (22%) | 9 (16%) | 10 (16%) | 11 (18%) |
| Grade≥2 | 3 (9%) | 2 (6%) | 5 (14%) | 1 (3%) | 6 (10%) | 0 | 0 | 8 (13%) |
| Myalgia | 5 (14%) | 5 (15%) | 7 (19%) | 4 (12%) | 9 (15%) | 9 (17%) | 13 (22%) | 10 (16%) |
| Grade≥2 | 1 (3%) | 2 (6%) | 7 (19%) | 0 | 3 (5%) | 1 (2%) | 1 (2%) | 5 (8%) |
| Headache | 7 (20%) | 3 (9%) | 11 (31%) | 7 (21%) | 17 (28%) | 8 (15%) | 15 (25%) | 13 (22%) |
| Grade≥2 | 2 (6%) | 0 | 6 (17%) | 0 | 6 (10%) | 0 | 4 (7%) | 5 (9%) |
| Nausea | 4 (12%) | 3 (9%) | 3 (9%) | 3 (9%) | 8 (13%) | 4 (8%) | 6 (10%) | 6 (10%) |
| Grade≥2 | 1 (3%) | 1 (3%) | 3 (9%) | 1 (3%) | 2 (3%) | 2 (4%) | 2 (3%) | 2 (3%) |
| Injection site symptoms | ||||||||
| ≥1 symptom | 6 (17%) | 3 (9%) | 10 (28%) | 7 (21%) | 21 (32%) | 21 (38%) | 34 (42%) | 25 (40%) |
| Grade ≥2 | 1 (3%) | 0 | 0 | 0 | 2 (3%) | 0 | 1 (2%) | 1 (2%) |
| Pain | 4 (12%) | 1 (3%) | 7 (19%) | 3 (9%) | 10 (16%) | 7 (13%) | 15 (25%) | 13 (22%) |
| Grade ≥2 | 1 (3%) | 0 | 0 | 0 | 2 (3%) | 0 | 1 (2%) | 1 (2%) |
| Tenderness | 6 (17%) | 1 (3%) | 7 (19%) | 6 (18%) | 13 (22%) | 10 (18%) | 24 (39%) | 19 (32%) |
| Grade ≥2 | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) |
| Redness | 2 (6%) | 1 (3%) | 0 | 1 (3%) | 10 (17%) | 11 (20%) | 13 (21%) | 0 |
| Grade ≥2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Swelling | 2 (6%) | 0 | 0 | 0 | 7 (12%) | 8 (15%) | 7 (11%) | 0 |
| Grade ≥2 | 0 | 0 | 0 | 0 | ||||
Abbreviation: HA, hemagglutinin.
Fever indicates a self-measured oral temperature of at least 38°C.
Feverishness indicates the participants’ self-reported sense of feverishness.
A total of 146 nonserious unsolicited adverse events were reported by 84 study participants during the 21-day period after both vaccinations. Four of these events were considered to be severe: headaches due to sinus congestion and allergies, exacerbation of sciatica pain after a fall, 1 episode of gastroenteritis, and 1 episode of vomiting due to food poisoning. No severe adverse event was considered to be associated with vaccination.
Fourteen serious adverse events in 13 participants were reported during the 7-month study period: 3 episodes of pneumonia, 2 episodes of gastritis, and 1 eachof cellulitis, perineal abscess, ectopic pregnancy, suicidal ideation, C7 radiculopathy, left-sided numbness, pericarditis, seizure, and chronic obstructive pulmonary disease exacerbation. Eight of these events occurred in the 15-μg HA group, and 6 were in the 30-μg HA group. None of these events were considered to be associated with vaccination. Six new-onset chronic medical conditions were diagnosed in 5 participants: diabetes mellitus (n = 3), asthma (n = 1), hypertension (n = 1), and glucose intolerance and steatohepatitis (n = 1). Two of these participants were in the 15-μg HA group, and 3 were in the 30-μg HA group. None of these events were judged to be associated with vaccination.
There were no appreciable changes in CD4 cell counts or proportion of participants with undetectable VL between baseline and study day 31 (Table 1).
