Abstract
Ascorbate (Asc, vitamin C) was quantified in the human brain noninvasively using two different 1H NMR spectroscopy methods: short-echo time STEAM and MEGA-PRESS homonuclear editing. Taking advantage of increased sensitivity and chemical shift dispersion at 7 T, Asc was quantified with increased reliability relative to our previous study accomplished at 4 T. Asc concentration quantified from short-echo time spectra measured from the occipital lobe of eight healthy subjects ([Asc] = 1.1 ± 0.3 µmol/g, mean ± SD) was in excellent agreement with Asc concentration quantified from the same volume of interest using homonuclear editing ([Asc] = 1.2 ± 0.2 µmol/g). This agreement indicates that at 7 T, Asc can be reliably quantified in the human brain simultaneously with 15 other metabolites. Additional advantages of the short-echo time approach were: shorter measurement time than homonuclear editing and minimal effect of T2 relaxation on Asc quantification. High magnetic field was also beneficial for Asc quantification with MEGA-PRESS because increased chemical shift dispersion enabled editing with full efficiency, which resulted in a supra-linear gain in signal-to-noise ratio relative to 4 T.
Keywords: ascorbate, brain, human, MEGA-PRESS, MRS, noninvasive, STEAM, 7 T
INTRODUCTION
Ascorbate (Asc, vitamin C) is one of the two most concentrated nonenzymatic antioxidants in the human central nervous system (1). Homeostatic maintenance of millmolar concentration (2) is evidence of an important role in the brain (3), which is particularly susceptible to oxidative damage (4). Several studies indicate that oxidative stress contributes to compromised brain function in normal aging (5) and constitutes a risk factor for neurodegenerative disease (6). The ability to assay human brain Asc concentration noninvasively would enable the investigation of how this antioxidant changes throughout aging, neurodegenerative disease, and therapy.
Asc resonances in in vivo 1H NMR spectra of the human brain were previously assigned using homonuclear editing (7). Close chemical shift proximity of Asc resonances required high-frequency selectivity (i.e. prolonged duration) of the editing RF pulse, which led to a long-echo time (TE = 112 ms). Thus, transverse (T2) relaxation had a detrimental impact on signal-to-noise ratio (SNR) and the reliability of Asc quantitation. Nevertheless, the 1.3 µmol/g human brain Asc concentration quantified from the edited resonances was in agreement with post mortem data (8), supporting validity of the assumption that effects of T2 relaxation on Asc quantification were negligible, when Cr was used as an internal reference (9). Quantification of Asc using editing with long TE is dependent on the reasonable assumption that factors in the chemical environment impacting T2 are similar across experimental conditions.
Asc concentrations have recently been quantified simultaneously with other brain metabolites in rat and mouse brain using short-echo time 1H NMR spectra measured at 9.4 T (9,10). Significant changes in Asc concentration were measured with increasing rat pup age (9). Key advantages of detecting at short-echo time were high SNR, negligible influence of T2 on measured concentration, and the potential to quantify Asc and 16 additional neurochemicals at the same time. Because the small Asc resonances were not visually resolved from strong neighboring glutathione (GSH), glutamine (Gln), and glutamate (Glu) resonances, quantification relied on spectral deconvolution (9,11–13). Nevertheless, measured Asc concentrations and their changes throughout development were in agreement with ex vivo data (1) and with concentrations measured using edited spectroscopy (9).
The goal of the current study was to validate Asc concentrations quantified from short-echo time spectra against those measured via homonuclear editing in the human brain at 7 T. The motivation for this project was to achieve: high SNR, T2 insensitive quantitation, and simultaneous detection of Asc with the entire neurochemical profile. The experimental design was to quantify Asc from both short-echo time and homonuclear edited spectra measured from identical volumes of interest (VOI). A complementary goal of this study was to improve editing efficiency at 7 T relative to that previously achieved at 4 T (7).
EXPERIMENTAL
Protocol
Eight normal volunteers (3 male, 5 female, average age 28 years) gave informed consent for this study, which was conducted according to procedures approved by our institution. All experiments were performed with a 7 T, 90-cm horizontal bore magnet (Magnex/Varian Scientific, Yarnton, UK) equipped with a gradient system (40 mT/m, 500 ms rise time) and a powerful second-order shim system (14). The 7 T magnet was interfaced to a Varian INOVA console (Varian, Palo Alto, CA, USA). Subjects were positioned supine inside the magnet and a quadrature half-volume RF coil with an elliptical loop design (11 and 15 cm diameters) was used for transmission and detection (14). The protocol for each volunteer began with T1 weighted TURBOFLASH images to select a cubic VOI (2 × 2 × 2 cm3) centered on the midsagittal plane and calcarine fissure in the occipital lobe (Fig. 1). Shimming of all first- and second-order shims was achieved using FASTMAP with EPI readout (15,16), which resulted in water line widths of 14 ± 1 Hz (mean ± SD). Short-echo time and homonuclear edited spectra were measured consecutively in each subject from identical VOI. The overall scan time was less than 1.5 h per subject.
