Abstract
Background. Anthrax-associated shock is closely linked to lethal toxin (LT) release and is highly lethal despite conventional hemodynamic support. We investigated whether protective antigen–directed monoclonal antibody (PA-mAb) treatment further augments titrated hemodynamic support.
Methods and Results. Forty sedated, mechanically ventilated, instrumented canines challenged with anthrax LT were assigned to no treatment (controls), hemodynamic support alone (protocol-titrated fluids and norepinephrine), PA-mAb alone (administered at start of LT infusion [0 hours] or 9 or 12 hours later), or both, and observed for 96 hours. Although all 8 controls died, 2 of 8 animals receiving hemodynamic support alone survived (median survival times 65 vs 85 hours, respectively; P = .03). PA-mAb alone at 0 hour improved survival (5 of 5 animals survived), but efficacy decreased progressively with delayed treatment (9 hours, 2 of 3 survived; 12 hours, 0 of 4 survived) (P = .004 comparing survival across treatment times). However, combined treatment increased survival irrespective of PA-mAb administration time (0 hours, 4 of 5 animals; 9 hours, 3 of 3 animals; and 12 hours, 4 of 5 animals survived) (P = .95 comparing treatment times). Compared to hemodynamic support alone, when combined over PA-mAb treatment times (0, 9, and 12 hours), combination therapy produced higher survival (P = .008), central venous pressures, and left ventricular ejection fractions, and lower heart rates, norepinephrine requirements and fluid retention (P ≤ .03).
Conclusions. PA-mAb may augment conventional hemodynamic support during anthrax LT-associated shock.
Mortality in patients with shock during the 2001 US anthrax outbreak was high [1, 2]. Although the mechanisms and optimal management of this shock remain unclear, the risk of anthrax persists, as demonstrated by recent cases among injection drug abusers in Europe [3, 4]. Seventeen of 52 patients from this outbreak died, many with progressive shock despite standard intensive care unit (ICU) support.
Anthrax bacilli produce 2 toxins: lethal toxin (LT) and edema toxin (ET) [5, 6]. Each is composed of a toxic moiety (lethal factor [LF] or edema factor [EF], respectively) and protective antigen (PA), a component necessary for LF or EF uptake by host cells. LF is a zinc endopeptidase that cleaves mitogen-activated protein kinase kinases (MAPKK), whereas EF is a calmodulin-dependent adenyl cyclase. Of the two, LT is the more lethal and thought to play a central role in the pathogenesis of anthrax shock [7–20].
Despite the contribution of LT-induced shock to the pathogenesis of anthrax, its treatment has received little study. Conventional therapy for septic shock (which would include hemodynamic support with fluid and vasopressor administration titrated to physiologic endpoints like mean arterial pressure) has not been investigated in models of anthrax. In rats, fixed (nontitrated) doses of either normal saline or norepinephrine (NE) administered as 24-hour infusions were beneficial against lipopolysaccharide (LPS) or Escherichia coli challenge, but not LT challenge [20, 21]. Notably, however, inability to titrate these therapies in the LT-challenged rodent model may have produced adverse effects that limited benefit. Besides hemodynamic support, toxin inhibition has also been proposed for the treatment of life-threatening anthrax infection. Both polyclonal (Anthrax Immune Globulin Intravenous [AIGIV], Cangene Corp, Winnipeg, Canada) and monoclonal (raxibacumab; Human Genome Sciences, Rockville, MD) antibody preparations, directed in part or solely against PA, have been added to the US Strategic National Stockpile. However, whether such preparations have additive benefit when combined with conventionally titrated hemodynamic support is not clear [21–27]. Defining the effects of titrated hemodynamic support, alone or together with protective antigen–directed monoclonal antibody (PA-mAb), for LT-associated shock is important.
We previously developed a sedated, instrumented, and mechanically ventilated canine model in which LT, infused over 24 hours to simulate toxin release during infection, produced progressive hypotension and organ dysfunction [16]. We employed this model to investigate the effects of titrated hemodynamic support and PA-directed monoclonal antibody (raxibacumab) alone or together, for LT-induced shock. Although hemodynamic support was available (if indicated) from the start of toxin infusion, PA-mAb was administered either early at the initiation of toxin (0 hours), or later at 9 or 12 hours to investigate the effect of delayed treatment as might occur clinically.