Vaccine Immunogenicity
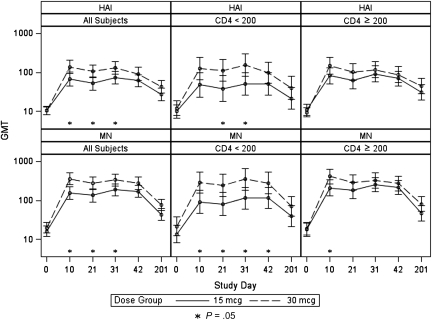
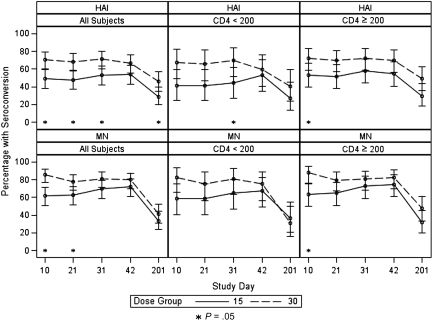
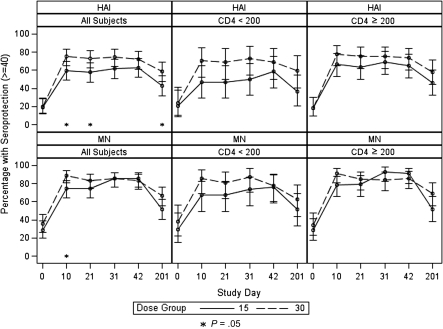
Overall, we found that vaccinating HIV-infected individuals with 30 μg HA resulted in statistically significantly higher HAI GMTs, compared with vaccinating with 15-μg HA dose, on day 10 (139.0 vs 51.9; P = .01), day 21 (106.7 vs 51.9; P = .001), and day 31 (130.0 vs 73.7; P = .03) but not on day 42 (91.8 vs 61.6; P = .11). MN GMTs were also statistically significantly higher after receipt of 30 μg HA than after receipt of the 15-μg dose (day 10: 361.5 vs 155.3 [P = .001]; day 21: 273.7 vs 134.3 [P = .008]; day 31: 334.0 vs 188.9 [P = .02]), but the difference on day 42 only reached borderline statistical significance (276.0 vs 171.0; P = .06) (Figure 2). The percentage of participants who reached the putative seroprotective HAI titer of 40 was higher in the 30-μg HA group than in the 15-μg HA group at all time points, and the difference was statistically significant on days 10 (75% vs 59.6%; P = .03), 21 (72.8% vs 57.6%; P = .04), and 201 (58.6% vs 42.5%; P = .048). The percentage of participants who achieved a MN seroprotective titer of 40 was higher in the 30-μg HA group on days 10 (89.0% vs 74.5%; P = .01), 21 (83.5% vs 74.7%; P = .2), and 201 (66.7% vs 51.7%; P = .06) but was similar between the 2 groups on day 31 (85.4% vs 85.4%; P = 1.00) and lower in the 30-μg HA group on day 42 (83.1% vs 85.4%; P = .8) (Figure 3). The HAI seroconversion rates were also higher in the 30-μg HA group at all time points, with the difference reaching statistical significance on days 10 (70.7% vs 48.9%; P = .003), 21 (68.5% vs 47.8%; P = .007), 31 (71.1% vs 52.8%; P = .01), and 201 (46.0% vs 28.7%; P = .03). Similar findings were found for seroconversion by MN antibody assay, with the increase in seroconversion rates reaching statistical significance on days 10 (85.7% vs 61.7%; P = .0002) and 21 (78.0% vs 62.6%; P = .03) (Figure 4).
Figure 2.
Geometric mean titer (GMT) and 95% confidence interval of hemagglutination inhibition (HAI) and microneutralization (MN) antibodies after vaccination with 15 μg or 30 μg hemagglutinin (HA), stratified by CD4 cell count. P value reflects the comparison between the antibody GMT after receipt of 15 μg or 30 μg HA at the specified time point.
