Abstract
Background. Hepatitis C virus (HCV) readily establishes chronic infection with exhaustion of HCV-specific T cells and escape from neutralizing antibodies. Spontaneous recovery from chronic infection is rare and has never to our knowledge been studied immunologically.
Methods. We prospectively studied, from prior to infection through >2 years of follow-up, cytokines, HCV-specific T cells, and antibodies, as well as viral sequence evolution in a white male who spontaneously cleared HCV genotype 1a after 65 weeks.
Results. Significant alanine aminotransferase and plasma cytokine elevation and broad HCV-specific T-cell responses did not result in HCV clearance in the acute phase. Frequency and effector function of HCV-specific T cells decreased thereafter, and HCV titers stabilized as is typical for the chronic phase. HCV clearance after 65 weeks followed the appearance of neutralizing antibodies at week 48 and was associated with reversal of HCV-specific T-cell exhaustion, as evidenced by reduced programmed death–1 (PD-1) expression and improved T-cell function. Clearance occurred without inflammation or superinfection with hepatitis B virus, human cytomegalovirus virus, influenza, and Epstein-Barr virus.
Conclusions. T-cell exhaustion is reversible at least in the first 2 years of chronic HCV infection, and this reversion in conjunction with neutralizing antibodies may clear HCV. These findings are relevant for immunotherapy of chronic infections.
The propensity of the hepatitis C virus (HCV) to cause chronic infection has made it one of the leading causes of liver cirrhosis and hepatocellular carcinoma in the world [1]. Although antiviral therapy in the form of pegylated interferon α and ribavirin is effective in achieving sustained HCV clearance in approximately 55% of chronically infected patients [2, 3], the attendant toxicity and high costs render it inaccessible to a considerable percentage of the infected patient population. In this context, the immunological mechanisms that mediate spontaneous HCV clearance continue to sustain great interest.
Epidemiological data suggest that 25% of HCV-infected adults clear the virus in the first 6 months of infection [4], but resolution of HCV infection without therapy after establishment of persistence is rare [5, 6]. A single causal event cannot be attributed to HCV clearance in the chronic phase. Individual cases of HCV clearance have been reported after superinfection with hepatitis A [7], hepatitis B [8, 9], or hepatitis D [10] viruses, orthotopic liver transplantation [11], surgery [12], or in the absence of precipitating events [5], but the underlying immunological mechanisms of HCV clearance in these instances have never to our knowledge been studied.
Chronic HCV infection is defined as HCV persistence for >6 months after infection and is characterized by relatively stable HCV RNA titers, low levels of liver inflammation, weak HCV-specific T-cell responses, and strong neutralizing antibody responses that drive HCV sequence evolution [13, 14]. Spontaneous recovery after acute HCV infection is associated with vigorous HCV-specific CD4 and CD8 T-cell responses (for a review, see [14]) that are maintained for decades after HCV clearance [15]. In contrast, in chronic infection, HCV-specific CD4 and CD8 T cells are focused on few HCV epitopes and decline to such low numbers that they are often undetectable in the blood [15, 16]. The few HCV-specific T cells that are detectable display an exhausted phenotype with decreased ability to proliferate, to kill infected target cells, or to produce cytokines [16]. This T-cell dysfunction, initially identified in the mouse model of lymphocytic choriomeningitis virus (LCMV) infection, has been attributed to high levels of persisting viral antigen [17] that drive the expression of the inhibitory receptor-programmed death–1 (PD-1) on virus-specific T cells. Its ligand, PD-L1, is constitutively expressed on intrahepatic sinusoidal endothelial cells, Kupffer cells, and stellate cells and is upregulated on hepatocytes in an interferon (IFN)-α– and IFN-γ–dependent manner. Interaction of PD-L1 with PD-1 on liver-infiltrating T cells results in inhibition of antiviral effector functions [18]. Accordingly, PD-1+ HCV-specific T cells display a dysfunctional phenotype in the blood [19] and liver [20].
