Abstract
Therapeutic vaccines, when used alone or in combination therapy with antileishmanial drugs, may have an important place in the control of a variety of forms of human leishmaniasis. Here, we describe the development of an adenovirus-based vaccine (Ad5-KH) comprising a synthetic haspb gene linked to a kmp11 gene via a viral 2A sequence. In nonvaccinated Leishmania donovani–infected BALB/c mice, HASPB- and KMP11-specific CD8+ T cell responses were undetectable, although IgG1 and IgG2a antibodies were evident. After therapeutic vaccination, antibody responses were boosted, and IFNγ+CD8+ T cell responses, particularly to HASPB, became apparent. A single vaccination with Ad5-KH inhibited splenic parasite growth by ∼66%, a level of efficacy comparable to that observed in early stage testing of clinically approved antileishmanial drugs in this model. These studies indicate the usefulness of adenoviral vectors to deliver leishmanial antigens in a potent and host protective manner to animals with existing L. donovani infection.
The leishmaniases result from infection with protozoan parasites of the genus Leishmania. Spread across 88 countries worldwide, the leishmaniases are regarded as neglected tropical diseases [1, 2], a status reflected in underfunding of drug and vaccine development programs [3]. Antimonial drugs remain the mainstay of chemotherapy, although in the major endemic foci of human visceral leishmaniasis (HVL) in India, drug resistance has made these drugs of limited value. Only amphotericin B, paromomycin, and miltefosine are recognized alternatives [4]. Currently, there are no vaccines licensed for use in humans [5].
Vaccination against leishmaniasis has long been held to be achievable [6]. Self-cure provides long-lasting protection and is the basis of leishmanization. Epidemiological evidence supports a degree of cross-protection between Leishmania species. Prophylactic immunization in rodents, dogs, and primates has been partially successful using killed or attenuated parasites, crude parasite extracts, recombinant proteins, DNA or viral delivery, and peptide immunization [5]. To date, vaccines have had poor efficacy in clinical trials [7], and concerns remain about the ability of experimental models to predict protection against natural, sandfly-transmitted infection [5]. The latter issue is of less significance for therapeutic vaccination and immunotherapy studies, and despite the immunosuppression associated with established disease, both clinical and experimental data confirm the potential of these approaches [8–10]. Therapeutic vaccination may add to the few tools currently available to treat patients, shortening drug regimens and/or reducing dosage and reducing relapse rate [11]. In comparison with prophylaxis, it may provide an attractive proposition for vaccine development, given simpler clinical trial design [12].
Here, we report on the proof-of-concept stage of the development of a therapeutic vaccine for HVL. We demonstrate that 2 Leishmania antigens (HASPB and KMP11), delivered by a single dose of a recombinant adenoviral vector, significantly reduce parasite burden in a stringent mouse model. Vaccination was accompanied by newly detectable vaccine antigen-specific CD8+ T cell responses and boosting of pre-existing CD4+ T cell–dependent antibody responses, and vaccine efficacy benefited from the inherent adjuvant activity of the viral vector.
MATERIALS AND METHODS
Mice and Infections
Female BALB/c mice (Charles River) were maintained at the University of York under specific pathogen-free conditions and used at 6–12 weeks of age. L. donovani (MHOM/ET/67/L28/LV9) was maintained in B6.RAG1-/- mice, and amastigotes were isolated as described elsewhere [13]. Mice were infected intravenously with 2–3 × 107 amastigotes and were randomized to receive Ad5-KH (see below) or Ad5-GFP (Vector BioLabs) in 20–50 μL saline either subcutaneously at the base of the tail or intradermally in the footpad. Mice were killed 10 days after vaccination, and spleens were removed for assessment of parasite burden, as represented by Leishman Donovan Units (LDU; representing the number of amastigotes/1000 host cells × organ weight [13]) and for immunological analysis. Distribution of Ad-GFP was monitored by fluorescent stereomicroscopy [14]. All experiments were approved by the University of York Ethical Review Panel and were performed under UK Home Office license.
Recombinant Adenovirus
A synthetic haspb gene comprising the conserved N and C termini bordering 10 selected HASPB repeats was generated and linked to the coding region of kmp11 by the tetravirus TaV 2A sequence (RAEGRGSLLTCGDVEENPG; kindly provided by Prof. M. Ryan, St. Andrews, UK). The final sequence was back-translated using Gene Designer DNA2.0 software with codons optimized for human expression and selected to minimize DNA repeat structures. The construct was flanked by Kozak sequence 5′ of the ATG and a SV40-derived polyadenylation sequence to improve translation initiation and allow mRNA processing, respectively. The final synthetic gene, called huKMP11_HASPB_consensus, was synthesized under contract by Geneart. The gene was inserted into an E1/E3 deleted Ad5 viral vector supplied by Vector Biolabs. The viral particle to plaque-forming unit (pfu) ratio of the viruses used was ∼20–25:1. Protein expression was confirmed by Western blot using lysates from P815 cells transduced with virus (multiplicity of infection, 100:1) 24–36 hours earlier (data not shown, [15]).