Figure 3.
Microneutralization (MN) and hemagglutination inhibition (HAI) seroconversion rates and 95% confidence interval after vaccination with 15 μg or 30 μg hemagglutinin (HA) for the study population and stratified by CD4 cell count. P value reflects the comparison between the seroconversion rates following receipt of 15 μg or 30 μg HA at the specified time point.
Figure 4.
Microneutralization (MN) and hemagglutination inhibition (HAI) seroprotection rates and 95% confidence interval after vaccination with 15 μg or 30 μg hemagglutinin (HA) for the study population and stratified by CD4 cell count. P value reflects the comparison between the seroprotection rates following receipt of 15 μg or 30 μg HA at the specified time point.
When analyzed by CD4 cell count stratum, the difference in antibody responses to the 15-μg and 30-μg HA doses reached statistical significance in the CD4 cell count <200 cells/mL stratum on day 10 (MN GMT of 91.3 and 289.0, respectively; P = .01), on day 21 (HAI GMT of 11.3 and 38.8, respectively [P = .03], and MN GMT of 81.7 and 246.8, respectively [P = .02]), on day 31 (HAI GMT of 51.1 and 153.4, respectively [P = .02], and MN GMT of 114.3 and 352.8, respectively [P = .01]), and on day 42 (MN GMT of 115.5 and 281, respectively; P = .046) (Figure 2). In the CD4 cell count ≥200 cells/mL stratum, the HAI and MN GMTs were higher in participants who received 30 μg HA than in those who received standard-dose vaccine at all time points, but the difference reached statistical significance only on day 10 (MN GMT of 413.1 and 209.9, respectively; P = .03) (Figure 2). The percentage of participants who reached the putative seroprotective titer of 40 or those who seroconverted was also higher in the 30-μg HA group than in the 15-μg HA group at all time points in both study strata, with the difference reaching statistical significance on day 31 (seroconversion, 69.7% vs 44.1%; P < .05, by HAI) in the CD4 cell count <200 cells/mL stratum and on day 10 (seroconversion, 72.4% and 53.3% [P = .04, by HAI]; 87.7% and 63.3% [P = .001 by MN]) in the CD4 cell count ≥200 cells/mL stratum (Table 3, Figures 3 and 4).
Table 3.
Immune Responses to 1 and 2 Doses of the 2009 H1N1 Vaccine at 15 μg or 30 μg Hemagglutinin per Dose, Using the Hemagglutination Inhibition and Microneutralization Assays
| CD4 <200 cells/mL |
CD4 ≥200 cells/mL |
|||
| 15 μg HA | 30 μg HA | 15 μg HA | 30 μg HA | |
| Response | (n = 35) | (n = 36) | (n = 60) | (n = 61) |
| Prevaccination | ||||
| Percentage with HAI titer ≥40 (95% CI) | 21 (9–38) | 24 (11–41) | 18 (10–30) | 18 (9–30) |
| 10 days post–dose 1 (day 10) | ||||
| Percentage with HAI titer ≥40 (95% CI) | 47 (30–65) | 71 (53–85) | 67 (53–78) | 78 (65–87) |
| Percentage with seroconversion (95% CI) | 41a (25–59) | 68a (49–83) | 53 (40–66) | 72 (59–83) |
| 21 days post–dose 1 (day 21) | ||||
| Percentage with HAI titer ≥40 (95% CI) | 47 (30–65) | 69 (50–84) | 64 (50–76) | 75 (62–85) |
| Percentage with seroconversion (95% CI) | 41a (25–59) | 66a (47–81) | 52b (38–65) | 70b (57–81) |
| 10 days post–dose 2 (day 31) | ||||
| Percentage with HAI titer ≥40 (95% CI) | 50 (32–68) | 73 (54–87) | 69 (55–81) | 75 (62–86) |
| Percentage with HAI seroconversion (95% CI) | 44 (27–62) | 70 (51–84) | 58 (44–71) | 72 (58–83) |
| 21 days post–dose 2 (day 42) | ||||