The contribution of the humoral immune response to the clearance of HCV infection is less clear. Paradoxically, HCV can be cleared in the acute phase of infection without contribution of antibodies (eg, in hypogammaglobulinemic patients) [21] but is able to persist in the chronic phase in the presence of neutralizing antibodies. Indeed, neutralizing antibodies typically appear after chronic HCV infection is established [22]. They fail to clear the virus at this stage, but exert selection pressure on viral variants and contribute to the evolution of the HCV envelope sequences through decades of chronic HCV infection [23].
Here, we identified an individual who progressed from acute to chronic HCV genotype 1a infection, but cleared HCV after >65 weeks without therapy. The subject was a participant in the Swan Project, a prospective, longitudinal cohort study of young, uninfected injection drug users [24], the population at highest risk of acquiring new HCV infection in the United States. Uninfected study participants are followed prospectively, and humoral and cellular immune responses are analyzed from the time they point they were diagnosed HCV RNA positive. To investigate the cellular and humoral events underlying this rare phenomenon, we performed a detailed prospective analysis that included IFN-γ enzyme-linked immunospot (ELISPOT) assays, phenotyping of HCV-specific T cells, plasma cytokine estimations, neutralizing antibody assays, and HCV sequencing.
MATERIALS AND METHODS
Patient Enrollment, Sample Collection, and HCV Antibody and HCV RNA Testing
The Swan Project is a longitudinal study of young persons (aged 18–35) who inject illicit drugs and are recent initiates to injection drug use (≤5 years of use) living in New York [24]. Study participants who test negative for HCV antibody (HCV 3.0 enzyme-linked immunosorbent assay [ELISA], Ortho Clinical Diagnostics, Raritan, NJ) undergo prospective biweekly follow-up, including risk behavior interviews, harm reduction counseling, referrals to needed services, and HCV RNA testing by discriminatory HCV transcription-mediated amplification assay (Procleix HIV-1/HCV, Chiron, Emeryville, CA). Once participants test positive for HCV RNA, blood samples are drawn weekly for alanine aminotransferase (ALT) and HCV RNA quantitation (Cobas Taqman HCV, Roche Diagnostics, Indianapolis, IN). Plasma and peripheral blood mononuclear cells (PBMCs) are separated as described elsewhere [25] from whole blood specimens drawn biweekly and stored until use at −80°C and −180°C, respectively. Participants who do not clear infection within 90 days are offered antiviral treatment through a unique multidisciplinary program. The study was approved by the institutional review boards of Weill Cornell Medical College and State University of New York Downstate College of Medicine. All participants provided written informed consent.
ELISPOT Assays
IFN-γ ELISPOT assays were performed as described elsewhere [25] using 600 15-mer peptides with 10-amino-acid overlap representing the complete HCV 1a proteome in a 43-peptide pool matrix array in addition to pools of 6 cytomegalovirus (CMV) and 15 Epstein-Barr virus (EBV) epitopes. The final peptide concentration in the assay was 1 μg/mL, and the dimethyl sulfoxide content was 0.2%.
Tetramer Synthesis and Staining
HLA-A3/peptide monomers, containing the CORE-51(KTSERSQPR) epitope, a dominant CD8 T-cell target in this subject were synthesized at the National Institute of Allergy and Infectious Diseases Tetramer Facility of the National Institutes of Health AIDS Research and Reference Reagent Program, Atlanta, GA, and assembled into tetramers by step-wise addition of allophycocyanin (APC)–conjugated streptavidin (Invitrogen Corp., Camarillo, CA). In total, 3–4 × 106 PBMCs were stained with 5 μg/mL ethidium bromide monoazide (Sigma-Aldrich Corporation, St. Louis, MO) under fluorescent light for 8 minutes, washed twice, and stained with 1 μL tetramer (1:100 dilution) for 20 minutes at room temperature. Cells were washed, stained with anti-CD14-Phycoerythrin(PE)-Cy5 (clone 61D3, Abd Serotec, Raleigh, NC), anti-CD3-Alexa 700 (cloneUCHT1), anti-CD19-PE-Cy5 (clone HIB19), anti-CD8-Pacific Blue (clone RPA-T8), anti-HLA-DR-APC-Cy7 (clone L243; all from BD Biosciences, San Jose, CA) anti-PD-1-PE (clone H12.2H7, Biolegend, San Diego, CA), anti-CD127-FITC (clone eBioRDR5), anti-CD38-PE-Cy7 (clone HIT2; both from eBioscience, San Diego, CA) and analyzed on a BD LSRII cytometer using FACS Diva version 6.1.3 (BD Biosciences) and FlowJo (version 8.8.6) software (Tree Star Inc, Ashland, OR). Controls consisted of samples stained with all antibodies except for antibodies against either PD-1, CD38, HLA-DR, CD27, CD28, and CD127, respectively.