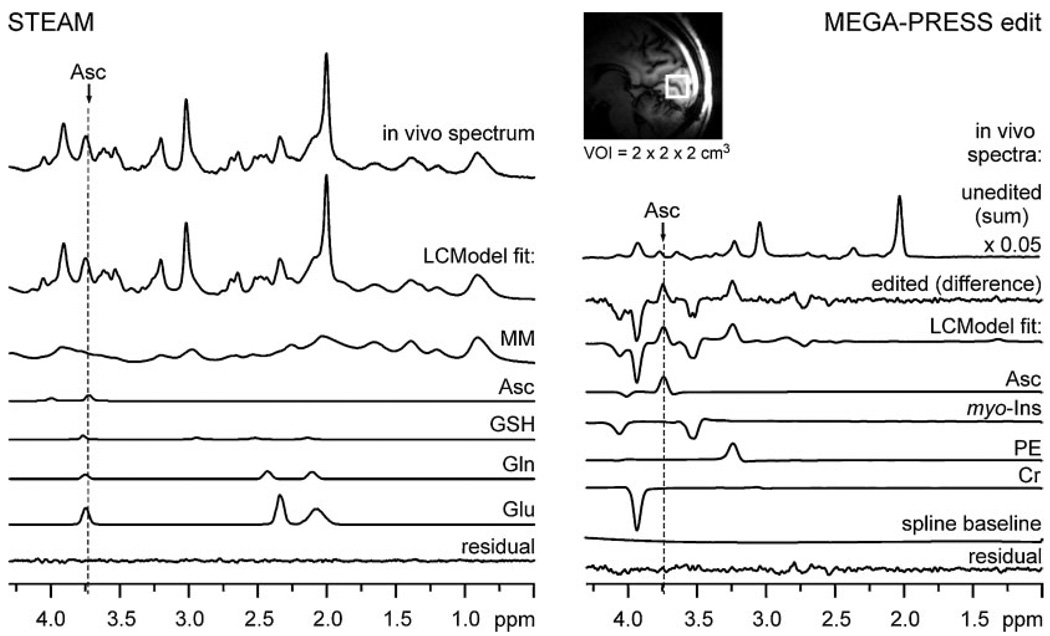
Figure 1.
Human brain 1H NMR spectra measured via ultra-short-echo-time STEAM and MEGA-PRESS editing and their LCModel analysis. Left panel: in vivo STEAM spectrum (TE = 6 ms, TR = 5 s, NT = 160) and metabolite spectra fitted via LCModel which overlap with the Asc resonance at 3.73 ppm. Right panel: in vivo MEGA-PRESS homonuclear unedited spectrum (sum of ‘on’ and ‘off’ scans), in vivo edited spectrum (TE = 112 ms, TR = 5 s, NT = 512), and edited spectra of metabolites fitted via LCModel. Inset: sagittal MRI with the typical location of the volume of interest in the occipital lobe.
Short-echo time spectroscopy
Short-echo time STEAM (TE = 6 ms, TM = 32 ms, TR = 5 s, number of transients (NT) = 160) localization was utilized as previously described (14) and preceded by outer volume suppression (OVS) and water suppression (VAPOR, variable power RF pulses with optimized relaxation delays) (17,18). Brain metabolite concentrations were quantified using LCModel (12,13). The LCModel basis set included simulated spectra of the following brain metabolites: alanine (Ala), Asc, aspartate (Asp), creatine (Cr), γ-aminobutyric acid (GABA), glucose (Glc), Glu, Gln, GSH, glycerophosporylcholine (GPC), glycine (Gly), myo-inositol (myo-Ins), scyllo-inositol (scyllo-Ins), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphorylcholine (PC), phosphorylethanolamine (PE), taurine (Tau), and the experimentally measured spectrum of fast-relaxing macromolecules (MM) as previously described (14,18). Briefly, an average MM spectrum was measured from five subjects using metabolite nulling inversion-recovery experiments with a short repetition time (TR = 2 s, inversion time TI = 0.675 s). The residual signal of the fast relaxing methylene group of PCr at 3.93 ppm was removed from the MM spectrum and the high-frequency noise was suppressed by a Gaussian filter (σ = 0.05 s) (14). The LCModel parameter that controls node spacing of the spline function used for fitting the baseline (DKNTMN, LCModel parameter for the node spacing of the spline function) was set to 0.20 ppm. For all spectra, each single free induction decay (FID) was stored in memory and then frequency and phase were corrected prior to summation of all FIDs (NT = 160). All brain metabolites included in the LCModel basis set except Ala, Glc, Gly, and PC were quantified with Cramer–Rao lower bounds (CRLB) <15%. The sum of GPC + PC was fit with CRLB ≤6%. The unsuppressed water signal measured from the VOI without OVS was used as an internal concentration reference for quantification assuming 80% water content (14).