ELISPOT Analysis
A truncated peptide library (PepSet) spanning the huKMP11_HASPB_consensus protein using 11mers with an overlap of 10 and offset of 1 was generated (Mimotopes). The 444 individual peptide sets (each containing a 11mer plus its respective truncated 10, 9, and 8 mers) were used in contiguous pools of 10 for initial epitope mapping studies or individually for fine mapping; 9-mer peptides were custom synthesized by ProImmune. ELISPOT plates (Mabtech) were coated with anti-IFNγ overnight, washed, and then blocked with complete RPMI 1640 (30 minutes at room temperature); 2.5 × 105 spleen cells or 105 purified CD8+ T cells (with 2 × 105 naive spleen cells) were seeded per well with or without peptides (2 μg/mL). After overnight culture, plates were washed, and numbers of IFN-γ spot-forming cells were detected by conventional methods. The detection limit for epitope-specific responses was conservatively estimated as 20 000 IFNγ+ CD8+ T cells per uninfected spleen and 25 000 IFNγ+ CD8+ T cells per infected spleen.
Flow Cytometry and Cytokine Analysis
Splenocytes (2 × 106 cells/well) were restimulated in vitro for 7 hours at 37°C in the presence of peptides (1 μg/mL) with Brefeldin A (10 μg/mL) added for the last 4 hours. Cells were surface stained (with CD3/CD90-PE-CY7, CD8 APC-Cy7/APC-H7, and CD4-PERCP), fixed (2% PFA, 10 minutes on ice), washed (FACS buffer + 0.5% saponin), and stained with IFNγ (XMG1.2)-PB, IL-10 (JES5-16E3)-APC, IL-2 (JES6-5H4)-PE, and TNF (MP6-XT22)-FITC. Isotype control antibodies were used to set markers for analysis and determination of the frequency of cytokine producing cells (using a CyAn analyser and Summit software; Beckman Coulter). The number of peptide-specific cells per spleen was calculated from the absolute number of CD3+CD8+ cells per spleen (obtained from count beads) that produced IFNγ after stimulation with KMP11 or HASPB minus the number of IFNγ-producing cells observed in the no antigen control samples.
Generation of Recombinant Proteins
His-tagged recombinant huKMP11 and rhuHASPB_consensus were purified by sequential nickel chelation chromatography (HisTrap), anion exchange chromatography (HiTrapQ), and gel filtration (Superdex 200 16/60 or Superdex75 16/60, for HASPB and KMP11, respectively) from French Press Escherichia coli lysates clarified by centrifugation and filtration [15]. Purifications were performed on an AKTApurifier100, with GE Healthcare columns. Proteins were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and concentrated for storage.
Antibody Responses
Ninety-six–well Maxisorp plates (Nunc) were coated overnight with 10 μg/mL of rhuKMP11 or rhuHASPB in carbonate buffer (pH, 9.6). Plates were washed and blocked with PBS/0.05% Tween/1% BSA for 2 hours at room temperature, and serum samples were added in 2-fold serial dilutions (2 hours, room temperature). Bound antibody was detected with peroxidase goat anti-mouse IgG1 or IgG2a (2 hours, room temperature) and quantified at 410 nm using ABTS substrate [13].
Statistical Analysis
With 8 mice per group, we calculated a 90% power to detect an inhibition of parasite burden of 50%, with a significance level of 0.05 (GraphPad Software). Data were analyzed by 1-way analysis of variance and Tukey’s Multiple Comparison Test, by paired Student’s t test or by χ2 test, as appropriate.
RESULTS
Development of a Synthetic Vaccine Gene
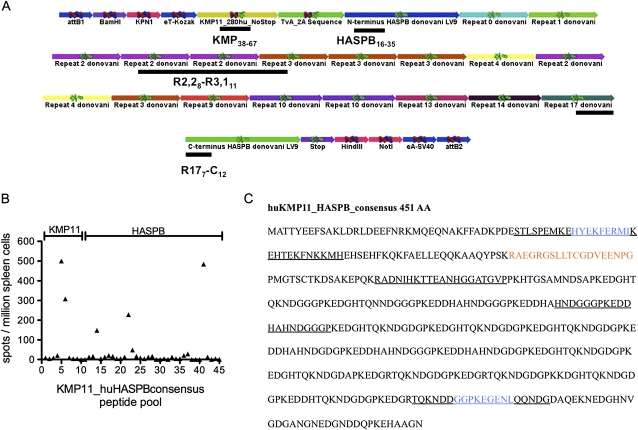
HASPB contains multiple polymorphic 11–14 AA repeats [15–17], and DNA sequencing has identified isolate-specific HASPB variants, with different numbers, composition, and relative order of repeats (Table 1). We designed a synthetic haspb gene (HASPB_consensus) encoding the conserved N and C termini bordering 10 of the repeats identified to date. To include also a conserved protein, we produced a synthetic gene with kmp11 (LinJ.35.2260/2270; Database version TriTrypDB 04.05.2011) and HASPB_consensus linked by a 2A sequence derived from the tetravirus Thosea asigna virus (TaV) [18] (Figure 1A). The fully synthetic gene with humanized codon use was cloned into an E1/E3-deficient adenovirus serotype 5 (Ad5) to generate the recombinant viral vaccine Ad5-huKMP11_HASPB_consensus (referred to as Ad5-KH).
Table 1.