HCV Sequence Analysis
A 5.2-kilobase (kb) HCV gene product spanning the 5′UTR to the NS3/NS4A region was amplified by reverse-transcription polymerase chain reaction (RT-PCR) as described elsewhere [26]. The PCR product was purified using a QIAquick PCR purification kit (Qiagen, GmbH) and cloned with the TOPO XL PCR cloning kit (Invitrogen, Carlsbad, CA). In total, 10–20 colonies were subjected to a colony PCR with conditions identical to the one described for the nested PCR [26]. Overlapping parts of the HCV genome were sequenced on a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA), aligned with Codon Code Aligner (Version 2.0.2, Codon Code Corp., Dedham, MA), and analyzed using the ClustalW2 multiple sequence alignment program (www.ebi.ac.uk/Tools/clustalw2).
Quantitation of Serum Cytokines
Bead arrays (BD Biosciences) were used to quantitate plasma CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CXCL9 (MIG), CXCL10 (IP-10), CXCL11 (I-TAC), IFN-α, IFN-γ, tumor necrosis factor (TNF), TNFRII, interleukin (IL)-8, IL-10, IL12p70, IL-13, FasL, and GM-CSF. Enzyme immunoassays (EIAs) were used to quantitate plasma TRAIL (CD253, BD Opt EIA, San Jose, CA), CXCL6, CCL19, IL-15, IL-18 and IL-22 (R&D Systems, Minneapolis, MN).
Antibody Assays
To detect HCV E2 antibodies Galanthus nivalis lectin (1 μg/mL, Sigma) coated plates were incubated with 0.25 μg each of recombinant HCV E2 genotype 1a (ImmunoDiagnostics, Wilburn, MA) and 1b protein (eEnzyme, Montgomery Village, MD) for 2 hours at room temperature. Fifty microliters serum from uninfected blood donors or the study participant were added at a 1:100 dilution with 5% bovine serum albumin in phosphate-buffered saline (PBS). After 1 hour at room temperature, plates were washed 3 times with PBS, and the captured antibodies were detected with mouse antihuman immunoglobulin G (IgG) horseradish peroxidase (Serotec; 1:20 000 dilution) for 1 hour. After 3 washes with PBS, tetramethylbenzidine super-sensitive l-C (BioFX Laboratories, Owings Mill, MD) was added, and optical density was determined at 450 nm with a microplate reader (POLAR Star, BMG Labtech, Cary, NC).
To detect neutralizing antibodies [25], serial 1:200–1:3200 dilutions of heat-inactivated plasma were incubated in duplicates for 1 hour at 37°C with 100 immunostaining units/well of HCV with genotype 1a (H-NS2/NS3-J virus [27]) or genotype 2a structural proteins (JFH-1 [28]). The virus–plasma mixture was then incubated with Huh7.5.1 cells for 4 hours at 37°C in 96-well plates. Unbound virus was removed with a single wash and cells were incubated with fresh Dulbecco’s modified Eagle’s medium with 3% fetal calf serum for 96 hours (H-NS2/NS3-J virus) or 72 hours (JFH1), followed by methanol fixation and HCV NS5A staining [29]. The neutralization was presented as reciprocal 50% inhibitory dilution (ID50) calculated by linear interpolation using the formula [(50% − low percentage)/(high percentage − low percentage)] × (high dilution − low dilution) + low dilution [30].