Homonuclear editing
1H homonuclear difference editing for the Asc protons resonating at 3.73 ppm via coupled protons resonating at 4.01 ppm was adapted from the originally developed pulse sequence for noninvasive detection of Asc in the human brain at 4 T (7). Increased spectral dispersion made it possible to edit with full efficiency at 7 T. The 3.73 ppm 6CH2 resonance of Asc was refocused during every other scan by applying a 40ms 180° Gaussian RF pulse (full width at half maximum = 38 Hz) at 4.01 ppm. The edited spectrum (TE = 112 ms, TR = 5 s, NT = 512) was obtained by subtracting spectra measured with the editing pulse applied (‘on’) from those measured without it (‘off’). Scans with the editing pulse ‘on’ and ‘off’ were acquired in an interleaved manner. Highly frequency selective (i.e. long) editing RF pulses were necessary due to close proximity of the Asc resonances at 3.73 and 4.01 ppm. Thus, the shortest echo time feasible was 106 ms. To optimize editing efficiency, spectra were measured from a solution of Asc at TE ranging from 106 to 160 ms and filtered to match in vivo line widths. Maximal intensity of the edited Asc resonance was observed at TE = 112 ms. Improvement in editing efficiency was not observed with shorter editing pulses accommodated via shifting their carrier frequency off-resonance from 4.01 ppm to avoid excitation of the Asc resonance at 3.73 ppm (7). RF power of the editing pulses was optimized by placing their offset at the water frequency and incrementing until water signal was minimized (7). The bandwidth of the 180° pulse was sufficiently narrow to avoid exciting resonances in the ±20 Hz rage centered at 3.73 ppm (the chemical shift of the 6CH2 resonance of Asc), leaving an adequate margin for potential B0 drift during in vivo studies (7). B0 drift was monitored, and the frequencies of RF pulses were reset whenever drift exceeded 12 Hz from the optimal setting. As with the short-echo time data, each acquisition (NT = 1) was stored separately in memory and then frequency and phase were corrected prior to summation. Frequency correction was never more than 13 Hz. The VAPOR water suppression and OVS that preceded MEGA-PRESS were identical to those preceding STEAM. Although the spin systems of MM are unlikely to contribute resonances in the vicinity of the edited 6CH2 Asc multiplet (3.73 ppm), a metabolite nulled spectrum (19,20) was measured to assess MM contributions. An inversion pulse was applied before the MEGA-PRESS editing scheme, and the time between the inversion pulse and MEGA-PRESS (TI = 0.675 s) was set experimentally to the value where the strongest brain metabolite resonances (NAA, Cr, and Cho) were nulled in unedited (i.e. the sum of spectra measured with editing pulses ‘on’ and ‘off’) spectra. To minimize the retention of metabolite resonances with slightly different T1, a shorter repetition time (TR = 2 s) was used.
Asc concentration was quantified using LCModel (12,19). Basis spectra for LCModel were measured with the MEGA-PRESS sequence using the same parameters as for Asc editing. Spectra were collected from phantoms containing pure Asc, Asp, Cr, myo-Ins, Lac, NAA, and PE in pH buffer (pH = 7.1, 37°C). LCModel fitting was performed over the spectral range from 1.0 to 4.4 ppm. The phase and chemical shift of edited spectra were set manually based on corresponding unedited spectra then automatically relative to the Cr basis spectrum (constrained to within 20° and 0.3 ppm). The node spacing for the spline function (DKNTMN) was set to 10 ppm, forcing a very flat baseline. The Lac and NAA resonances were not found to be present in the in vivo spectra (CRLB = 999%). Concentrations of Asc were calculated based on the following equation:
| (1) |
where SIAsc corresponds to signal intensity of Asc measured by LCModel, and the constant k was determined from a phantom experiment in which concentrations of Cr ([Cr] = 16 mM) and Asc ([Asc] = 2 mM) were known (9,19). The constant k accounted for both editing efficiency and T2 relaxation. Calculation of in vivo Asc concentration was based on the assumption that differences between T2 relaxation of Cr and T2 relaxation of Asc had the same impact on resonance intensity in vitro and in vivo, i.e. the ratio exp(−TE/T2(Cr))/exp(−TE/T2(Asc)) was approximately the same under in vitro and in vivo conditions. Deviation from this assumption would result in systematic overestimation or underestimation of measured Asc concentration for all subjects. Phantom spectra were line broadened to match line widths encountered in vivo. The integral intensity of the singlet at 3.03 ppm in the unedited spectrum, corresponding to the methyl group of total Cr, was used as an internal reference for quantification (SICr, signal intensity of Cr). The in vivo concentration of total Cr, 8.55 µmol/g, was based on the average concentration of Cr + PCr measured using STEAM.
RESULTS
Representative spectra measured using short-echo time STEAM and MEGA-PRESS editing from the same occipital lobe VOI are shown in Fig. 1. This figure also illustrates how LCModel was used to quantify Asc. Experimental spectra were fit as a liner combination of metabolite (model) spectra comprising the LCModel basis set. LCModel fit residuals were mostly consistent with white noise, without evidence of unassigned resonances, which indicates that the LCModel basis set included all metabolites with a detectable contribution to the experimental spectrum.