Analysis of Repeat Type Frequency in Different Leishmania Species/Strains Causing Visceral Disease
|
Leishmania donovani Indian Isolates |
LV9a |
Leishmania chagasi |
L. infantum |
||||||||
| HASPB Amino Acid Repeats | Repeat Type | BHU3c34 | BHU3c32-6 | BHU55c65 | AG83c1 | AG83c6 | AG83c3 | LV9-11809 | LV9-11810 | K26 | LiJ23V3-1220 |
| PKEDGHTQKNDGGG | 0 | 1 | 1 | 1 | |||||||
| PKEDGHTQNNDGGG | 1 | 1 | 1 | ||||||||
| PKEDDHAHNDGGG | 2 | 6 | 5 | 5 | 2 | 6 | 1 | ||||
| PKEDGHTQKNDGDG | 3 | 2 | 2 | 1 | 5 | 2 | 22 | 3 | 3 | ||
| PKEDDHAHNDGDG | 4 | 3 | 2 | 3 | 2 | ||||||
| PKEDDHAHNDGNG | 5 | 1 | |||||||||
| PKEDDHAHNDGGC | 6 | 1 | |||||||||
| PEEDGHTQKNDGDG | 7 | 1 | |||||||||
| PKEDDHAHSDGGG | 8 | 1 | 1 | ||||||||
| PKEDGHTQKNDGDA | 9 | 1 | 1 | 1 | |||||||
| PKEDGRTQKNDGDG | 10 | 1 | 4 | 4 | |||||||
| PKEDGRTQRNDGDG | 11 | 1 | |||||||||
| PKEDGHTQKND | 12 | 1 | |||||||||
| PKKDGHTQKNDGDG | 13 | 1 | 1 | ||||||||
| PKEDDHTQKNDGDG | 14 | 1 | 1 | ||||||||
| PKKDDHAHNDGDG | 15 | 1 | |||||||||
| PKEDGHTQKNDDGG | 16 | 1 | |||||||||
| PKEDGRTQKNDDGG | 17 | 1 | 1 | ||||||||
| PKEDGRTQKNNGDG | 18 | 1 | |||||||||
| PKEDGRTQKNDGGG | 19 | 1 | |||||||||
LV9: MHOM/ET/67/L28/LV9 laboratory challenge strain.
Figure 1.
Epitope mapping of huKMP11_HASPB_consensus. A, Schematic diagram of huKMP11_HASPB_consensus. Each colored segment is annotated for clarity. Black underscore bars indicate approximate positions of epitopes defined in (B). Subscript numbers refer to repeat number (if appropriate) and respective AA position. B, Naive BALB/c mice were vaccinated subcutaneously with 108 pfu Ad5-KH. Peptide-specific responses to huKMP11_HASPB_consensus were assessed by ELISPOT assay of pooled spleen cells. Data reflect one experiment of three performed that gave similar results. C, Amino acid sequence of huKMP11_HASPB_consensus indicating TaV 2A sequence (red), regions containing dominant epitopes (underline) and confirmed KMP11 and HASPB C terminus epitopes (blue).
Immunogenicity and CD8+ T Cell Epitope Mapping of the Response to Ad5-KH
To determine immunogenicity and map CD8+ T cell epitopes, we used synthetic peptides that spanned the KH sequence in 20 AA segments. Uninfected BALB/c mice were vaccinated subcutaneously with 108 pfu Ad5-KH, and after 10 days, ELISPOT revealed a limited number of CD8+ T cell epitopes (Figure 1B). In HASPB, recognition was targeted to one 20mer region in the conserved N-terminus: one spanning the last repeat region and the conserved C-terminus and one spanning 2 repeats. For KMP11, recognition was limited to a 30 amino acid stretch (KMP1138–67). The location of these CD8+ T cell epitopes is indicated in Figure 1A and 1C.
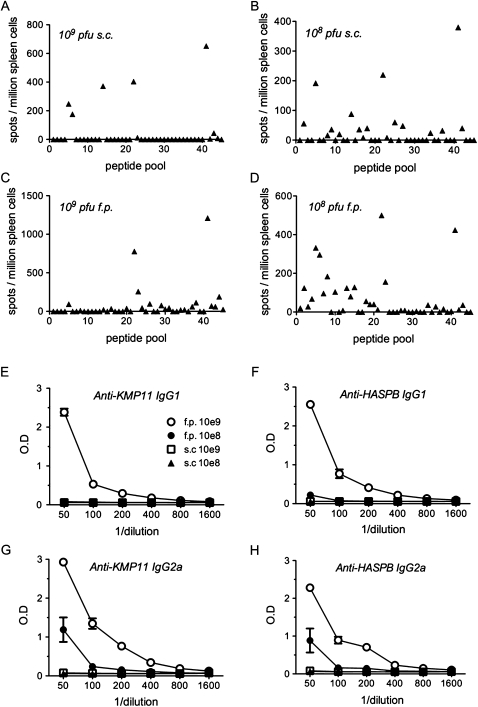
We next evaluated whether route and dose influenced the breadth and magnitude of the response (Figure 2). Increasing the dose by 10-fold had little effect on epitope selection. However, whereas immunogenicity of the HASPB epitopes increased by 2- and 4-fold, this was not the case for KMP11 responses (Figure 2A and 2B). Altering the route of vaccination also affected epitope selection and immunogenicity. The response to the HASPB C-terminal epitope was 2-fold greater when 109 pfu were administered in the footpad instead of subcutaneously (Figure 2C). Conversely, the response to the HASPB N-terminal epitope and to KMP11 was selectively absent (Figure 2C). Surprisingly, 108 pfu administered in the footpad gave the broadest epitope coverage, although at the expense of potency of individual epitopes (Figure 2D). Epitope selection was not influenced to any appreciable extent by the use of a poly-protein construct, because when KMP11 and HASPB were independently expressed in Ad5 and used for vaccination, similar epitopes were identified (data not shown). Thus, epitope selection is subtly influenced by both dose and route of administration, with combined responses of >2000 IFNγ+ CD8+ T cells per 106spleen cells induced by single dose vaccination.
Figure 2.