To detect neutralizing antibodies against autologous HCV sequences, lentiviral pseudoparticles encoding autologous HCV E1E2 sequences and a luciferase reporter gene were generated. Briefly, HCV E1E2 expression plasmids were generated by amplifying parts of the 5.2-kb PCR products (Table 1) with genotype 1a–specific primers [31]. Purified E1E2 PCR products (GenBank Accession number: JQ268595, JQ268596, JQ268597, JQ268598) were used for the generation of HCV pseudoparticles (HCVpp) as described elsewhere [31]. HCVpp were incubated with serial dilutions of heat-inactivated plasma for 1 hour at 37°C and added in quadruplicate cultures of Hep3B hepatoma cells in a 96-well plate. After removing unbound pseudoparticles after 5 hours, cells were incubated with fresh media for 72 hours followed by measurement of pseudoparticle infectivity as relative light units (RLUs). Percent neutralization was calculated as follows: 100 × [1 − (RLUtest/RLUcontrol)] and the ID50 was calculated as described previously in this section.
Table 1.
Amino Acid Changes in the HCV E2 Sequence During the Course of HCV Infection
 |
To exclude coinfections, serum samples were tested for antibodies to hepatitis B virus (HBV) core antigen (Anti-HBc Assay, Ortho-Clinical Diagnostics, Rochester, NY), and to human immunodeficiency virus type 1 (HIV-1; HIV-1/HIV-2 Plus O EIA assay, Bio-Rad, Hercules, CA). IgG and immunoglobin M (IgM) antibodies to influenza A virus were quantitated in serum samples from weeks 2, 4, 12, 16, 18, 22, 25, 30, 48, and 78 (IBL-America, Minneapolis, MN).
RESULTS
Course of HCV Infection in a Subject Who Spontaneously Cleared Chronic Hepatitis C
We studied prospectively from prior to infection through more than 2 years of follow-up, a white male who developed high HCV titers of 359672 IU/mL (genotype 1a) within 3 weeks of infection via injection drug use. Anti-HCV seroconversion was seen by week 7. He subsequently developed acute hepatitis with ALT levels of 603 U/L 11–12 weeks after infection (Figure 1A) and increased levels of inflammatory cytokines (IL-18, CXCL10) 6 weeks after infection (Figure 1B). Serum levels of TNFRII, CCL2, CCL5, CXCL9, CXCL11, and TRAIL peaked shortly before ALT did, and CCL5 levels correlated inversely with the ALT peak (Figure 1B). Acute hepatitis resulted in a decrease in HCV titers by about 2 log10, but HCV titers rebounded within a week. By week 20, the subject had settled into a typical chronic phase of hepatitis with high, relatively stable HCV titers (Figure 1A) and a low degree of inflammation (ALT range, 84–181 U/L). CCL2, CXCL9, CXCL11, and TRAIL concentrations in the serum returned to normal levels, but TNFRII, CCL5, IL-18, and CXCL10 levels remained increased in the chronic phase of HCV infection, indicating low-level continuing inflammation (Figure 1B). At week 48, ALT levels increased slightly to 210 U/L, then decreased and stayed within normal range for the remainder of the study. Following ALT normalization at week 65, HCV RNA became undetectable at week 78, and remained undetectable until week 122 (Figure 1) and a year thereafter (not shown). HCV clearance was not associated with any cytokine increase.
Figure 1.
Clinical course and serological biomarkers of HCV infection in a subject who spontaneously cleared HCV after established chronic infection. A, Serum ALT levels (red line) and HCV titer (black line) are shown over time. The upper limit of normal for ALT at the testing laboratory is 45 U/L. The lower limit for detection of the quantitative HCV RNA assay is 20–28 IU/mL. Anti-HCV: HCV antibodies were determined by HCV 3.0 ELISA. B, Serum cytokine concentrations throughout the course of infection. CCL3, CCL4, interleukin (IL)-6, IL-8, IL-10, IL-12p70, IL-13, IL-15, TNF, FasL, GM-CSF, interferon (IFN)-α, and IFN-γ levels did not change throughout the course of infection (not shown). The horizontal bars indicate the acute phase (red), chronic phase (orange), and HCV clearance phase (green) as described in panel A and the results section.