METHODS
Study Design
The National Institutes of Health Clinical Center Animal Care and Use Committee approved all experiments. Four purpose-bred beagles (weighing 11–14 kg) with venous and pulmonary and systemic arterial catheters, urinary catheter, and tracheostomy tube were studied every week, as described elsewhere [16]. Sedation, maintenance fluids, and mechanical ventilation were similarly applied to all groups using standardized ICU protocols. After stabilization and starting at 0 hours, all animals were challenged with a 24-hour LT infusion. During each of the first 5 experiments (weeks 1–5), 1 animal from each group of 4 was allocated at 0 hours to 1 of the following: (1) no treatment (placebo alone at 0 hours)—controls, (2) hemodynamic support alone (plus placebo at 0 hours), (3) PA-mAb alone at 0 hours, or (4) hemodynamic support together with PA-mAb at 0 hours. Thus, each therapy was studied concurrently during each week of study. During study weeks 6–8, animals were again allocated to each of the same 4 treatment groups, but PA-mAb or placebo were administered 9 hours after starting LT. It was evident after 8 experiments that controls (no treatment) were associated with uniform lethality, and hemodynamic support alone had only limited benefit. From an animal welfare perspective, we were not justified in investigating these 2 groups further. The primary question at that point was whether additional delay in treatment with PA-mAb (either alone or with hemodynamic support) would further influence its efficacy. Therefore, during weeks 9 and 10, the 4 animals studied weekly were allocated to only 2 groups: PA-mAb administered alone at 12 hours, or PA-mAb at 12 hours with hemodynamic support. Hemodynamic monitoring was started as indicated (see below) at 0 hours and continued until the end of the study (96 hours) for all 10 experiments. Cardiopulmonary and other laboratory measures were obtained immediately before and at regular intervals after initiation of toxin, as described elsewhere (Figure 1) [16]. Whereas blood pressure, heart rate (HR), temperature, and oxygen saturation were continuously monitored, central venous pressure (CVP) was recorded every 2 hours, and pulmonary artery opening pressure (PAOP) was measured every 2 hours for the first 8 hours, and every 4 hours thereafter for the rest of the study. Arterial blood gases (ABGs) were obtained every 2 hours for the first 8 hours, and every 8 hours or as needed based on protocol thereafter. Left ventricular ejection fraction (LVEF) was measured by echocardiography at 24-hour intervals starting at 0 hours. Total fluid intake was recorded every 2 hours, whereas urine output was recorded every 24 hours and at time of death. Surviving animals were euthanized at 96 hours after recording measurements.
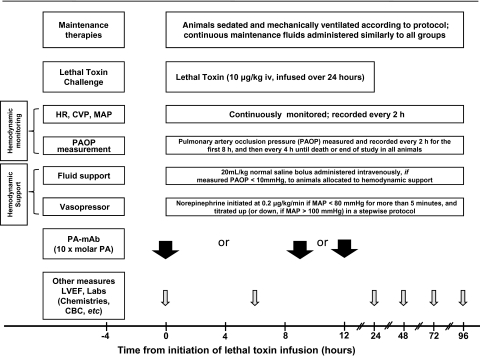
Figure 1.
Timeline of experimental interventions, measurements, and treatments. As outlined in “Methods,” at the initiation of 24-h lethal toxin infusions, animals were randomized to receive hemodynamic support alone, protective antigen–directed monoclonal antibody (PA-mAb) at the time of or 9 or 12 h after starting toxin infusion, hemodynamic support combined with PA-mAb (administered at 0, 9, or 12 h), or no treatment (control). Hemodynamic support included a single bolus of 20 mL/kg of normal saline if the pulmonary artery occlusion pressure (PAOP, checked every 2 h for the first 8 h and every 4 h thereafter) was <10 mmHg. Also, if at any time the mean arterial blood pressure (MAP) decreased to <80 mmHg for >5 min, a norepinephrine (NE) infusion was initiated at 0.2 μg/kg/min and, if necessary, increased in a stepwise fashion every 5 min to 0.6, 1, or a maximum of 2 μg/kg/min. NE was titrated down in a stepwise fashion if MAP was >100 mmHg for >5 min. Abbreviations: ABG, arterial blood gas; CBC, complete blood count; CVP, central venous pressure; HR, heart rate; LVEF, left ventricular ejection fraction (measured with echocardiography).
Toxin and Treatments
Lethal toxin was prepared as described elsewhere (20 μg/kg PA + 10 μg/kg LF) [16]. The dose employed was designed to produce a control lethality rate >50% when infused over 24 hours. The PA-mAb dose (10 × molar amount of PA administered to each animal) was administered as a single 2 mL intravenous injection at 0, 9, or 12 hours as described elsewhere [28]. Animals not assigned to PA-mAb treatment (ie, control and hemodynamic support alone groups) received a similar volume of placebo (an inactive, nonspecific monoclonal antibody) concurrently.
To ensure that all animals entered the study with similar preloads, PAOP was measured prior to randomization, and if PAOP was <10 mmHg, 1 to 3 boluses (20 mL/kg) of normal saline were administered until a PAOP of at least 10 mmHg was achieved. Thereafter, animals assigned to groups receiving hemodynamic support were administered a single bolus of 20 mL/kg of normal saline if the PAOP (checked every 2 hours for the first 8 hours and every 4 hours thereafter) was found to be <10 mmHg. Additionally, if at any time the mean arterial blood pressure (MAP) decreased to <80 mmHg for >5 minutes, NE infusion was initiated at 0.2 μg/kg/min and, if necessary, increased in a stepwise fashion every 5 minutes to 0.6, 1, or a maximum of 2 μg/kg/min (and similarly titrated down if MAP was >100 mmHg for >5 min). Amounts of fluid and NE received by each animal were recorded every 2 hours. Animals in the control and the PA-mAb only groups also had hemodynamic measurements performed and recorded, but did not receive fluid boluses or NE. All supportive therapies were administered by technicians blinded to PA-mAb allocation.
Ventilator management, temperature control, and sedation with midazolam, fentanyl, and dexmedetomidine were managed uniformly for all groups on the basis of previously reported protocols [29]. Stepwise ventilator adjustments were made to FiO2, positive end–expiratory pressure, and respiratory rate on the basis of continuous pulse oximetry and ABGs performed at regular intervals. All animals received maintenance fluids throughout (Normasol-M at 3 mL/kg/h for the first 36 hours, 2 mL/kg/h for the next 36 hours, then 1 mL/kg/h until the end of the study) [29]. Additional care for all animals included gastrointestinal and deep venous thrombosis prophylaxis, plus ceftriaxone to prevent catheter-related infections as described elsewhere [29].