Response to vaccination with Ad5-KH is dose and route dependent. A–D, Naive BALB/c mice were vaccinated either subcutaneously (s.c.; A, B) or into the footpad (f.p.; C, D) with 109 pfu (A, C) or 108 pfu (B, D) Ad5-KH. At 10 days after vaccination splenocytes were prepared and restimulated with pooled pepsets and assayed by IFNγ ELISPOT assay. One of 2 experiments giving similar results is shown. E–H, IgG1 (E, F) and IgG2a (G, H) responses to recombinant KMP11 (E, G) and HASPB (F, H) were determined by ELISA in serum from mice vaccinated with 109 pfu f.p. (open circle), 109 pfu s.c (open square), 108 pfu f.p. (closed circle), or 108 pfu s.c. (closed triangle). Data represent mean ± standard error of the mean derived from 5 mice per group.
We next evaluated the IgG subclass response in vaccinated mice (Figure 2E–H), as indirect evidence for an underlying CD4+ T cell response. Footpad vaccination induced clear and dose-dependent IgG1 and IgG2a responses, but we failed to detect any response after subcutaneous vaccination, again pointing to route of administration as a determinant of the breadth of the host response. To establish whether differential virus distribution might account for these diverse responses, we evaluated the distribution of Ad5-GFP. After subcutaneous injection, GFP+ cells were readily detected in inguinal lymph nodes (LNs). In contrast, after footpad injection, intense GFP expression was observed in popliteal LNs, with GFP+ cells also detected in inguinal LNs and spleen (Supplementary Figure 1). Although qualitative, these data suggest that footpad immunization may lead to greater viral infection and systemic spread.
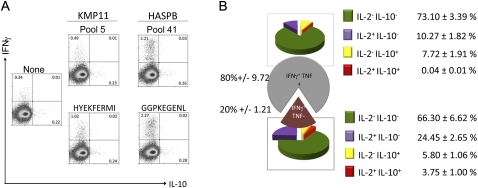
To more readily monitor CD8+ T cell responses, we fine-mapped the HASPB C terminus and the KMP11 epitopes (data not shown). We identified only one candidate epitope in each region, HASPBGGPKEGENL (where positions 1 and 2 derive from repeat 17 and positions 3–9 are derived from the C terminus) and KMP11HYEKFERMI (Figure 1C). Synthesized single peptides recalled responses similar to the pools from which they were identified (Figure 3A). The response to HASPBGGPKEGENL was associated with a greater intensity of IFNγ secretion detected by flow cytometry than that stimulated by KMP11HYEKFERMI, and we confirmed the poly-functionality of the HASPBGGPKEGENL CD8+ T cell response with respect to IFNγ, TNF, IL-2, and IL-10 (Figure 3B) [19].
Figure 3.
Fine mapping of KMP11 and HASPB epitopes. A, Naive BALB/c mice were vaccinated in the footpad with 109 pfu Ad5-KH and 10 days later splenocytes were restimulated with either the pooled pepsets from which the epitope was derived or synthetic peptides corresponding to predicted epitopes. After 3 h, Brefeldin A was added for an additional 4 h. Cells were surface-stained for CD3 and CD8 and then stained for intracellular IFNγ and IL-10. The percentage of cytokine positive cells is indicated in each quadrant. B, CD8+ T cells from mice vaccinated as above were restimulated with HASPB peptide, surface-stained for CD3 and CD8, and stained for intracellular cytokines. HASPB-specific CD8+ T cells were initially separated into IFNγ+TNF-or IFNγ+TNF+ subsets and then further sub-divided on the basis of IL-2 and IL-10 production. Pie charts reflect the relative contribution of the different cytokine-producing CD8+ T cell populations and percentage of each population is indicated. Data are mean ± standard error of the mean (5 mice).
CD8+ T cell Response of L. donovani–Infected Mice to HASPB and KMP11
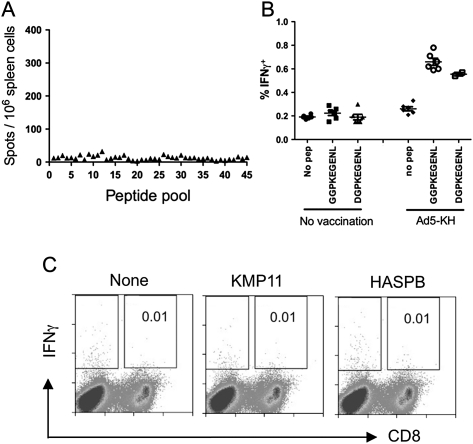
We next asked whether L. donovani–infected mice had detectable CD8+ T cell responses to these antigens. However, no CD8+ T cell responses could be observed in infected mice (Figure 4A). We studied 2 possible mechanisms that might account for this lack of recognition. First, in the dominant HASPBGGPKEGENL epitope, a HASPB repeat 17 provides position 1, whereas the LV9 challenge strain had a D for G substitution at this position (Table 1). However, synthetic peptides with either amino acid at position 1 had identical activity (Figure 4B), and HASPBDGPKEGENL also failed to stimulate CD8+ T cells in infected mice (data not shown). Second, to determine whether the lack of detectable response might reflect clonal exhaustion/suppression because of analysis at a relatively late stage of infection [20], we tested mice infected with L. donovani for only 7 days, when immune responses are relatively intact. These mice also failed to recognize the respective peptides (Figure 4C; data not shown). These data suggest that HASPB and KMP11 either fail to prime detectable numbers of CD8+ T cells or induce a very rapidly exhausted response during normal infection.
Figure 4.