| Percentage with HAI titer ≥40 (95% CI) | 59 (41–75) | 69 (50–84) | 65 (51–78) | 74 (60–84) |
| Percentage with seroconversion (95% CI) | 53 (35–70) | 59 (41–76) | 55 (41–68) | 70 (57–82) |
| 180 days post–dose 2 (day 201) | ||||
| Percentage with HAI titer ≥40 (95% CI) | 36 (20–55) | 59 (41–76) | 46 (33–60) | 58 (44–71) |
| Percentage with serconversion (95% CI) | 27 (13–46) | 41 (24–59) | 30a (18–44) | 49a (35–63) |
| Prevaccination | ||||
| Percentage with MN titer ≥40 (95% CI) | 29 (15–47) | 38 (22–56) | 28 (17–41) | 34 (23–48) |
| 10 days post–dose 1 (day 10) | ||||
| Percentage with MN titer ≥40 (95% CI) | 68 (49–83) | 85 (69–95) | 78 (66–88) | 91 (81–97) |
| Percentage with seroconversion (95% CI) | 59b (41–75) | 82b (65–93) | 63 (50–75) | 88 (76–95) |
| 21 days post–dose 1 (day 21) | ||||
| Percentage with MN titer ≥40 (95% CI) | 68 (49–83) | 81 (64–93) | 79 (66–89) | 85 (73–93) |
| Percentage with seroconversion (95% CI) | 59 (41–75) | 75 (57–89) | 65 (51–77) | 80 (67–89) |
| 10 days post–dose 2 (day 31) | ||||
| Percentage with MN titer ≥40 (95% CI) | 74 (56–87) | 88 (71–96) | 93 (82–98) | 84 (72–93) |
| Percentage with seroconversion (95% CI) | 65 (46–80) | 81 (64–93) | 73 (59–84) | 81 (68–90) |
| 21 days post–dose 2 (day 42) | ||||
| Percentage with MN titer ≥40 (95% CI) | 76 (59–89) | 78 (60–91) | 91 (80–97) | 86 (74–94) |
| Percentage with seroconversion (95% CI) | 68 (49–83) | 75 (57–89) | 75 (61–85) | 82 (70–91) |
| 180 days post–dose 2 (day 201) | ||||
| Percentage with MN titer ≥40 (95% CI) | 52 (34–69) | 63 (44–79) | 52 (38–66) | 69 (55–81) |
| Percentage with seroconversion (95% CI) | 36 (20–55) | 31 (16–50) | 31 (20–46) | 47 (34–61) |
Bold faced values represent P < .05 for the comparison between the 15-μg and 30-μg HA groups.
Abbreviations: CI, confidence interval; HA, hemagglutinin; HAI, hemagglutination inhibition assay; MN, microneutralization assay.
P = .05 for comparison of the variables between the 15-μg and 30-μg HA groups.
P = .06 for comparison of the variables between the 15-μg and 30-μg HA groups.
The effect of a second dose on augmenting the immune response was variable. Using a general estimating equation model, we found that, for the HAI antibodies, there was no significant increase in GMT, seroconversion, or seroprotection rates after a second dose. For the MN antibodies, although there was not a statistically significant increase in GMT after the second dose, the seroconversion and seroprotection rates were significantly higher (P = .01 for both comparisons) (Table 3).
The results of the linear regression models fit separately for each covariate indicate that the following variables have a statistically significant positive association with antibody GMT after vaccination: receiving 30 μg HA vaccine (P < .05 for HAI on day 21 and MN on days 21 and 201, without adjusting for any other covariate), receipt of antiretroviral therapy (P < .05 for HAI and MN on day 201), low VL (P < .05 for MN on days 21 and 42), and baseline HAI titer (P < .0005 for HAI and MN on days 21, 42, and 201). There was a negative association with low VL (compared with VL less than the limit of detection) for MN on days 21 and 42 (P < .05). The dose effect on HAI GMTs at days 21 and 42 was not significantly different between participants with and without hepatitis C virus coinfection, adjusting for CD4 cell count, VL, and age (data not shown).