Statistical Methods
Survival times were compared between treatment groups using exact log rank tests, and the association between survival times and PA-mAb starting times were assessed using Tarone-Ware trend test (StatXact, Cytel Software Corp, Cambridge, MA) [30]. For the remaining variables (log10-transformed if the distribution was right-skewed), the change from baseline values for individual animals were averaged for each 24-hour period. Data for individual treatment groups were averaged across weekly experiments where appropriate. To evaluate shock reversal, we standardized MAP and NE using z scores and then calculated a “shock reversal” score (designated as shock index [SI]) using the difference of the MAP z score and NE z score, with a higher score indicating improved hemodynamics. Linear mixed models (SAS PROC Mixed, SAS version 9.2, Cary, NC) were used to compare the change from baseline values of different treatments for each 24-hour period [31]. Repeated measurements of each animal and the actual pairing of animals within each week were accounted for in the model. Standard residual diagnostics were used to check model assumptions. Two-sided P values of .05 or less were considered significant without adjusting for multiple comparisons.
RESULTS
Survival
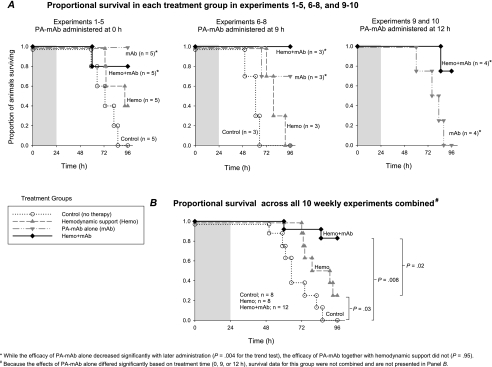
Over weeks 1–5 and 6–8, all 8 control animals (ie, receiving neither hemodynamic support nor PA-mAb) died following LT challenge, while 2 of 8 animals receiving hemodynamic support alone survived (Figure 2A). In animals receiving PA-mAb alone, 5 of 5 treated at 0 hours, 2 of 3 treated at 9 hours, and none of the 4 treated at 12 hours survived. In animals receiving hemodynamic support and PA-mAb, 4 of 5 treated with PA-mAb at 0 hours, 3 of 3 treated at 9 hours, and 3 of 4 treated at 12 hours survived. When analyzed across the 3 treatment times, PA-mAb was significantly less effective with later treatment when used alone (P = .004 for the trend test comparing 0- vs 9- vs 12-hour treatment), but not when combined with hemodynamic support (P = .95 comparing the 3 treatment times). Across all experiments (excluding the PA-mAb alone group, since survival differed significantly across the 3 treatment times), compared with controls, hemodynamic support alone or with PA-mAb increased survival significantly (P = .03 and .008, respectively; Figure 2B). Furthermore, combination therapy was significantly more effective than hemodynamic support alone (P = .02). Finally, when examining experiments in which PA-mAb was administered at 9 or 12 hours compared with combination therapy, PA-mAb alone was associated with significantly reduced survival (P = .03).
Figure 2.
Proportion of animals surviving over time after a 24-h lethal toxin challenge (shaded area) and treated with conventional hemodynamic support alone (Hemo) or protective antigen–directed monoclonal antibody (PA-mAb) alone (mAb), or both (Hemo + mAb) or neither (placebo alone, control). The 3 panels in Figure 2A show experiments in which PA-mAb or placebo was administered at 0, 9, or 12 h, respectively. The efficacy of PA-mAb alone decreased significantly with delay in administration (P = .004 for the trend test) but not when it was combined with hemodynamic support (P = .95, trend test). Figure 2B displays proportional survival in animals in the 3 groups shown combined over all 10 experiments, irrespective of timing of PA-mAb. Because the effects of PA-mAb alone differed significantly with time of administration, survival data from this group could not be combined over experiments and are not presented in panel B. Two-group comparison P values labeled in Figure 2B were based on exact log-rank tests.
On the basis of these survival results, other laboratory data were analyzed to first understand why combining hemodynamic support and PA-mAb together was more beneficial than hemodynamic support alone. This analysis compared controls and animals receiving hemodynamic support alone or with PA-mAb. Data were combined across all experiments (regardless of PA-mAb treatment times) because effect of therapy on survival did not vary significantly by treatment time. Then, to investigate why PA-mAb alone later (at 9 and 12 hours) was less effective than combination therapy, we compared early (0 hours) or later (9 and 12 hours) treatment with PA-mAb alone to combination therapy. Time trend analysis showed that decreases in the efficacy of PA-mAb alone from early (0 hours) to later (9 or 12 hours) treatment were highly significant (P = .004), consistent with prior studies in rats testing the effects of PA-mAb [28, 32]. Previous studies in this canine model had also shown that hemodynamic dysfunction develops within 4 to 8 hours of the initiation of LT challenge [16]. These findings, in combination with the fundamental difference in methodology comparing treatment at the start versus following the initiation of challenge, provided the basis for analyzing the 9- and 12-hour PA-mAb–alone groups together.