HASPB- and KMP11-specific CD8+ T cells are not detectable in Leishmania donovani–infected mice. A, BALB/c mice were infected with 3 × 107 L. donovani amastigotes and at day 31 after infection, splenic CD8+ T cells were analyzed for responses to huKMP11_HASPB_consensus using pooled pepsets and ELISPOT assay. B, CD8+ T cell responses in day 21–infected BALB/c mice immunized with Ad5-KH were evaluated for IFNγ production by flow cytometry after restimulation with GGPKEGENL (contained in Ad5-KH) or DGPKEGENL (as found in the LV9 challenge strain). C, BALB/c mice infected for 7 days with L. donovani were tested for CD8+ T cell responses using KMP11HYEKFERMI and HASPBGGPKEGENL.
Therapeutic Vaccination Using Ad5-KH
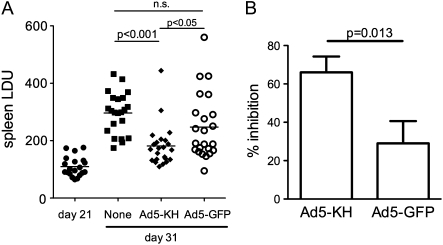
To evaluate the therapeutic efficacy of Ad5-KH, mice infected for 21 days were vaccinated and splenic parasite burden was assessed 10 days later. In preliminary studies, we failed to observe any significant protection using Ad5-KH administered subcutaneously (data not shown) and, therefore, focused on vaccination with 109 pfu Ad5-KH administered in the footpad. Splenic parasite burden in control nonvaccinated mice increased from day 21 to day 31 after infection, as expected [13, 21], whereas in vaccinated mice parasite burden at day 31 was significantly lower (P < .001; n = 23) (Figure 5A). Compared with unvaccinated mice, the increase in spleen parasite burden from day 21 to day 31 was inhibited by 66% ± 8% in mice vaccinated with Ad5-KH (vs 29% ± 12% in Ad5-GFP vaccinated mice; P = .013) (Figure 5B). With use of the lower value of the 95% confidence interval of the mean parasite burden of unvaccinated mice as a defining threshold, 91% (21/23) of Ad5-KH vaccinated mice were defined as protected. Mice vaccinated with Ad5-GFP had significantly higher parasite burdens than those vaccinated with Ad5-KH (P < .05) (Figure 5A), which were not significantly different from unvaccinated mice. However, Ad5-GFP vaccinated mice did show a trend indicative of a degree of nonspecific protection compared with unvaccinated mice, at least in some mice (65% responders; P < .03 vs. Ad5-KH vaccinated) (Figure 5A). Thus, Ad5-KH constitutes an effective therapeutic intervention in this experimental model of infection, with a component of protection likely being attributable to nonspecific effects of the viral vector.
Figure 5.
Therapeutic vaccination using Ad5-KH. BALB/c mice were infected with Leishmania donovani and at day 21 after infection, immunized in the footpad with 109 pfu Ad5-KH. A, Parasite burden was evaluated at day 31 from Giemsa-stained impression smears and is expressed in Leishman Donovan Units (LDU; [13]). Data are pooled from three independent experiments (20–23 mice per group). Mean ± standard error of the mean parasite burden is indicated. Significant differences in parasite load as determined by ANOVA are shown in horizontal bars. B, Percentage inhibition of parasite growth at day 31 was calculated for mice vaccinated with Ad5-KH and Ad5-GFP, according to the formula: (LDU d31 unvaccinated - LDU d21) - (LDU d31 vaccinated - LDU d21)/(LDU d31 unvaccinated - LDU d21) × 100 [22]. Data were analysed by Student’s t test.
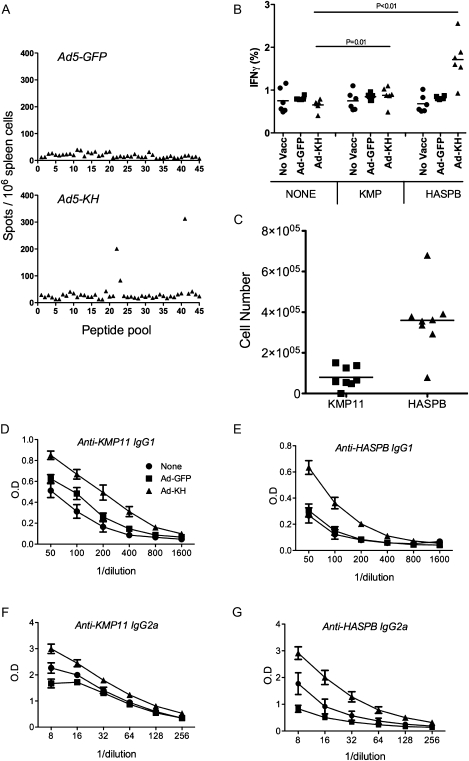
We then asked whether therapeutic vaccination stimulated HASPB- and/or KMP11-specific CD8+ T cells in infected mice. Similar to nonvaccinated controls, infected mice vaccinated with Ad5-GFP did not respond to any peptides tested, indicating that nonspecific immune stimulation could not recover potentially underlying CD8+ T cell responses (Figure 6A and Figure 4A). In contrast, Ad5-KH–vaccinated mice responded to HASPB with the same breadth of response seen in uninfected, vaccinated mice (Figure 6A and Figure 2C), with an estimated 200 000 IFNγ+ CD8+ T cells per infected spleen. As also noted in uninfected mice, we detected no response to KMP11 in vaccinated infected mice by ELISPOT (Figure 6A). However, by intracellular flow cytometry, a significant although weak response against KMPHYEKFERMI could be identified in vaccinated mice (Figure 6B). This equated to ∼100 000KMPHYEKFERMI-specific IFNγ+ CD8+ T cells per spleen, compared with ∼400 000 IFNγ+ CD8+ T cells responding to HASPBGGPKEGENL (Figure 6C). The discrepancy between the magnitude of the response determined by ELISPOT and flow cytometry is likely attributable to different thresholds for scoring positive responses (electronic gating vs. manual scoring based on spot size).