Abbreviations: ALT, alanine aminotransferase; ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus.
HCV-Specific Humoral Immune Responses
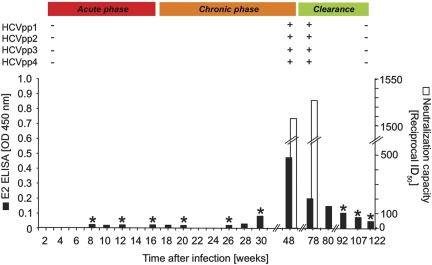
HCV-specific antibodies were first detected by standard clinical EIA 7 weeks after infection. Because this assay does not include HCV envelope proteins as antigens, we tested serum samples from selected time points in a lab-based EIA for the presence of HCV E2–specific antibodies, which appeared week 30 after infection and peaked at week 48 (Figure 2). To evaluate HCV neutralization, we tested the capacity of plasma to block infection of human hepatoma cells by an HCV strain with genotype 1a structural proteins (H-NS2/NS3-J virus) [27]. Neutralizing antibodies appeared at week 48, and their neutralizing capacity increased further by week 78 after infection (Figure 2). These antibodies were specific for the infecting HCV genotype because the genotype 2a JFH-1 HCV strain was not neutralized (not shown), and they were short-lived because they were undetectable from week 92 onward (Figure 2).
Figure 2.
HCV envelope-specific humoral immune response. Black bars represent the titer of HCV E2-specific antibodies as tested at the indicated time points by EIA. White bars represent the reciprocal serum dilution that is able to neutralize chimeric HCV genotype 1a strain by 50% (ID50). Asterisks indicate time points at which neutralizing antibodies were not detected. HCVpp with autologous HCV E2 sequences indicated in Table 1. Symbols + and – indicate the presence or absence of antibodies neutralizing the indicated HCVpp as described in the text. A positive value is defined as >200 reciprocal ID50. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus; HCVpp, HCV pseudoparticles; ID50, 50% inhibitory dilution; OD, optical density.
The appearance of neutralizing antibodies at week 48 coincided with the de novo appearance of several nonsynonymous mutations in the E2 hypervariable region 1 in the majority of sequenced HCV clones (Table 1). This region encompasses a conformational epitope with the sequence TAGRETAGFASFF, which is recognized by the monoclonal antibody 9/27 with a strong neutralization capacity [32]. To evaluate whether these sequences represented HCV escape mutants, we generated 4 HCVpp bearing the most frequent mutated HCV envelope sequences (Table 1). Plasma samples obtained at weeks 3, 48, 78, and 122 were tested for the presence of neutralizing antibodies that blocked the entry of these HCVpp into hepatoma cells. Whereas no neutralization was detected in the week 3 sample, all HCVpp were neutralized by weeks 48 and 78, with higher than 200 reciprocal ID50 (Figure 2). These mutations may therefore represent an unsuccessful bid by HCV to evade humoral immune responses. The neutralizing antibody response was transient and was undetectable at week 122.
HCV-Specific T-Cell Responses
To study a possible contribution of T-cell responses to HCV clearance, we first performed IFN-γ ELISPOT assays. A multispecific T-cell response was observed at weeks 8 and 12 (Figure 3), coinciding with the rise in ALT levels and a temporary decrease in HCV titer. However, both the magnitude and the breadth of the T-cell response decreased by week 25 and week 30 as typical for chronically evolving hepatitis C. To investigate whether the failure to clear HCV in the acute phase was due to viral escape mutations, we sequenced a 5.2-kb fragment from the HCV 5′UTR to the NS3/NS4A region at weeks 3, 6, 8, 12, and 30. No mutations were found.
Figure 3.
Interferon γ (IFN-γ) production by HCV-specific T cells. Bars represent the cumulative IFN-γ ELISPOT response of PBMCs to overlapping peptides spanning the indicated HCV sequences. Responses against p7, NS2, and the NS4A sequence are not shown because the respective peptides were not tested in separate peptide pools.