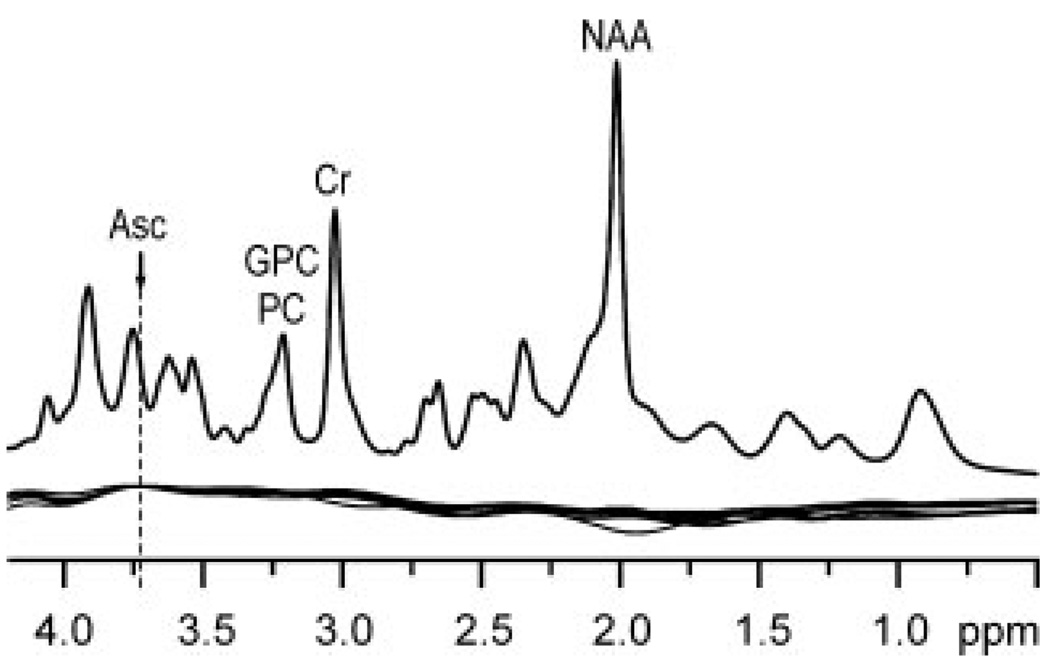
Estimated errors of Asc quantification from STEAM spectra, defined by the CRLB, were never more than 14% in any single subject, even though the Asc resonance at 3.73 ppm was strongly overlapped by resonances of GSH, Gln, Glu, and MM (Fig. 1). Artificial broadening of our measured spectra up to 20 Hz revealed that in spite of strong spectral overlap, reliable quantitation of Asc from STEAM spectra with larger line widths is possible, provided the range of convolution in data fitting, defined by the LCModel parameter RFWHM is increased from its default value of 1.8 to 3.0 ppm. Correlation coefficients from the standard nonlinear least-squares covariance matrix (21,22) between Asc and the other neurochemicals were never stronger than −0.2. Absence of distortion at chemical shifts near water and lipid resonances demonstrates high localization performance and excellent water suppression. Sixteen neurochemicals including Asc were reliably quantified in all subjects with average CRLB below 14% (Table 1). The largest average measurement error (CRLB) was 13%, i.e. for scyllo-Ins. The spline functions used to model the baselines of all 8 spectra (11) are illustrated in Fig. 2. The spline baseline characterizes contributions not attributable to any of the basis spectra, such as residual partially suppressed lipid resonances and signals from low concentration metabolites not included in the basis set, and corrects for small imperfections in the experimentally measured MM basis spectrum (11). Nearly model-free constrained regularization allows only complexity demanded by the data to be described by the spine baseline (11). High inter-individual reproducibility of the spline baseline (Fig. 2), especially in the proximity of the predominant Asc resonance (3.73 ppm), demonstrates minimal confounding of Asc quantification by a potential excessive degree of freedom in the spline function.
Table 1.
Average brain metabolite concentrations measured from short-echo-time STEAM spectra (n = 8), standard deviation (SD), and average Cramer–Rao lower bounds (CRLB)
| Metabolite | Concentration (µmol/g) |
SD (µmol/g) |
CRLB (%) |
|---|---|---|---|
| Asp | 2.0 | 0.4 | 11 |
| Asc | 1.1 | 0.3 | 11 |
| GPC + PC | 1.0 | 0.3 | 4 |
| Cr | 4.6 | 0.3 | 4 |
| GABA | 0.9 | 0.1 | 11 |
| Glu | 8.9 | 0.3 | 2 |
| Gln | 2.5 | 0.2 | 3 |
| GSH | 0.7 | 0.1 | 9 |
| myo-Ins | 6.3 | 0.9 | 3 |
| scyllo-Ins | 0.3 | 0.1 | 13 |
| Lac | 0.7 | 0.1 | 9 |
| NAA | 12.4 | 0.7 | 1 |
| NAAG | 1.2 | 0.2 | 6 |
| PCr | 3.9 | 0.5 | 4 |
| PE | 2.1 | 0.2 | 6 |
| Tau | 1.7 | 0.2 | 7 |
| Cr + PCr | 8.6 | 1.2 | 1 |
Figure 2.
Top: representative in vivo short-echo-time STEAM spectrum (TE = 6 ms, TR = 5 s, NT = 160). Apodization: Gaussian multiplication (σ = 0.1 s). Bottom: overlaid spline baseline functions for LCModel analysis of the 8 short-echo time STEAM spectra measured from the occipital lobes of healthy subjects.