Figure 6.
Immune responses induced by vaccination with Ad5-KH in Leishmania donovani–infected mice. A, CD8+ T cell responses evaluated by ELISPOT in mice vaccinated with Ad5-GFP (top) or Ad5-KH (bottom). B and C, Peptide-specific IFNγ responses to KMP11HYEKFERMI and HASPBGGPKEGENL were evaluated by flow cytometry and presented as frequency of responding CD8+ T cells (B) and as absolute number of peptide specific CD8+ T cells per spleen (C). Values of individual mice are plotted. Data are derived from one of the 3 experiments included in Figure 5 (in which inhibition of parasite growth induced by Ad5-KH was 96% ± 6% and by Ad5-GFP was 56% ± 10%; n = 8; P < .01). D–G. IgG1 (D and E) and IgG2a (F and G) antibody responses to KMP11 (D and F) and HASPB (E and G) were determined by ELISA using serum from unvaccinated infected mice (circles) and infected mice vaccinated with Ad5-GFP (squares) or Ad5-KH (triangles). Data represent mean ± standard error of the mean derived from 6–8 mice per group from one experiment.
The poor recognition of HASPB and KMP11 observed in nonvaccinated L. donovani–infected mice did not extend to the CD4+ T cell and B cell compartment, because IgG1 and IgG2a responses to both antigens were readily detectable. These responses were boosted by therapeutic vaccination with Ad5-KH but not by Ad5-GFP (Figure 6D–G). Together, these data indicate that successful therapeutic vaccination with Ad5-KH was accompanied by the appearance of detectable vaccine antigen-specific CD8+ T cell responses, with bias toward HASPB and boosting of antibody responses to both vaccine antigens.
DISCUSSION
This study demonstrates that an adenovirus-based vaccine can provide significant therapeutic effects in mice infected with L. donovani. With the caveat of the sensitivity of in vitro recall assays, both of HASPB and KMP11 appear to be examples of cryptic antigens in terms of CD8+ T cell recognition (ie, they failed to stimulate detectable responses after infection, but are nevertheless immunogenic when used for vaccination) [23].
Our strategy to develop a therapeutic vaccine was driven by a 5-point product profile. First, we sought to show efficacy in a selected animal model. We chose inhibition of spleen parasite growth in mice, because this organ fails to spontaneously clear infection and is relatively refractory to drug treatment (reviewed in [24]). Furthermore, we studied the impact of therapeutic vaccination when parasite burden was increasing at its maximal rate and when tissue breakdown and immunosuppression were established [14, 20, 25]. Although it is unlikely that a therapeutic vaccine would be used alone [11], we set our criterion for success as inhibition of parasite growth of 50%. This level of response equates favorably to that seen following single dose therapy using 4 mg/kg Ambisome [26] or administration of sodium stibogluconate [27], although better responses are achievable with longer chemotherapy [26, 28]. Second, for clinical practicality, we aimed for efficacy using a single vaccination. Adenoviral vectors have been reported to provide single dose protection against Plasmodium berghei in mice [29]. We have not yet studied heterologous prime-boost regimens, although greater efficacy might be anticipated [30–32]. Third, we chose a delivery system that would benefit from inherent adjuvant activity, because a degree of protection due to nonspecific proinflammatory responses is well documented [33–36]. In this respect, marked similarities in host gene expression signatures induced by adenovirus [37] and L. donovani [38] have been noted. It remains to be determined whether this adjuvant effect operates directly [38] or is secondary to a reduction in parasite burden (as might also be anticipated in sequential chemo-immunotherapy). Fourth, with limited resources for clinical trials, we sought to develop a vaccine based on a platform with established safety and immunogenicity data. Adenoviruses are widely accepted as one of the most potent means to induce CD8+ T cell priming, along with CD4+ T cell and antibody responses [40]. Although some caution remains about the use of Ad5 in humans [41], new generation simian adenoviruses appear to be safe and to circumvent existing anti-Ad5 immunity [42]. Fifth, adenovirus can be stored in a thermostable manner [43], facilitating delivery in countries where the disease is endemic.
The selection of HASPB was based on prophylactic vaccination studies indicating good protection associated with the development of CD8+ T-cell responses in mice [13, 21] and immunogenicity in dogs [44]. Although HASPB in our laboratory strain of L. donovani has multiple identical repeats, field isolates show extensive divergence in repeat sequence and arrangement. Such repeat diversity may suggest immune selective pressure [45]. We therefore developed a synthetic HASPB gene in which the sequence and composition of 10 repeat units was chosen (1) to preserve the relative order of repeats that were present in >1 clinical isolate, (2) to conserve some of the repeat reiteration found in natural isolates (eg, strings of repeat 2), and (3) to maintain the overall protein length to that found in natural isolates. Our data showing that, at least in BALB/c mice, CD8+ T cell recognition is largely focused on the N and C termini of HASPB and that HASPB is weakly if at all recognized by CD8+ T cells during normal infection argue that repeat variation may not be under CD8+ T cell selective immune pressure. The IFNγ+CD8+ T cell responses against HASPB induced in infected mice by vaccination (∼400,000 sfc per spleen for HASPBGGPKEGENL alone) were also similar or better than those reported for single dose vaccination in other systems, including Ad5-L. donovani A2 [29, 46]. Neither published data nor the current study have directly addressed whether CD8+ T cells alone are sufficient for protection, and indeed antibody and CD4+ T cell responses are present under all conditions examined. Nevertheless, it is notable that adoptive transfer of only 106 CD8+ T cells recognizing an ovalbumin epitope expressed in transgenic L. donovani afforded therapeutic protection in a similar model [47].