In multivariate linear regression models, a statistically significant positive association was found between HAI antibody titer response and receiving 30 μg HA vaccine (P < .05 for days 21, 42, and 201), baseline HAI antibody titer (P < .001 for days 21, 42, and 201), and clinical study site (P = .0003 on day 21, P = .007 on day 42, and P = .01 on day 201). A borderline statistically significant negative association was found between HAI antibody titer and age on day 201 (P = .09). For the MN antibody titers, a statistically significant positive association was found with receiving 30 μg HA vaccine (P < .05 for days 21 and 201), baseline MN titer (P < .001 for days 21, 42, and 201), and clinical study site (P = .002 for day 21; P = .01 for day 42).
DISCUSSION
Our findings revealed that, in HIV-infected patients, increasing the antigen content of the inactivated influenza vaccine results in improved immune responses, especially in patients with CD4 cell count <200 cells/mL. A second vaccine dose had little effect on improving the immune response. Statistically significant determinants of response to vaccine in our population were receipt of 30 μg HA vaccine, receipt of antiretroviral treatment, baseline HAI titers, and clinical study site.
HIV-infected patients responded poorly to influenza vaccines containing seasonal or pandemic 2009 H1N1 virus antigens [10, 11, 19]. Unsuccessful attempts to improve the immune responses to influenza vaccines included giving a second dose of seasonal inactivated influenza and adding AS03 adjuvant to 2009 H1N1 vaccine [14, 20]. Conversely, Bickel et al [15] demonstrated improved immunogenicity with 2 doses of 2009 H1N1 vaccine adjuvanted with AS03, and Soonawala et al [21] demonstrated that a single dose of MF59-adjuvanted 2009 H1N1 vaccine resulted in immune responses in HIV-infected patients that were comparable to those in healthy adults. Currently, MF59 and AS03 adjuvants are not licensed in the United States. Moreover, use of 2 doses of influenza vaccine to increase its immunogenicity is a rather important hurdle to the success of vaccination campaigns when only limited time is available to achieve high coverage. Increasing the antigen content of seasonal influenza vaccines is a licensed method to improve the immunogenicity of seasonal influenza vaccines in older persons in the United States [22, 23]. In our study, we revealed that HAI and MN GMT responses are significantly improved in HIV-infected participants by doubling the HA dose of a single dose of influenza vaccine and that the seroprotection and seroconversion rates also are improved. The improved seroprotection and seroconversion rates observed in our cohort in association with the higher dose did not reach the levels observed in response to H1N1 vaccination in HIV-seronegative healthy adults in the literature [10, 13]. Of note, the currently licensed high-dose influenza vaccine for use in the older population contains 60 μg HA per antigen, which is twice the dose used in our study. On the basis of the published findings in older individuals, incremental increases in the HA concentration up to 60 μg results in further improvement of vaccine immunogenicity [23]. Because the immunosenescence in older individuals shares many parallels with the immune dysfunction seen with HIV infection and the response to 2009 H1N1 vaccination, in general, in special populations has been similar to the one seen with seasonal influenza, we hypothesize that using 60 μg HA per antigen will result in significant improvement of the immunogenicity of seasonal influenza vaccine in HIV-infected individuals [24].
Predictors of a good immune response to influenza vaccines in HIV-infected patients have varied among studies, but a correlation with the general immune status of the patient as reflected by baseline CD8 cell count, nadir CD4 cell count, baseline HAI titer ≥40, younger age, and higher CD4 cell count is generally found [10, 11, 14, 15, 25]. In the aforementioned studies, participants had high median CD4 cell counts, and most were receiving treatment. In our study, with a relatively high proportion of participants with low CD4 cell counts, we found a significant positive association between improved HAI GMT and higher HA concentration, receipt of antiretroviral therapy, and baseline HAI titers. Therefore, our study confirms that the baseline immune status of the patient is an important determinant of the response, as gauged by receipt of therapy. We also noted significant differences in HAI and MN GMT responses between sites. Because of the low parameter estimates, it is not likely that the difference is clinically significant (all the estimates were <2, indicating that the difference in the HAI/MN between the sites is a <4-fold increase; data not shown).
