Abstract
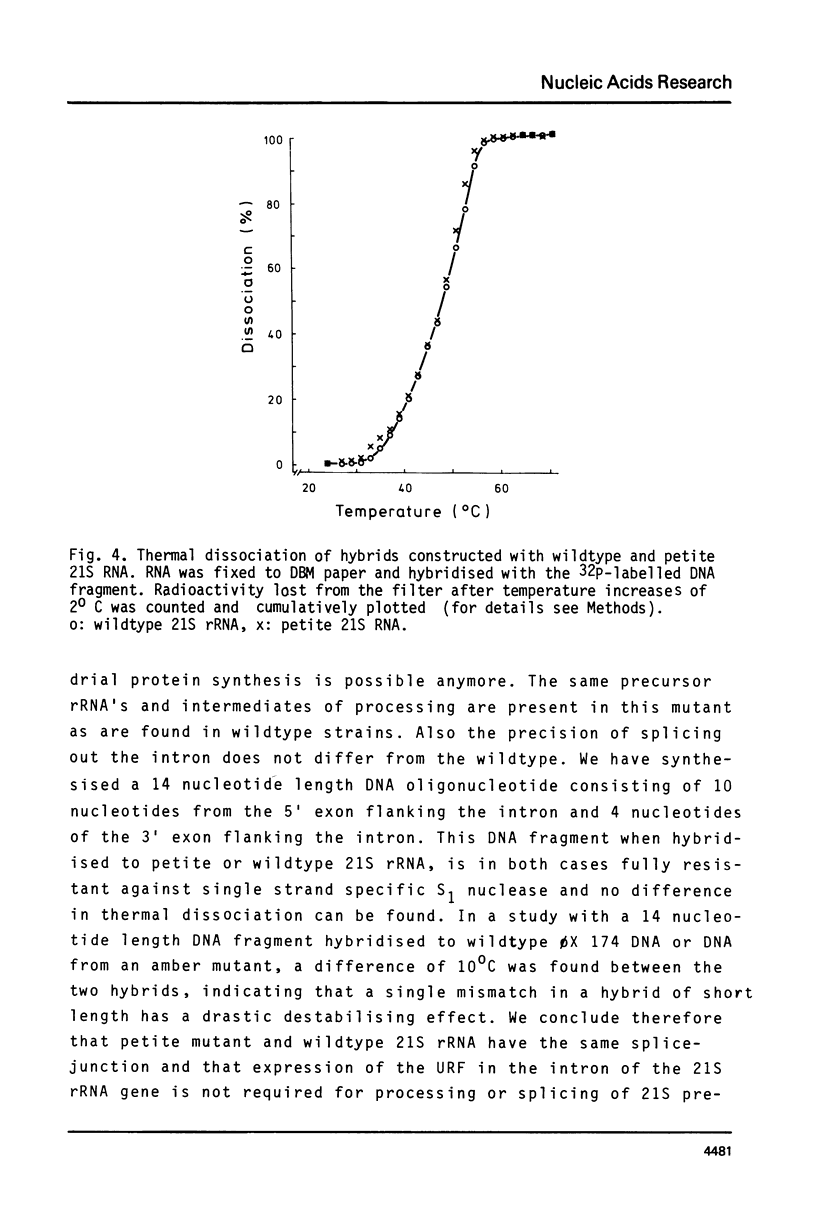
In some strains of Saccharomyces cerevisiae the mitochondrial gene coding for 21S rRNA is interrupted by an intron of 1143 bp. This intron contains a reading frame for 235 amino acids: Unassigned Reading Frame (URF). In order to check whether expression of this URF is required for proper splicing of precursors to 21S rRNA, the precision of RNA splicing was analysed in a petite mutant, where no mitochondrial protein synthesis is possible anymore. We have devised a new assay to monitor the precision of the splicing event. The method is of general application, provided that the sequence of the splice boundaries is known. In the case of the 21S rRNA it involves the synthesis of the DNA oligonucleotide d(CGATCCCTATTGTC( complementary to the 5' d(CGATCCCTAT) and 3' d(TGTC) borders flanking the intron in the 21S rRNA gene. The oligonucleotide is labelled with 32p at the 5'-end, hybridised to RNA and subsequently subjected to digestion with S1 nuclease. Resistance to digestion will only be observed if the correct splice-junction is made. The petite mutant we have studied contains a 21S rRNA with the same migration behaviour as wildtype 21S rRNA. In RNA blotting experiments, using an intron specific hybridisation probe, the same intermediates in splicing are found both in wild type and petite mutant. Finally the synthetic oligonucleotide hybridises to petite 21S rRNA and its thermal dissociation behaviour is indistinguishable from a hybrid formed with wildtype 21S rRNA. We conclude that expression of the URF, present in the intron of the 21S rRNA gene, is not required for processing and correct splicing of 21S ribosomal precursor RNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Borst P., Grivell L. A. One gene's intron is another gene's exon. Nature. 1981 Feb 5;289(5797):439–440. doi: 10.1038/289439a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Hecht N. B., Tabak H. F., Van Ommen G. J., Borst P. Splice point sequence and transcripts of the intervening sequence in the mitochondrial 21S ribosomal RNA gene of yeast. Cell. 1980 May;20(1):207–214. doi: 10.1016/0092-8674(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., Borst P. Isolation of yeast mitochondrial ribosomes highly active in protein synthesis. Biochim Biophys Acta. 1971 Sep 30;247(1):91–103. doi: 10.1016/0005-2787(71)90811-2. [DOI] [PubMed] [Google Scholar]
- Heyting C., Menke H. H. Fine structure of the 21S ribosomal RNA region on yeast mitochondrial DNA. III. Physical location of mitochondrial genetic markers and the molecular nature of omega. Mol Gen Genet. 1979 Jan 11;168(3):279–291. doi: 10.1007/BF00271498. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Kreike J., Bechmann H., Van Hemert F. J., Schweyen R. J., Boer P. H., Kaudewitz F., Groot G. S. The identification of apocytochrome b as a mitochondrial gene product and immunological evidence for altered apocytochrome b in yeast strains having mutations in the COB region of mitochondrial DNA. Eur J Biochem. 1979 Nov;101(2):607–617. doi: 10.1111/j.1432-1033.1979.tb19755.x. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten S., Synenki R. M., Locker J., Christianson T., Rabinowitz M. Processing of precursors of 21S ribosomal RNA from yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1417–1421. doi: 10.1073/pnas.77.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman A. F., Grivell L. A., Lamie F., Smits H. L. Identification of mitochondrial gene products by DNA-directed protein synthesis in vitro. Biochim Biophys Acta. 1978 Apr 27;518(2):351–365. doi: 10.1016/0005-2787(78)90192-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Ullrich A., Baxter J. D., Goodman H. M. Nucleotide sequence of part of the gene for human chorionic somatomammotropin: purification of DNA complementary to predominant mRNA species. Cell. 1977 Sep;12(1):157–165. doi: 10.1016/0092-8674(77)90193-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M., Schatz G. Mutations in putative intervening sequences of the mitochondrial cytochrome b gene of yeast produce abnormal cytochrome b polypeptides. J Biol Chem. 1979 Oct 10;254(19):9331–9334. [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]