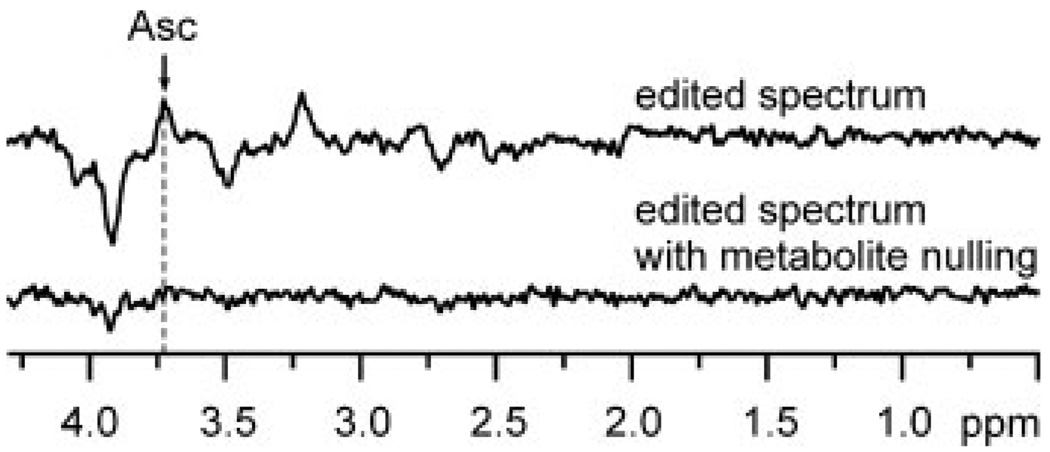
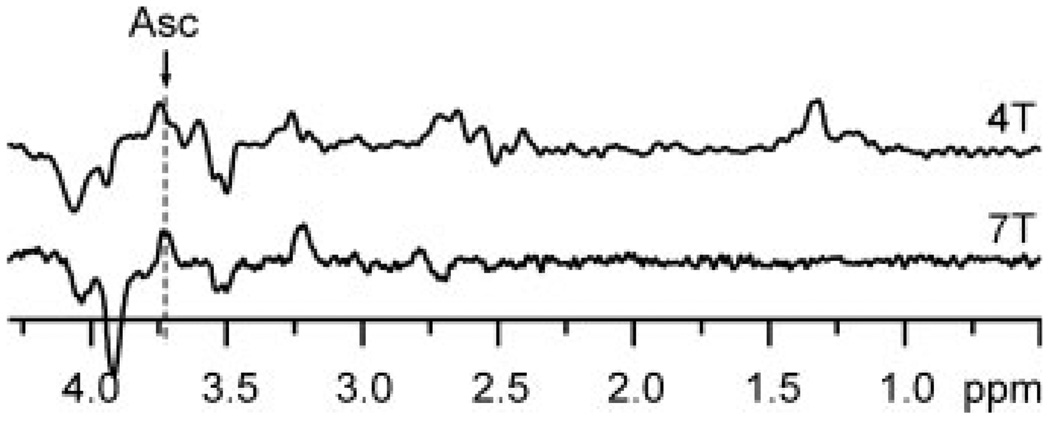
MEGA-PRESS edited Asc resonances were detected with high sensitivity and resolved from co-edited resonances of metabolites (Fig. 1). All brain metabolites with J-coupled protons resonating in the bandwidth of the Asc editing pulse and with sufficient concentration (23) contributed co-edited resonances of anticipated intensity and pattern. All of the resonances appearing in the edited spectra were assigned. With no contributions in the vicinity of 2.009 ppm, the chemical shift of the NAA methyl resonance verifies the absence of subtraction artifact. Asc was quantified with CRLB ≤8% in all subjects. The undistorted, flat baseline over the entire spectral range demonstrates high localization performance and excellent water suppression. The improvement in SNR of the edited Asc resonance measured at 7 T relative to that previously achieved in the same brain region at 4 T (9) was 33% more than anticipated based on a linear gain with field strength (and linear reduction with VOI size). MM did not contribute any co-edited resonances (Fig. 3). The only resonance in the MM spectrum was a small, narrow peak at 3.93 ppm, corresponding to the fast-relaxing CH2 resonance of PCr. Improvements in the spectral selectivity of editing achieved at 7 T relative to that reported previously at 4 T are illustrated in Fig. 4. The Asc resonance at 3.73 ppm comprises overlapping contributions from two nonequivalent 6CH2 protons with chemical shifts of 3.72 and 3.75 ppm (24), which leads to a slightly different shape of the Asc signal at different field strengths. The concentration measured at 7 T agreed with that previously measured at 4 T (9,24), and with those measured ex vivo (8,25). Asc concentration was measured at 7 T in the 8 cm3 VOI with a smaller measurement error (CRLB = 6%) than previously measured at 4 T in a 27 cm3 VOI (CRLB = 9%) in the same brain region.
Figure 3.
MEGA-PRESS spectra of the human brain edited for ascorbate. Top: standard editing for Asc, same parameters as in Fig. 1, but TR = 2 s. Bottom: the metabolite nulled spectrum, i.e. with an inversion pulse prior to MEGA-PRESS, TI = 0.675 s. These spectra were measured in an interleaved fashion.
Figure 4.