Effects of Hemodynamic Support, Alone or in Combination with PA-mAb
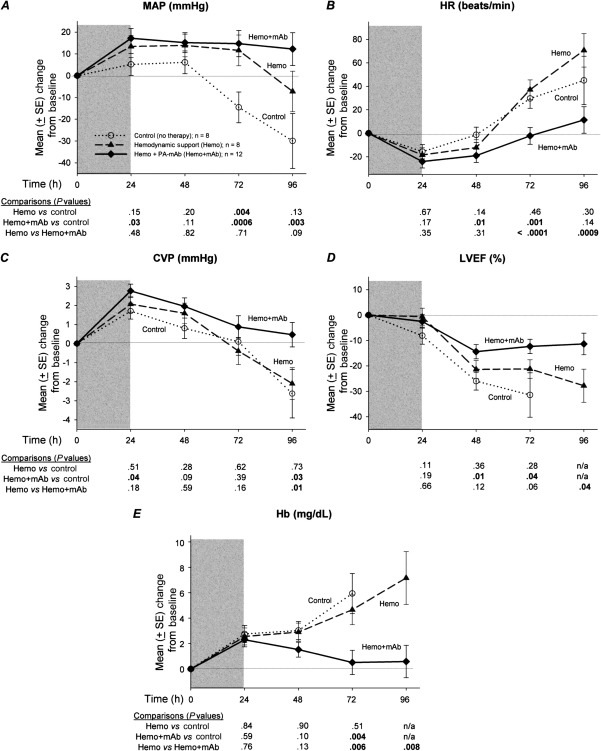
Compared with no treatment (controls), hemodynamic support alone (experiments 1–8) increased MAP, and this was significant at 72 hours (P = .004; Figure 3). However, this treatment did not significantly alter any other hemodynamic parameter measured throughout the study.
Figure 3.
Mean (± standard error [SE]) changes from baseline over the study period in mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), hemoglobin (Hb), and left ventricular ejection fraction (LVEF, as measured by echocardiography) in canines challenged with a 24-h infusion of lethal toxin (shaded area) and treated with either conventional hemodynamic support alone (Hemo) or in combination with protective antigen directed monoclonal antibody (Hemo + mAb) compared with placebo-treated controls (no therapy). Except for LVEF, these parameters were measured either continuously or multiple times every 24 h, and data shown at various time points are daily averages of values recorded in the preceding 24-h period. Number of animals from which data are gathered every day may be determined from the survival plots in Figure 2. Data shown are least-square means, and comparisons are made between groups every 24 h; corresponding P values (from linear mixed models) are shown below each graph, with P ≤ .05 considered significant.
When averaged over the 3 PA-mAb treatment times and all experiments, compared with controls, combination therapy with hemodynamic support and PA-mAb resulted in higher MAP and CVP, smaller declines in LVEF, and lower HR, that reached significance at ≥1 time points (24, 48, 72, or 96 hours) (P ≤ .04; Figure 3). Compared with hemodynamic support alone, combination therapy produced higher CVP (P = .01) and smaller decreases in LVEF (P = .04) at 96 hours, and lower HR at 72 and 96 hours (P < .0009). Consistent with higher CVP, hemoglobin concentrations were lower with combined treatment compared with either controls at 72 hours or hemodynamic support alone at 72 and 96 hours (P ≤ .008; Figure 3).
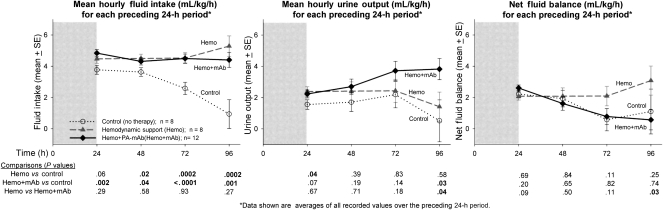
As designed, compared with controls administered maintenance fluid only, animals receiving titrated hemodynamic support (alone or with PA-mAb) received more fluid through most of the study (P ≤ .04; Figure 4). However, animals receiving hemodynamic support alone did not have significantly higher urine outputs than did controls (except at 24 hours, P = .04), whereas animals receiving both hemodynamic support and PA-mAb did (significant at 96 hours, P = .03). Thus, although both groups treated with hemodynamic support received similar amounts of fluid, the combination therapy group had higher urine output and retained less fluid, and these differences were significant at 96 hours (P ≤ .03).
Figure 4.
Mean (± standard error [SE]) fluid intake, urine output, and net fluid balance over each 24-h period of the study in lethal toxin (LT)–challenged canines randomized to no therapy (control), conventional hemodynamic support alone (Hemo), or in combination with protective antigen–directed monoclonal antibody (Hemo + mAb). The format for this figure is similar to the previous ones. Shaded areas represent time over which LT was infused, and number of animals contributing to data at each time point may be determined from survival plots in Figure 2. All P values were based on linear mixed models, and P ≤ .05 were considered significant.