Abbreviations: E1/2, envelope regions 1/2; ELISPOT, enzyme-linked immunospot; HCV, hepatitis C virus; NS, nonstructural; PBMCs, peripheral blood mononuclear cells.
At week 48, a small but noticeable restoration of the T-cell response was observed, which was further amplified by week 78, resulting in a 9-fold increase in the cumulative number of IFN-γ–producing cells compared with week 30. Responses against each structural and nonstructural HCV antigen were detectable at this time point (Figure 3 and not shown), but elevations in ALT or in cytokine levels were not observed. The change in T-cell responsiveness was HCV-specific because IFN-γ responses to 6 CMV and 15 EBV epitopes did not noticeably increase (not shown).
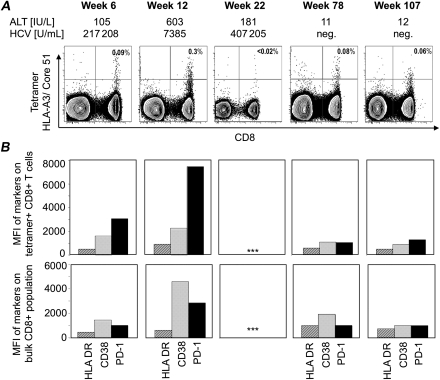
Using an HLA-A3 tetramer presenting the HCV core-51 epitope, HCV-specific CD8+ T cells were detected as early as 6 weeks after infection and peaked at week 12 (Figure 4A), the time of maximal ALT levels and a temporary decrease in HCV titer of >2 log10. Tetramer-positive T cells expressed high levels of PD-1 at this time, and a smaller increase in expression of the activation markers HLA-DR and CD38 (Figure 4B). In comparison, the bulk CD8 T-cell population displayed significantly lower PD-1 levels consistent with the interpretation that PD-1 induction was the result of HCV-specific T-cell stimulation. Consistent with the waning ELISPOT responses, the frequency of HCV-CORE-51–specific CD8+ T cells decreased to undetectable levels by week 22. Surprisingly, however, it was restored at weeks 78 and 107, reaching almost the same level as at week 6 but without significant increase in HLA-DR, CD38, and PD-1 expression.
Figure 4.
Frequency and phenotype of HCV-specific T cells. A, Frequency of HLA-A3/core-51–positive T cells during the acute and chronic phase of hepatitis C and shortly after resolution of infection. B, Changes in the expression level of HLA-DR, CD38, and PD-1 on HCV-specific, tetramer HLA-A3/core-51–positive CD8 T cells (upper panel) and on bulk CD8 T cells (lower panel) at the time points indicated in panel A. Abbreviations: HCV, hepatitis C virus; MFI, mean fluorescence intensity.
DISCUSSION
In this study, we investigated the sequence of immunological events that culminated in the spontaneous clearance of HCV 65–78 weeks after infection and >1 year after the acute phase of hepatitis subsided. Consistent with previous studies [33], the HCV-specific T-cell response in this subject was strongest during the acute stage of infection when ALT levels peaked, but despite strong T-cell and cytokine responses, HCV clearance was not achieved in the acute phase.
What are the mechanisms of HCV clearance in the chronic phase of infection? The first immunological event was the neutralizing antibody response that appeared at week 48. The effectiveness of this antibody response was evidenced by the >1 log decline in viral titer and the concomitant viral mutations in all HCV clones obtained at this time point. These viral variants were efficiently countered by neutralizing antibodies and thus were not able to facilitate escape from humoral surveillance. This is unusual because the appearance of neutralizing antibodies in chronic hepatitis C typically does not result in HCV clearance but in selection of HCV escape mutants [23].