Comparison of MEGA-PRESS edited spectra at 4 and 7 T. Top: editing for Asc at 4 T (from our previous study (7), VOI = 3 × 3 × 3 cm3, TE = 112 ms, TR = 4.5 s, NT = 512, 40 ms editing pulses at 4.13 ppm). Bottom: editing for Asc at 7 T (this study, VOI = 2 × 2 × 2 cm3, TE = 112 ms, ms, TR = 5 s, NT = 512, 40 ms editing pulses at 4.01 ppm). Both spectra were measured from the human brain occipital lobe using quadrature half-volume RF coils and post processed with a Gaussian filter, σ = 0.1 s.
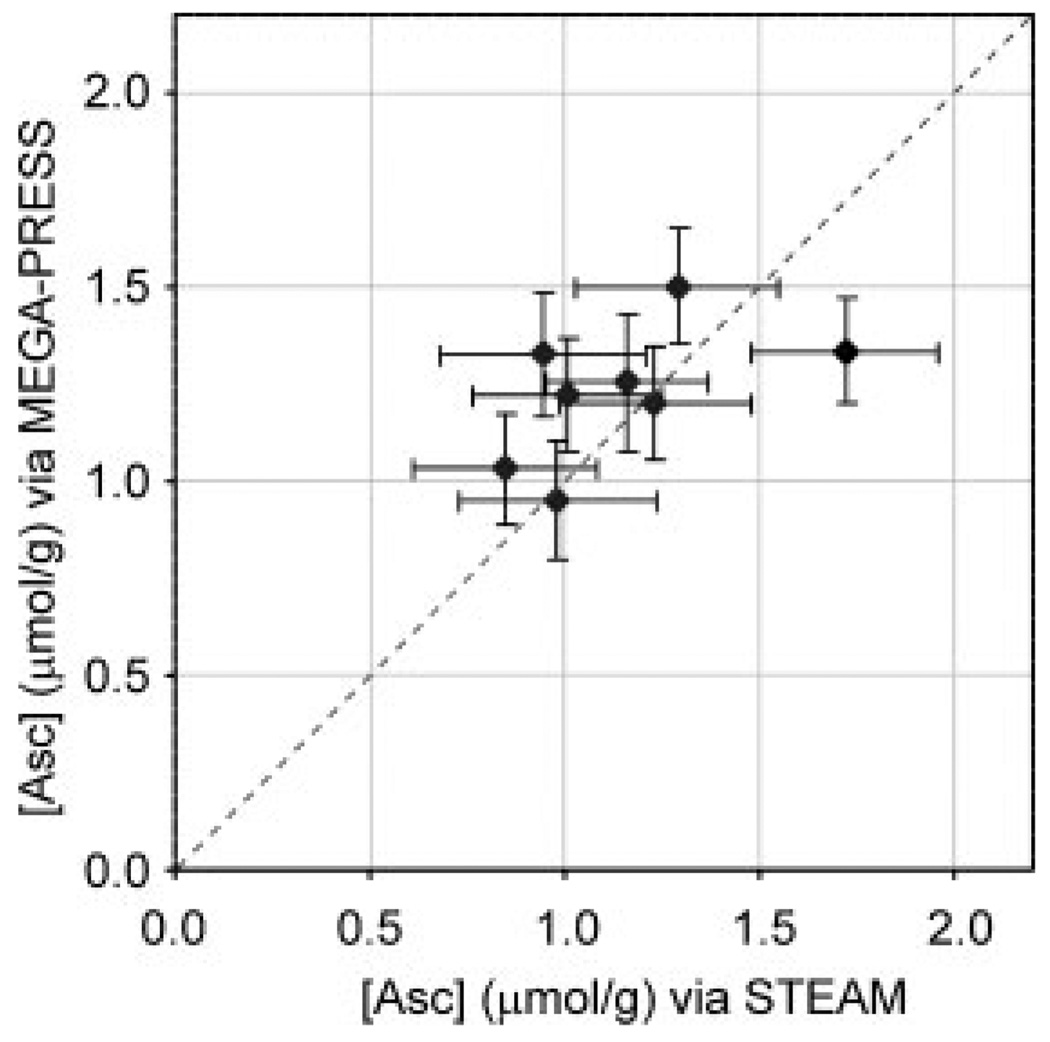
Asc concentrations measured with short-echo time STEAM (1.1 ± 0.3 µmol/g, mean ± SD, n = 8) and MEGA-PRESS (1.2 ± 0.2 µmol/g, n = 8) were in very good agreement. The average estimated error of Asc quantification (CRLB) was higher for STEAM (11%) than for MEGA-PRESS (6%). Figure 5 illustrates Asc concentrations measured using the two methods in each individual subject.
Figure 5.
Comparison of Asc concentrations quantified from short-echo-time STEAM versus MEGA-PRESS edited spectra measured at 7 T from the occipital lobe of eight healthy subjects. Both spectra were acquired during the same session for each individual. Error bars illustrate estimated errors of LCModel analysis (CRLB).
DISCUSSION
Good agreement between Asc concentrations measured using short-echo time STEAM spectroscopy and those measured using MEGA-PRESS homonuclear editing indicates that at 7 T in the human brain, Asc can be quantified simultaneously with 15 other brain metabolites directly from short-echo-time spectra. Agreement between these two quantification methods does not necessarily prove that calculated Asc concentrations are accurate, because both quantification methods are susceptible to bias. However, the probability of bias of the same sign and magnitude is low because the common sources of bias in these two techniques are not generally related. For example, an incomplete set of metabolite spectra in the LCModel basis set can cause systematic errors in quantification of short-echo-time spectra (9). On the other hand, the intensity of the edited Asc signal is dependent on T2, which is not easy to measure in vivo. However, newly emergent methodology for measuring T2 (26–31) may allow for more accurate quantification of concentration from spectra measured at long TE.