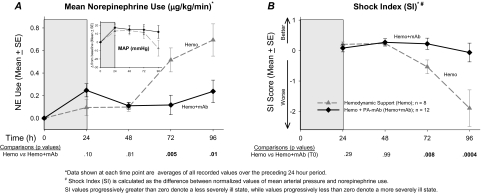
Controls received no NE during the study and were not used to analyze this treatment in other groups. Animals that received hemodynamic support alone required progressively higher NE doses. In comparison, animals that received combination therapy required less NE at 72 and 96 hours (P ≤ .01; Figure 5). Because MAP and NE use were interdependent (ie, NE was titrated based on MAP), we combined the 2 parameters into a composite score termed the shock index (SI; see “Methods”) to further evaluate hemodynamic stability. A higher score represents greater hemodynamic stability (ie, higher blood pressure with lower NE requirement). Compared with animals receiving hemodynamic support alone, those also receiving PA-mAb had higher SI at 72 and 96 hours (P ≤ .008; Figure 5).
Figure 5.
A, Mean (± standard error [SE]) amounts of norepinephrine (NE) usage averaged over each 24-h period required to maintain mean arterial blood pressure (MAP) ≥80 mmHg in the 2 groups of LT-challenged animals randomized to this treatment—Hemo and Hemo + mAb. Inset shows corresponding changes form baseline in MAP in the 2 groups, similar to Figure 3A. The Hemo group required a significantly higher amount of NE to maintain similar MAP levels compared with the Hemo + mAb group over the second half of the study (P values for comparisons below figure). B, Because MAP and NE usage are interdependent, we present a combined score, termed the shock index (SI), by calculating the difference between normalized values of MAP and NE usage. Values progressively greater than zero denote a less severely ill state, whereas values less than zero denote more severe illness. The Hemo + mAb group did significantly better than the Hemo group over the second half of the study period (P ≤ .008). All P values were based on linear mixed models, and P ≤ .05 were considered significant.
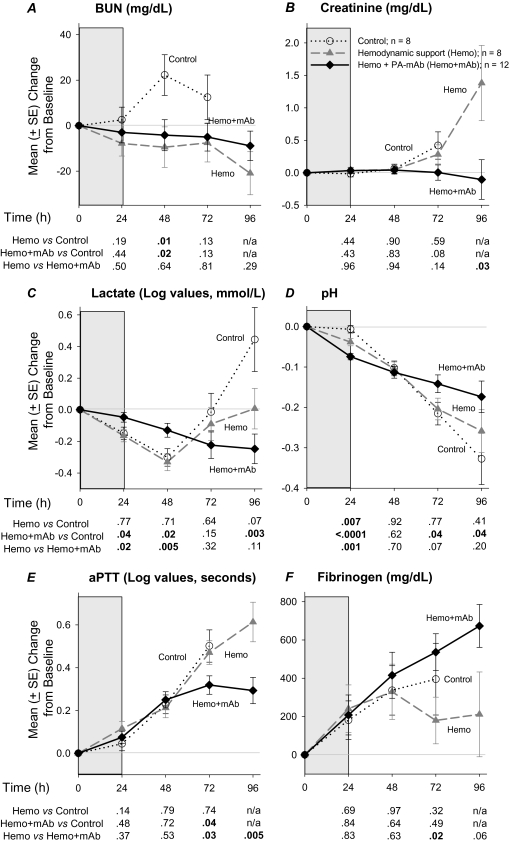
Compared with controls, blood urea nitrogen levels in both groups receiving hemodynamic support decreased (significant at 48 hours; P ≤ .02). However, compared with hemodynamic support alone, combination treatment with PA-mAb was also associated with lower creatinine at 96 hours (P = .03; Figure 6). Compared with controls, hemodynamic support alone did not alter lactate significantly but did transiently decrease pH at 24 hours (P = .007). Lactate levels with combination therapy, although higher at 24 and 48 hours (P ≤ .04), were significantly lower than controls at 96 hours (P = .003). Consistent with this, with combination therapy, although pH decreased more than in controls at 24 hours, it was higher later (P ≤ .04). Compared with hemodynamic support alone, the combination produced higher lactates at 24 and 48 hours (P ≤ .02) and lower pH at 24 hours (P = .001), but the reverse at 72 and 96 hours. Compared with controls, hemodynamic support alone did not alter activated partial thromboplastin time (aPTT) or fibrinogen significantly. Combination therapy resulted in smaller increases in aPTT compared to controls at 72 hours and to hemodynamic support alone at 72 and 96 hours, and increased fibrinogen compared with the latter at 72 hours (P ≤ .05 for all). Finally, compared with controls, hemodynamic support alone increased glucose at 24 hours, whereas combination therapy increased glucose at 24 and 72 hours, PaCO2 at 24 hours, and calcium at 72 hours (P ≤ .05 for all; data not shown). Compared with hemodynamic support alone, the combination increased PaCO2 at 24 hours and glucose at 72 hours (P ≤ .05; data not shown).
Figure 6.
Mean (± standard error [SE]) changes from baseline over study period in blood urea nitrogen (BUN), creatinine, lactate, pH, activated partial thromboplastin time (aPTT), and fibrinogen (F) measurements in canines challenged with a 24-h infusion of lethal toxin (shaded areas) and treated with placebo (control), or conventional hemodynamic support alone (Hemo) or in combination with protective antigen–directed monoclonal antibody (Hemo + mAb). Data shown are least square means; number of animals contributing data at each time point may be determined from the survival plots in Figure 2. Comparisons between groups are made using averaged data at 24-h intervals; corresponding P values are shown below each figure. Note that for lactate and aPTT, values plotted as change from baseline are log values. All P values were based on linear mixed models, and P ≤ .05 were considered significant.