KMP11 has been shown to be protective in murine L. donovani infection when administered as a DNA vaccine [48], and multiple human epitopes have been mapped [49], suggesting that it would be immunogenic in humans. Under the immunization regimen chosen for studies of protection here, we did not observe an appreciable ELISPOT response to KMP11 after vaccination of either uninfected or infected wild-type mice, although a weak response was detectable by flow cytometry. This limited recognition was vaccine dose- and route-dependent, arguing against problems in KMP11 recognition because of expression as a self-cleaving polyprotein or inherent lack of immunogenicity. The basis for this observation is under further study. Nevertheless, our data clearly demonstrate how subtle alterations in vaccine delivery alter epitope selection and indirectly highlight the difficulties in extrapolating from epitope recognition in vaccinated mice to vaccinated humans.
Finally, although CD8+ T cell responses to HASPB and KMP11 have yet to be studied in patients with any form of leishmaniasis, it is of interest that neither HASPB nor KMP11 have proved to be particularly good at inducing recall CD4+ T cells responses in patients recovered from HVL [50], although HASPB antibody responses have been shown to have diagnostic potential [51]. Our data suggest that there may be no a priori requirement for an antigen to be well recognized during natural infection for it to be effective when used for therapeutic (and perhaps prophylactic) vaccination. Only clinical trial will determine whether huKMP11_HASPB_consensus expressed in an adenoviral vector is immunogenic and effective as a tool for combined immunotherapy against HVL or other forms of leishmaniasis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank Prof. M. Ryan, for providing the tetravirus TaV virus 2A sequence; Dr. Lorna MacLean, for assistance with statistical analysis; Dr. Syamal Roy, for review of the manuscript; and the staff of the Biological Services Facility, for animal husbandry.
Financial support.
This work was funded by a Wellcome Trust Translation Award (085879 to P. M. K, C. N. J. L. and T. A) and by Volkswagen Stiftung (I/77 908 to P. W).
Potential conflicts of interest.
The gene encoding the recombinant poly-protein described in this manuscript is covered by PCT/GB2010/000815 to P. M. K, D. F. S, C. N. J. L., and T. A. (University of York and University of Edinburgh). The authors declare no other commercial interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Working to overcome the global impact of neglected tropical diseases. World Health Organization, Geneva; 2010. [Google Scholar]
- 3.Moran M, Guzman J, Henderson K, et al. G-Finder 2010: neglected disease research and development: is the global financial crisis changing R&D? London: Policy Cures; 2011. [Google Scholar]
- 4.van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect Dis. 2010;10:184–94. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 5.Costa CH, Peters NC, Maruyama SR, de Brito EC, Jr, de Miranda Santos IK The Working Group on Research Priorities for Development of Leishmaniasis V. Vaccines for the leishmaniases: proposals for a research agenda. PLoS Negl Trop Dis. 2011;5:e943. doi: 10.1371/journal.pntd.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modabber F. Leishmaniasis vaccines: past, present and future. Int J Antimicrob Agents. 2010;36(Suppl 1):S58–61. doi: 10.1016/j.ijantimicag.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Noazin S, Modabber F, Khamesipour A, et al. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine. 2008;26:6759–67. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 8.Badaro R, Lobo I, Munos A, et al. Immunotherapy for drug-refractory mucosal leishmaniasis. J Infect Dis. 2006;194:1151–9. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento E, Fernandes DF, Vieira EP, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–7. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 10.Trigo J, Abbehusen M, Netto EM, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine. 2010;28:3333–40. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musa AM, Noazin S, Khalil EA, Modabber F. Immunological stimulation for the treatment of leishmaniasis: a modality worthy of serious consideration. Trans R Soc Trop Med Hyg. 2010;104:1–2. doi: 10.1016/j.trstmh.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Kaye PM, Aebischer A. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–70. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 13.Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–71. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 14.Dalton JE, Maroof A, Owens BM, et al. Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest. 2010;120:1204–16. doi: 10.1172/JCI41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKean PG, Trenholme KR, Rangarajan D, Keen JK, Smith DF. Diversity in repeat-containing surface proteins of Leishmania major. Mol Biochem Parasitol. 1997;86:225–35. doi: 10.1016/s0166-6851(97)00035-2. [DOI] [PubMed] [Google Scholar]
- 16.Alce TM, Gokool S, McGhie D, Stager S, Smith DF. Expression of hydrophilic surface proteins in infective stages of Leishmania donovani. Mol Biochem Parasitol. 1999;102:191–6. doi: 10.1016/s0166-6851(99)00074-2. [DOI] [PubMed] [Google Scholar]
- 17.Depledge DP, MacLean LM, Hodgkinson MR, et al. Leishmania-specific surface antigens show sub-genus sequence variation and immune recognition. PLoS Negl Trop Dis. 2010;4:e829. doi: 10.1371/journal.pntd.0000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Felipe P, Luke GA, Hughes LE, Gani D, Halpin C, Ryan MD. E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006;24:68–75. doi: 10.1016/j.tibtech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Darrah PA, Hegde ST, Patel DT, et al. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207:1421–33. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7–H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stager S, Alexander J, Kirby AC, et al. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–92. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 22.Murray HW, Montelibano C, Peterson R, Sypek JP. Interleukin-12 regulates the response to chemotherapy in experimental visceral Leishmaniasis. J Infect Dis. 2000;182:1497–502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 23.Wolpert E, Franksson L, Karre K. Dominant and cryptic antigens in the MHC class I restricted T cell response across a complex minor histocompatibility barrier: analysis and mapping by elution of cellular peptides. Int Immunol. 1995;7:919–28. doi: 10.1093/intimm/7.6.919. [DOI] [PubMed] [Google Scholar]