The strengths of the study were its prospective, randomized design and stratification by CD4 cell count. Although the majority of our participants were receiving antiretroviral therapy according to the most current guidelines, the stratification design ensured that a sizable proportion had a CD4 cell count <200 cells/mL, thus generating a unique set of data in this important subpopulation. One limitation of the study was the timing of study enrollment, which occurred after the peak of 2009 H1N1 influenza activity. This may have resulted in some interference with the results; however, it is expected to have affected all groups similarly. Some variables that may have affected the immune response to vaccination (eg, smoking status, renal function, and intravenous drug use) were not collected or used in the multivariate model developed in this study. Other limitations include the small sample size, which may have resulted in the lack of statistical significance observed in some of the comparisons; the male predominance; and the open-label design.
In summary, an improvement in the immunogenicity of 2009 H1N1 influenza vaccines in HIV-infected persons can be achieved with increasing the HA content of the vaccine. This finding may have implications for improving the immunogenicity of seasonal influenza vaccines in this and other immunosuppressed populations.
Notes
Acknowledgments.
We thank Tena Knudsen, Thomas Street Health Center, Dinna Lozano, Terry Scott, Heather Hill, Sarah Jackson, Jenifer Baer, Selina McKenzie, the Cincinnati Infectious Diseases Center, Tara Foltz, Tammy Lewis-McCauley, Jesse LePage, Kristie Price, Tom Pacatte, Irene Graham, and Edwin Anderson.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; N01-AI-800002, N01-AI-800006, N01AI0800008, HHSN272200800004C, N01-AI25464, and HHSN272200800001C) and the NIH (UL1RR025014 and UL1RR024979).
Potential conflicts of interest.
H. M. E. S. has received research support from GlaxoSmithKline for an unrelated project. W. A. K. has received research support from Novartis for an unrelated project. C. J. has recceived employment at AiCuris GmbH for work unrelated to this project. C. L. has received support for development of educational presentations by the AIDS Education and Training Centers. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/us_overview.htm. Accessed 29 October 2010.
- 2.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–7. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 3.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–6. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- 4.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–7. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Safrin S, Rush JD, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–7. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JP, Macauley C. Susceptibility to influenza A in HIV-positive patients. JAMA. 1989;261:245. doi: 10.1001/jama.1989.03420020097023. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 8.Perez CM, Dominguez MI, Ceballos ME, et al. Pandemic influenza A (H1N1) in HIV-1-infected patients. AIDS. 2010;24:2867–9. doi: 10.1097/QAD.0b013e32833e92d5. [DOI] [PubMed] [Google Scholar]
- 9.Archer B, Cohen C, Naidoo D, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill. 2009;14:19369. doi: 10.2807/ese.14.42.19369-en. [DOI] [PubMed] [Google Scholar]
- 10.Crum-Cianflone NF, Eberly LE, Duplessis C, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis. 2011;52:147–9. doi: 10.1093/cid/ciq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–92. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 13.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 14.Bickel M, Wieters I, Khaykin P, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–5. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 15.Bickel M, von Hentig N, Wieters I, et al. Immune response after two doses of the novel split virion, adjuvanted pandemic H1N1 influenza A vaccine in HIV-1-infected patients. Clin Infect Dis. 2011;52:122–7. doi: 10.1093/cid/ciq003. [DOI] [PubMed] [Google Scholar]
- 16.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 17.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–56. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 18.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 19.Amendola A, Boschini A, Colzani D, et al. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001;65:644–8. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- 20.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–83. [PubMed] [Google Scholar]
- 21.Soonawala D, Rimmelzwaan GF, Gelinck LB, Visser LG, Kroon FP. Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination. PLoS One. 2011;6:e16496. doi: 10.1371/journal.pone.0016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 23.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 24.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7:4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS. 1994;8:469–76. doi: 10.1097/00002030-199404000-00008. [DOI] [PubMed] [Google Scholar]