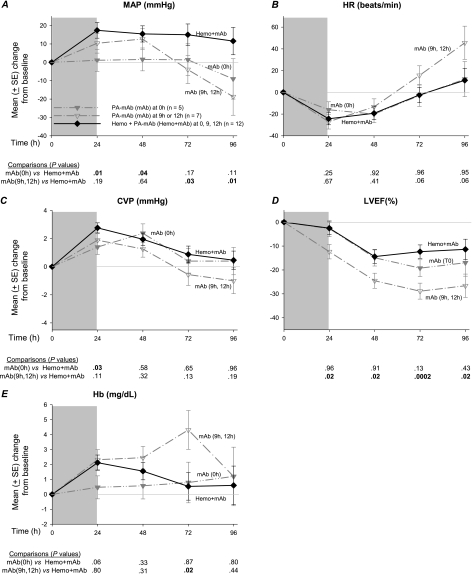
Effects of Early or Later PA-mAb Alone Compared With Combination Therapy
With respect to hemodynamic parameters, compared with combination therapy, early treatment (0 hours) with PA-mAb alone was associated with lower MAP at 24 and 48 hours and CVP at 24 hours (P ≤ .05; Figure 7). Later treatment (9 and 12 hours) with PA-mAb alone, however, was associated with lower MAP at 72 and 96 hours, and lower LVEF at all time points (ie, 24, 48, 72, and 96 hours). Although decreases in CVP were not significant with later PA-mAb alone, increases in hemoglobin at 72 hours were significant (P = .02). Animals that received PA-mAb alone did not receive NE or titrated fluids, and so measures of SI and net fluid balance for this group were not analyzed. For other data, compared with combination therapy, early PA-mAb treatment alone produced smaller increases in glucose at 24 hours, greater increases in aPTT at 72 and 96 hours, greater decreases in lactate at 48 hours and PT at 24 and 48 hours, and smaller decreases in pH at 24 hours (P ≤ .05; data not shown). Later treatment with PA-mAb alone produced lower PaCO2 and glucose at 24 hours, smaller decreases in HCO3 and pH at 24 hours, and greater increases in creatinine at 72 hours and aPTT at 96 hours (P ≤ .05; data not shown). Of all parameters measured, the 2 that differed significantly when comparing early versus late treatment with PA-mAb alone were LVEF and aPTT (P ≤ .004).
Figure 7.
Mean (± standard error [SE]) changes from baseline over the study period in mean arterial blood pressure (MAP), heart rate (HR), central venous pressure (CVP), left ventricular ejection fraction (LVEF), and hemoglobin (Hb) in canines challenged with a 24-h infusion of lethal toxin (shaded area) and treated with either protective antigen–directed monoclonal antibody (PA-mAb; mAb in the figure) alone or a combination of hemodynamic support and PA-mAb (Hemo + mAb). Data for the combination therapy group were combined across all PA-mAb administration times [0 h (n = 5), 9 h (n = 3), or 12 h (n = 4)]. Animals treated with PA-mAb alone are divided into 2 groups on the basis of early administration of treatment [0 h (n = 5)] or later administration [9 h and 12 h (n = 7)]. The format for this figure is similar to Figure 3. All P values were based on linear mixed models, and P ≤ .05 was considered significant.
DISCUSSION
In canines challenged with anthrax LT, the protective effect of PA-mAb alone on survival decreased significantly as treatment was delayed from 0 to 12 hours. However, when PA-mAb was added to hemodynamic support, whether given early or late, it was associated with improved survival compared with hemodynamic support alone. One explanation for these findings is that the pathogenic effects of LT contributing to death in the model—and potentially reversible with PA-mAb—progressed rapidly and were no longer preventable by PA-mAb alone at later time points. Hemodynamic support may have delayed these early events, or provided other benefit which PA-mAb augmented, even when administered later. Alternatively, cardiovascular or cellular dysfunction with LT may have compromised tissue delivery or the actions of delayed PA-mAb alone, but hemodynamic support may have prevented this dysfunction.
Both in vitro and in vivo studies have shown that LT can alter both peripheral vascular and myocardial function, and this dysfunction is associated with lethality in in vivo models [14–16, 33–36]. Therefore, inhibition of these LT-mediated effects provides one basis for combination therapy resulting in better survival than hemodynamic support alone. Although MAP did not differ between these groups, adding PA-mAb resulted in lower NE needs and a better SI than hemodynamic support alone. Better hemodynamics with combination therapy may have resulted from improved myocardial function (increased LVEF) or improved peripheral vascular function, either directly by protection of arterial function, or indirectly from improved venous capacitance (reflected by higher CVP). Increased preload (higher CVP) may in itself have increased LVEF. Notably, increases in CVP and LVEF with combination therapy were not related to excessive fluid replacement or fluid retention because urine output was actually greater, and net fluid balance lower, compared with hemodynamic support alone. Improved organ function and/or protective effects with combination therapy were also reflected at later time points by decreased creatinine and acidosis.