The reconstitution of the HCV-specific T-cell response was secondary to the neutralizing antibody response because the increase in HCV-specific T-cell responses was still small at week 48 and significantly increased only at the next study time point, week 78. Compared to the acute hepatitis phase (week 12), PD-1 and CD38 expression was considerably lower on HCV-specific CD8+ T cells, and the breadth of the IFN-γ effector response was superior. Although we did not evaluate other exhaustion markers due to limited cell numbers, it is notable that Tim-3, 2B4, CD160, and KLRG1 are typically coexpressed with PD-1 in HCV infection [34, 35]. Indeed, the degree of PD-1 expression on virus-specific T cells correlates directly with the extent of their dysfunction in HCV [19, 20, 36, 37] and in HIV infections [38–40]. Thus, it appears that hyperactivated HCV-specific T cells, as exemplified by CD38 and PD-1 expression, are associated with the induction of hepatitis but do not necessarily mediate disease resolution. In fact, successful HCV clearance in the acute phase is temporally associated with the appearance of CD38– CD8+ IFN-γ–producing T cells [41].
It is therefore conceivable that the neutralizing antibody response and the HCV-specific T-cell response, the latter “jumpstarted” by the temporary but significant decline in HCV titer, contributed to HCV clearance. Even if this spontaneous reversal of T-cell exhaustion was the effect rather than the cause of HCV clearance, this observation remains of interest because successful antiviral therapy in the acute phase is not associated with enhancement of T-cell responses [42, 43]. Although spontaneous reconstitution of impaired and exhausted T-cell responses has not yet been reported in viral infections in humans, our findings are consistent with the following related observations: In one study on chronic HBV infection, reduction of HBV titers by antiviral therapy resulted in a transient recovery of HBV-specific T-cell function that lasted for approximately 6 months [44, 45]. Unfortunately, the contribution of the improved T-cell function on HBV clearance could not be evaluated separately in that study due to the concomitant effect of the antiviral therapy. In another study using the mouse model of chronic LCMV infection, it was reported that mice with low viral titer responded better to therapeutic vaccination than those with high viral titer, as evidenced by improved vaccine-induced IFN-γ responses of LCMV-specific T cells [46]. These studies underscore the importance of viral titer in determining T-cell responsiveness and suggest that the lowering of viral load may be the primary prerequisite for immunotherapeutic approaches targeting chronic viral infections.
Despite strong evidence that both humoral and cellular responses were involved in HCV clearance, the actual trigger that kick-started the immune response leading to HCV clearance remains a matter of conjecture. The possibility of heterologous viral infections in triggering HCV-specific T-cell responses has previously been suggested by us [47] and others [48]. However, we ruled out HBV and HIV-1 infections in this subject and found no significant change in EBV- and CMV-specific T-cell responses and in influenza A virus–specific T-cell and antibody responses, rendering this scenario unlikely.
We believe that this is the first detailed immunological report of HCV clearance more than a year after the acute phase of HCV infection ended. Our data provide proof-of-principle that “exhaustion” of CD8+ T cells is not an irreversible phenomenon, at least in the first 2 years of persistent HCV infection. These findings may provide fresh impetus to research on immunotherapeutic options, such as the possibility of combining novel antivirals [49] with targeted blockade of PD-1/PD-L1 signaling [17] to rescue exhausted HCV-specific T cells.
Notes
Acknowledgments.
We thank Michael P. Busch and Leslie H. Tobler, Blood Systems Research Institute, San Francisco, CA, for diagnostic and quantitative HCV RNA testing; Marla Shuh, Center for the Study of Hepatitis C, Weill Medical College, NY, for assistance with study coordination; and the National Institute of Allergy and Infectious Diseases (NIAID) Tetramer Facility of the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Atlanta, GA, for tetramer synthesis.