- 24.Kaye PM, Svensson M, Ato M, et al. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–53. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 25.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–93. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen AB, Carter KC, Baillie AJ. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:2089–92. doi: 10.1128/aac.41.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter KC, Baillie AJ, Alexander J, Dolan TF. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J Pharm Pharmacol. 1988;40:370–3. doi: 10.1111/j.2042-7158.1988.tb05271.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob AgentsChemother. 1992;36:1630–4. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes-Sandoval A, Sridhar S, Berthoud T, et al. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–41. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- 30.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–78. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. Heterologous priming-boosting with DNA and modified vaccinia virus Ankara expressing tryparedoxin peroxidase promotes long-term memory against Leishmania major in susceptible BALB/c Mice. Infect Immun. 2007;75:852–60. doi: 10.1128/IAI.01490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vuola JM, Keating S, Webster DP, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 33.Dalton JE, Kaye PM. Immunomodulators: use in combined therapy against leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:739–42. doi: 10.1586/eri.10.64. [DOI] [PubMed] [Google Scholar]
- 34.Miranda-Verastegui C, Tulliano G, Gyorkos TW, et al. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Negl Trop Dis. 2009;3:e491. doi: 10.1371/journal.pntd.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okwor I, Uzonna JE. Immunotherapy as a strategy for treatment of leishmaniasis: a review of the literature. Immunotherapy. 2009;1:765–76. doi: 10.2217/imt.09.40. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder J, Brown N, Kaye P, Aebischer T. Single dose novel Salmonella vaccine enhances resistance against visceralizing L. major and L. donovani infection in susceptible BALB/c mice. PloS Negl Trop Dis. 2011;5:e1406. doi: 10.1371/journal.pntd.0001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffrey AP, Fawcett P, Nakai H, et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Molecular therapy: J Am Soc Gene Ther. 2008;16:931–41. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- 38.Phillips R, Svensson M, Aziz N, et al. Innate killing of Leishmania donovani by macrophages of the splenic marginal zone requires IRF-7. PLoS Pathog. 2010;6:e1000813. doi: 10.1371/journal.ppat.1000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller G, Lahrs S, Pillarisetty VG, Shah AB, DeMatteo RP. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 2002;62:5260–6. [PubMed] [Google Scholar]
- 40.Ledgerwood JE, Costner P, Desai N, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–13. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 41.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357–61. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill AV, Reyes-Sandoval A, O'Hara G, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 43.Alcock R, Cottingham MG, Rollier CS, et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Transl Med. 2010;2:19ra12. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- 44.Moreno J, Nieto J, Masina S, et al. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine. 2007;25:5290–300. doi: 10.1016/j.vaccine.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depledge DP, Lower RP, Smith DF. RepSeq—a database of amino acid repeats present in lower eukaryotic pathogens. BMC Bioinformatics. 2007;8:122. doi: 10.1186/1471-2105-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resende DM, Caetano BC, Dutra MS, et al. Epitope mapping and protective immunity elicited by adenovirus expressing the Leishmania amastigote specific A2 antigen: correlation with IFN-gamma and cytolytic activity by CD8+ T cells. Vaccine. 2008;26:4585–93. doi: 10.1016/j.vaccine.2008.05.091. [DOI] [PubMed] [Google Scholar]
- 47.Polley R, Stager S, Prickett S, et al. Adoptive immunotherapy against experimental visceral leishmaniasis with CD8+ T cells requires the presence of cognate antigen. Infect Immun. 2006;74:773–6. doi: 10.1128/IAI.74.1.773-776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhaumik S, Basu R, Sen S, Naskar K, Roy S. KMP-11 DNA immunization significantly protects against L. donovani infection but requires exogenous IL-12 as an adjuvant for comparable protection against L. major. Vaccine. 2009;27:1306–16. doi: 10.1016/j.vaccine.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Basu R, Roy S, Walden P. HLA class I-restricted T cell epitopes of the kinetoplastid membrane protein-11 presented by Leishmania donovani-infected human macrophages. J Infect Dis. 2007;195:1373–80. doi: 10.1086/513439. [DOI] [PubMed] [Google Scholar]
- 50.Kumar R, Goto Y, Gidwani K, Cowgill KD, Sundar S, Reed SG. Evaluation of ex vivo human immune response against candidate antigens for a visceral leishmaniasis vaccine. Am J Tro Med Hyg. 2010;82:808–13. doi: 10.4269/ajtmh.2010.09-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen AT, Gasim S, Moller T, et al. Serodiagnosis of Leishmania donovani infections: assessment of enzyme-linked immunosorbent assays using recombinant L. donovani gene B protein (GBP) and a peptide sequence of L. donovani GBP. Trans R Soc Trop Med Hyg. 1999;93:157–60. doi: 10.1016/s0035-9203(99)90291-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.