Low estimated measurement errors (CRLB) suggest that short-echo time 1H NMR spectroscopy at 7 T is sensitive enough to study how human brain [Asc] changes throughout normal and aberrant aging. The sensitivity of Asc quantification should also be high enough to detect responses to treatment. Improvements in SNR achieved at higher field can alternatively be utilized to shorten scan times or to focus on smaller volumes of interest.
The benefits of increased sensitivity and chemical shift dispersion at 7 T are particularly advantageous for the detection of weakly represented metabolites with coupled spin systems, such as Asc (29). Despite increased line width at 7 T relative to 4 T (32), separation of multiplets from coupled spin systems of differing metabolites significantly improves, because the overall signal widths of these multiplets are mainly determined by J-coupling constants, which are independent of B0 (32). Therefore the line-broadening effect of shorter T2 at higher field is less noticeable for multiplet than singlet resonances. Reliable quantification of Asc from 7 T STEAM spectra with the linewidth up to 20 Hz is possible, when other factors determining the spectral quality are maintained, such as high SNR, undistorted line shapes, flat baseline, highly efficient water suppression, and the elimination of signals arising outside of the VOI (subcutaneous lipids).
Previously we found that at 4 T, the average CRLB for Asc quantification from short-echo time spectra was 39% when measured using a similar protocol to this study (same VOI size and location, NT = 160, TR = 4.5 s, TE = 4 ms, n = 10). At 7 T, estimated quantification error of Asc quantification decreased below 14%, clearly demonstrating improvement with increasing magnetic field B0. Reliable quantification of Asc at 7 T was facilitated by several features of the short-echo time spectroscopy methodology. First, ultra-short TE minimized the signal attenuation due to T2 relaxation and thus preserved high SNR. Second, highly efficient water and outer volume suppression resulted in flat baseline of spectra, which consequently translated into flat and reproducible spline baseline correction in LCModel analysis (Fig. 2). Third, automated shimming by FASTMAP facilitated consistent line widths and line shapes. The dominant detectable signal of Asc at 3.73 ppm was overlapped by signals of GSH, Glu, and Gln (Fig. 1), which would prohibit Asc quantification if the deconvolution analysis was limited to a narrow spectral range centered at 3.74 ppm. However, a reliable deconvolution of the signals at 3.75 ppm into contributions from four metabolites was feasible when the whole spectral range of 0.5–4.2 ppm was used in the LCModel analysis. In other words, the reliable quantification of Asc from short-echo-time spectra required high spectral quality in the whole spectral range (0.5–4.2 ppm). When these features are not present, homonuclear editing remains a viable alternative for quantifying Asc.
Homonuclear editing for Asc is particularly difficult because the chemical shift of the resonance to be refocused (i.e. the 6CH2 multiplet at 3.73 ppm) is in close proximity to the chemical shift of the resonance excited by the editing pulse (i.e. the 5CH at 4.01 ppm). To avoid subtraction artifact arising from noncoupled resonances, the excitation profile of the editing pulse must not extend into the chemical shift of the resonance to be refocused. Despite using long-echo time (TE = 112 ms) for Asc editing at 4 T, there was not enough time between PRESS RF pulses for editing pulses of required frequency selectivity (i.e. duration). Therefore, editing pulses were displaced from their ideal chemical shift (7), which resulted in reduced editing efficiency, and consequently in reduced SNR at 4 T. Because spectral dispersion increases with increasing B0 field, the selectivity of an editing pulse of given length also increases with increasing B0. Due to increased chemical shift dispersion at 7 T, the frequency selectivity of a 40 ms editing pulse is high enough to allow setting its frequency directly on Asc resonance at 4.01 ppm without affecting the Asc resonance at 3.73 ppm. This substantially improved editing efficiency and thus SNR in this study. In addition, estimated measurement error from the LCModel analysis of edited spectra (CRLB) decreased at 7 T relative to that achieved at 4 T (7), even though the measured volume of interest (VOI) was substantially smaller at 7 T (VOI = 8 mL) than at 4 T (VOI = 27 mL). Furthermore, high-frequency selectivity of Asc editing at 7 T reduced the number and intensity of co-edited resonances, which resulted in increased precision and accuracy of Asc quantification.
Asc concentrations measured by ultra-short-echo time and homonuclear edited NMR spectroscopy were in excellent agreement, which indicates that Asc can be reliably quantified from short-echo time human brain spectra at 7 T despite highly overlapped Asc resonances. This means that Asc concentration can be quantified simultaneously with the whole neurochemical profile. Estimated error of Asc quantification (CRLB) at short-echo time was comparable to that achieved via editing, even though the total acquisition time for short TE spectra was only 30% of the time used to acquire edited spectra. For the edited Asc resonance, higher gain in SNR than attributable to increased sensitivity at 7 T relative to 4 T was achieved via increased editing efficiency at 7 T.