Financial support.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NIH intramural research program and by NIH grants R01DA16159, R01DA021550, M01RR000047, and UL1 RR 024996.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Scott JD, McMahon BJ, Bruden D, et al. High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives. Clin Infect Dis. 2006;42:945–52. doi: 10.1086/500938. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Saito T, Shinzawa H, et al. Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: a population-based cohort study. J Med Virol. 2003;71:56–61. doi: 10.1002/jmv.10448. [DOI] [PubMed] [Google Scholar]
- 7.Cacopardo B, Nunnari G, Nigro L. Clearance of HCV RNA following acute hepatitis A superinfection. Dig Liver Dis. 2009;41:371–4. doi: 10.1016/j.dld.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Jessner W, Strasser M, Graziadei I, Berr F, Vogel W. Sustained remission of chronic hepatitis C after acute hepatitis B superinfection. Scand J Infect Dis. 2006;38:818–21. doi: 10.1080/00365540600606523. [DOI] [PubMed] [Google Scholar]
- 9.Gruener NH, Jung MC, Ulsenheimer A, et al. Hepatitis C virus eradication associated with hepatitis B virus superinfection and development of a hepatitis B virus specific T cell response. J Hepatol. 2002;37:866–9. doi: 10.1016/s0168-8278(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 10.Deterding K, Pothakamuri SV, Schlaphoff V, et al. Clearance of chronic HCV infection during acute delta hepatitis. Infection. 2009;37:159–62. doi: 10.1007/s15010-007-7204-7. [DOI] [PubMed] [Google Scholar]
- 11.Suneetha PV, Mederacke I, Heim A, et al. Spontaneous clearance of chronic hepatitis C after liver transplantation: are hepatitis C virus-specific T cell responses the clue? Liver Transpl. 2008;14:1225–7. doi: 10.1002/lt.21559. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa M, Morimoto Y, Shiroi A, Yoshiji H, Kuriyama S, Fukui H. Spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C. Am J Gastroenterol. 2001;96:922–3. doi: 10.1111/j.1572-0241.2001.03650.x. [DOI] [PubMed] [Google Scholar]
- 13.Heller T, Rehermann B. Acute hepatitis C: a multifaceted disease. Semin Liver Dis. 2005;25:7–17. doi: 10.1055/s-2005-864778. [DOI] [PubMed] [Google Scholar]
- 14.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 16.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 17.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 18.Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152–60. doi: 10.1055/s-2007-979468. [DOI] [PubMed] [Google Scholar]
- 19.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semmo N, Lucas M, Krashias G, Lauer G, Chapel H, Klenerman P. Maintenance of HCV-specific T-cell responses in antibody-deficient patients a decade after early therapy. Blood. 2006;107:4570–1. doi: 10.1182/blood-2005-11-4522. [DOI] [PubMed] [Google Scholar]
- 22.Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101:10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–78. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Edlin BR, Shu MA, Winkelstein E, et al. More rare birds, and the occasional swan. Gastroenterology. 2009;136:2412–4. doi: 10.1053/j.gastro.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veerapu NS, Raghuraman S, Liang TJ, Heller T, Rehermann B. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology. 2011;140:676–85 e1. doi: 10.1053/j.gastro.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Netski DM, Mao Q, et al. Accurate representation of the hepatitis C virus quasispecies in 5.2-kilobase amplicons. J Clin Microbiol. 2004;42:4223–9. doi: 10.1128/JCM.42.9.4223-4229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol. 2007;81:629–38. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenbach BD, Evans MJ, Syder AJ, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 30.Lindenbach BD. Measuring HCV infectivity produced in cell culture and in vivo. Methods Mol Biol. 2009;510:329–36. doi: 10.1007/978-1-59745-394-3_24. [DOI] [PubMed] [Google Scholar]
- 31.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–86. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu M, Zhang J, Flint M, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271–6. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.McMahan RH, Golden-Mason L, Nishimura MI, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–57. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengsch B, Spangenberg HC, Kersting N, et al. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–53. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. doi: 10.1053/j.gastro.2008.02.033. 1937 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, et al. High level of PD-1 expression on hepatitis C virus (HCV)–specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–60. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 41.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman F, Heller T, Sobao Y, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 43.Lauer GM, Lucas M, Timm J, et al. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979–88. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boni C, Bertoletti A, Penna A, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968–75. doi: 10.1172/JCI3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boni C, Penna A, Ogg GS, et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology. 2001;33:963–71. doi: 10.1053/jhep.2001.23045. [DOI] [PubMed] [Google Scholar]
- 46.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wedemeyer H, Mizukoshi E, Davis AR, et al. Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–26. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 49.Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8:69–71. doi: 10.1038/nrgastro.2010.219. [DOI] [PubMed] [Google Scholar]