Different from small animal studies, our current findings in this canine model provide evidence that shock related to LT during anthrax infection is amenable to treatment with conventionally titrated hemodynamic support [20, 21]. Furthermore, such support and LT inhibition together may have additive benefit. However, the present findings do not support the use of LT inhibition without concomitant hemodynamic support in patients presenting later, with established anthrax and shock. Ultimately, however, in a mass casualty situation where the ability to administer intensive ICU support may be curtailed, administration of antibiotics and toxin inhibitors might be considered with fluid support alone, or possibly even without it. PA-mAb without hemodynamic support may deserve consideration if administered very early (eg, time of spore challenge and prior to onset of systemic effects).
Study of Class A biological agents such as Bacillus anthracis is hampered by the infrequency of clinical infection and biosafety challenges associated with in vivo bacterial experiments. Although therapies requiring infrequent dosing and little animal contact (eg, antimicrobials or antitoxins) can be investigated under such conditions, therapies like hemodynamic support, requiring frequent titration based on physiologic endpoints, cannot. Employing alternative models like ours, which recapitulate key pathogenic events during infection, but which are amenable to frequent manipulations, may provide additional insights into management. Such models are also more conducive to repetitive testing and can be better validated. Thus, our findings complement in vivo experiments assessing the effects of PA-mAb therapy alone in spore-challenged models [37]. Ultimately, however, it would be ideal to investigate the effects of a treatment like PA-mAb in bacteria-challenged models that permit the full complement of supportive therapies.
The PA-mAb employed in this study (raxibacumab), although reportedly safe when administered in normal humans, has not yet been tested clinically during infection [37, 38]. This preparation was highly effective when administered without antibiotics in spore-challenged nonhuman primates and rabbits [37]. Our findings provide additional data supporting the potential usefulness of this preparation.
This study has several limitations. First, the pathogenesis of shock with anthrax is likely related not only to LT but to other bacterial components such as ET and the peptidoglycan of anthrax cell wall [10, 16, 39–45]. Whether titrated hemodynamic support and PA-mAb would have additive benefit during live bacterial infection in which these other pathogenic mediators would have a role is unclear. Second, hemodynamic support in the present study was restricted to fluid and NE administration. Other vasopressors or inotropes such as phenylephrine, vasopressin, or dobutamine might also have beneficial effects and require study. Also, although our model simulates standard ICU care in the use of concomitant therapies (eg, heparin, famotidine, or ceftriaxone), these may have altered the pharmacokinetics of LT or PA-mAb. However, individually assessing their impact in a model such as the present one would be very difficult. Lastly, a large number of comparisons were made without adjustment for multiple comparisons. Therefore, the results should be considered exploratory in nature.
In conclusion, although hemodynamic support alone improved survival in this canine model, PA-mAb augmented this benefit, whether administered early or late. These findings add to others suggesting that inhibition of LT during anthrax infection may improve outcome, even in patients who have already developed evidence of shock and organ injury.
Notes
Financial support.
This work was supported by the National Institutes of Health (NIH) Clinical Center, and a Trans-NIH/Food and Drug Administration Biodefense grant.
Potential conflicts of interest.
Recombinant anthrax toxins and PA–monoclonal antibody were provided by Human Genome Sciences, Rockville, MD. T. M., G. M. S., and S. D. B. are employees or former employees of Human Genome Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–44. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Update: investigation of bioterrorism-related inhalational anthrax–Connecticut, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:1049–51. [PubMed] [Google Scholar]
- 3.Booth MG, Hood J, Brooks TJ, Hart A. Anthrax infection in drug users. Lancet. 2010;375:1345–6. doi: 10.1016/S0140-6736(10)60573-9. [DOI] [PubMed] [Google Scholar]
- 4.Anthrax outbreak among heroin users in the United Kingdom and Germany. http://www.emcdda.europa.eu/online/annual-report/2010/boxes/p83. Accessed 1 June 2011. [Google Scholar]
- 5.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 6.Sherer K, Li Y, Cui X, Eichacker PQ. Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy. Am J Respir Crit Care Med. 2007;175:211–21. doi: 10.1164/rccm.200608-1239CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brossier F, Weber-Levy M, Mock M, Sirard JC. Role of toxin functional domains in anthrax pathogenesis. Infect Immun. 2000;68:1781–6. doi: 10.1128/iai.68.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–7. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley JL, Smith H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961;26:49–63. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Li Y, Li X, et al. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis. 2007;195:572–80. doi: 10.1086/510856. [DOI] [PubMed] [Google Scholar]