Acknowledgements
The authors thank Pierre-Gilles Henry for developing software for LCModel analysis of edited spectra. We appreciate the efforts made by the CMRR staff to maintain spectrometer performance. This study was supported by the NIH (R21-AG029582, P41-RR008079, and P30-NS057091).
Contract/grant sponsor: NIH; contract/grant numbers: R21-AG029582; P41-RR008079; P30-NS057091.
Abbreviations used
- Ala
alanine
- Asp
aspartate
- Asc
ascorbate
- CRLB
Cramer–Rao lower bounds
- Cr
creatine
- FID
free induction decay
- Gln
glutamine
- Glu
glutamate
- Gly
glycine
- GPC
glycerophosporylcholine
- GSH
glutathione
- k
constant for quantification
- Lac
lactate
- MM
macromolecules
- myo-Ins
myo-inositol
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- NT
number of transients
- OVS
outer volume suppression
- PC
phosphorylcholine
- PCr
phosphocreatine
- PE
phosphorylethanolamine
- scyllo-Ins
scylloinositol
- SNR
signal-to-noise ratio
- Tau
taurine
- TI
inversion recovery time
- VAPOR
variable power RF pulses with optimized relaxation delays
- VOI
volume of interest
REFERENCES
- 1.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 2.Spector R, Lorenzo AV. Ascorbic acid homeostasis in the central nervous system. Am. J. Physiol. 1973;225:757–763. doi: 10.1152/ajplegacy.1973.225.4.757. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge J, editors. Free radicals in biology and medicine, 4th edn. New York: Oxford University Press; 2007. [Google Scholar]
- 4.Aoyamaya K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J. Pharmacol. Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 5.Foster TC. Biological markers of age-related memory deficits, treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J. Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpstra M, Gruetter R. 1H NMR detection of vitamin C in human brain in vivo. Magn. Reson. Med. 2004;51:225–229. doi: 10.1002/mrm.10715. [DOI] [PubMed] [Google Scholar]
- 8.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 9.Terpstra M, Tkac I, Rao R, Gruetter R. Quantification of vitamin C in the rat brain in vivo using short echo time 1H MRS. Magn. Reson. Med. 2006;55:979–983. doi: 10.1002/mrm.20854. [DOI] [PubMed] [Google Scholar]
- 10.Tkac I, Henry P-G, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn. Reson. Med. 2004;52:478–484. doi: 10.1002/mrm.20184. [DOI] [PubMed] [Google Scholar]
- 11.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 12.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 13.Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J. Magn. Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 14.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs7T. Magn. Reson. Med. 2009 doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 16.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Tkac I, Starcuk Z, Choi I, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn. Reson. Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Tkac I, Gruetter R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl. Magn. Reson. 2005;29:139–157. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn. Reson. Med. 2002;47:1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 20.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Bevington PR, Robinson DK. Multivariable correlations. In: Cotkin SJ, editor. Data Reduction and Error Analysis for the Physical Sciences. New York: McGraw-Hill Higher Education; 2003. pp. 201–207. [Google Scholar]
- 22.Provencher SW. Detailed output. LCModel & LCMgui User’s Manual. 2000:100. [Google Scholar]
- 23.Govindaraju G, Young K, Maudsley A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Terpstra M, Marjanska M, Henry P-G, Tkac I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn. Reson. Med. 2006;56:1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 25.Schaus R. The ascorbic acid content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am. J. Clin. Nutr. 1957;5:39–41. doi: 10.1093/ajcn/5.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Soher BJ, Pattany PM, Matson GB, Maudsley A. Observation of coupled 1H metabolite resonances at long TE. Magn. Reson. Med. 2005;53:1283–1287. doi: 10.1002/mrm.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larvaron P, Bielicki G, Boespflug-Tanguy O, Renou JP. Proton MRS of early post-natal mouse brain modifications in vivo. NMR Biomed. 2006;19:180–187. doi: 10.1002/nbm.997. [DOI] [PubMed] [Google Scholar]
- 28.Lei H, Zhang Y, Zhu X, Chen W. Changes in the proton T2 relaxation times of cerebral water and metabolites during forebrain ischemia in rat brain at 9.4 T. Magn. Reson. Med. 2003;49:979–984. doi: 10.1002/mrm.10490. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf RA, Brown PB, McIntyre S, Nixon TW, Behar KL, Rothman DL. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn. Reson. Med. 2006;56:386–394. doi: 10.1002/mrm.20946. [DOI] [PubMed] [Google Scholar]
- 30.Scheenen TW, Gambarota G, Weiland E, Klomp DW, Futterer JJ, Barentsz JO, Heerschap A. Optimal timing for in vivo 1H-MR spectroscopic imaging of the human prostate at 3T. Magn. Reson. Med. 2005;53:1268–1274. doi: 10.1002/mrm.20468. [DOI] [PubMed] [Google Scholar]
- 31.Lijing X, Gambarota G, Mlynarik V, Gruetter R. Proton T2 relaxation time of J-coupled cerebral metabolites in rat brain at 9.4 T. NMR Biomed. 2008;21:396–401. doi: 10.1002/nbm.1205. [DOI] [PubMed] [Google Scholar]
- 32.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn. Reson. Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]