- 11.Moayeri M, Crown D, Dorward DW, et al. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;5:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–81. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson LE, Kuo SR, Katki K, et al. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson LE, Mock J, Lal H, et al. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci. 2007;12:4670–5. doi: 10.2741/2416. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, Moayeri M, Li Y, et al. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney DA, Cui X, Solomon SB, et al. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis. 2010;202:1885–96. doi: 10.1086/657408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby JE. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect Immun. 2004;72:430–9. doi: 10.1128/IAI.72.1.430-439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turk BE. Manipulation of host signalling pathways by anthrax toxins. Biochem J. 2007;402:405–17. doi: 10.1042/BJ20061891. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Frucht DM. Bacillus anthracis: a multi-faceted role for anthrax lethal toxin in thwarting host immune defenses. Int J Biochem Cell Biol. 2007;39:20–4. doi: 10.1016/j.biocel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Cui X, Su J, et al. Norepinephrine increases blood pressure but not survival with anthrax lethal toxin in rats. Crit Care Med. 2009;37:1348–54. doi: 10.1097/CCM.0b013e31819cee38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherer K, Li Y, Cui X, et al. Fluid support worsens outcome and negates the benefit of protective antigen-directed monoclonal antibody in a lethal toxin-infused rat Bacillus anthracis shock model. Crit Care Med. 2007;35:1560–7. doi: 10.1097/01.CCM.0000266535.95770.A2. [DOI] [PubMed] [Google Scholar]
- 22.Walsh JJ, Pesik N, Quinn CP, et al. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis. 2007;44:968–71. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- 23.Anaraki S, Addiman S, Nixon G, et al. Investigations and control measures following a case of inhalation anthrax in East London in a drum maker and drummer, October 2008. Euro Surveill. 2008;13 :pii: 19076. [PubMed] [Google Scholar]
- 24.Klempner MS, Talbot EA, Lee SI, Zaki S, Ferraro MJ. Case records of the Massachusetts General Hospital. Case 25–2010. A 24-year-old woman with abdominal pain and shock. N Engl J Med. 2010;363:766–77. doi: 10.1056/NEJMcpc1003887. [DOI] [PubMed] [Google Scholar]
- 25.Grabenstein JD. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin Infect Dis. 2008;46:129–36. doi: 10.1086/523578. [DOI] [PubMed] [Google Scholar]
- 26.Health Protection Scotland. Interim clinical guidance for the management of suspected anthrax in drug users. http://www.hps.scot.nhs.uk/anthrax/documents/clinical-guidance-for-use-of-anthrax-immune-globulin-v12-1-2010-03-19.pdf. Accessed 1 June 2011. [Google Scholar]
- 27. doi: 10.2807/ese.15.02.19465-en. Ramsay CN, Stirling A, Smith J, et al, on behalf of the NHS GGC, on behalf of the Scottish National Outbreak Control Teams. An outbreak of infection with Bacillus anthracis in injecting drug users in Scotland. Euro Surveill 2010; 15:pii: 19465. [DOI] [PubMed] [Google Scholar]
- 28.Cui X, Li Y, Moayeri M, et al. Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis. J Infect Dis. 2005;191:422–34. doi: 10.1086/427189. [DOI] [PubMed] [Google Scholar]
- 29.Minneci PC, Deans KJ, Hansen B, et al. A canine model of septic shock: balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol. 2007;293:H2487–500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 30.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2nd ed. New York: Springer; 2003. Statistics for Biology and Health. [Google Scholar]
- 31.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Corrected edition. New York: Springer, 2000. Springer Series in Statistics. [Google Scholar]
- 32.Altaweel L, Chen Z, Moayeri M, et al. Delayed treatment with W1-mAb, a chimpanzee-derived monoclonal antibody against protective antigen, reduces mortality from challenges with anthrax edema or lethal toxin in rats and with anthrax spores in mice. Crit Care Med. 2011;39:1439–47. doi: 10.1097/CCM.0b013e3182120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolcome RE, 3rd, Chan J. Constitutive MEK1 activation rescues anthrax lethal toxin-induced vascular effects in vivo. Infect Immun. 2010;78:5043–53. doi: 10.1128/IAI.00604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozes Y, Moayeri M, Wiggins JF, Leppla SH. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect Immun. 2006;74:1266–72. doi: 10.1128/IAI.74.2.1266-1272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolando M, Stefani C, Flatau G, et al. Transcriptome dysregulation by anthrax lethal toxin plays a key role in induction of human endothelial cell cytotoxicity. Cell Microbiol. 2010;12:891–905. doi: 10.1111/j.1462-5822.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 36.Kandadi MR, Hua Y, Ma H, et al. Anthrax lethal toxin suppresses murine cardiomyocyte contractile function and intracellular Ca2+ handling via a NADPH oxidase-dependent mechanism. PLoS One. 2010;5:e13335. doi: 10.1371/journal.pone.0013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migone TS, Subramanian GM, Zhong J, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–44. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian GM, Cronin PW, Poley G, et al. A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Infect Dis. 2005;41:12–20. doi: 10.1086/430708. [DOI] [PubMed] [Google Scholar]
- 39.Hsu LC, Ali SR, McGillivray S, et al. A NOD2-NALP1 complex mediates caspase-1–dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–8. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popov SG, Villasmil R, Bernardi J, et al. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–55. doi: 10.1016/S0006-291X(02)00227-9. [DOI] [PubMed] [Google Scholar]
- 41.Triantafilou M, Uddin A, Maher S, et al. Anthrax toxin evades Toll-like receptor recognition, whereas its cell wall components trigger activation via TLR2/6 heterodimers. Cell Microbiol. 2007;9:2880–92. doi: 10.1111/j.1462-5822.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 42.Cui X, Su J, Li Y, et al. Bacillus anthracis cell wall produces injurious inflammation but paradoxically decreases the lethality of anthrax lethal toxin in a rat model. Intensive Care Med. 2010;36:148–56. doi: 10.1007/s00134-009-1643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dal Molin F, Tonello F, Ladant D, et al. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006;25:5405–13. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P, Ahuja N, Bhatnagar R. Anthrax edema toxin requires influx of calcium for inducing cyclic AMP toxicity in target cells. Infect Immun. 2002;70:4997–5007. doi: 10.1128/IAI.70.9.4997-5007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA. 1982;79:3162–6. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]