Abstract
Objective[mh2]
To evaluate the single dose pharmacokinetics of an intravenous dose of lorazepam in pediatric patients treated for status epilepticus (SE) or with a history of SE.
Study design
Ten hospitals in the Pediatric Emergency Care Applied Research Network (PECARN) enlisted patients 3 months to 17 years with convulsive SE (STATUS) or for a traditional PK study (ELECTIVE). Sparse sampling was used for STATUS and intensive sampling for ELECTIVE. Noncompartmental analyses were performed on ELECTIVE, and served to nest compartmental population PK analysis for both cohorts.
Results
48 STATUS and 15 ELECTIVE patients were enrolled. Median age was 7 years, 2 months. The population PK parameters were: clearance 1.2 mL/min/kg, half-life 16.8 hours, volume of distribution 1.5 L/kg. Based on the PK model, a 0.1 mg/kg dose is expected to achieve concentrations of approximately 100 ng/mL and maintain concentrations above 30–50 ng/mL for 6–12 hours. A second dose of 0.05 mg/kg would achieve desired therapeutic serum levels for approximately 12 hours without excessive sedation. Age-dependent dosing is not necessary beyond using a maximum initial dose of 4 mg.
Conclusions
Lorazepam PK in convulsive status epilepticus is similar to previous PK measured in pediatric patients with cancer, except for longer half-life and similar to adult PK parameters except for increased clearance.
It is estimated that 4–8 children per 1000 will experience an episode of status epilepticus (SE) before age 15 years.1 Benzodiazepines are the most effective agents for the initial treatment of SE, achieving lasting control in 80% of patients.2,3 Textbooks and expert opinion recommend both diazepam and lorazepam as initial therapy for children in SE and provide recommended doses that are commonly used in practice.4,5 In addition, midazolam has been used for initial therapy6,7 or for refractory status.8,9 Many experts support the use of lorazepam over diazepam as initial therapy for pediatric SE despite the fact that diazepam is FDA-approved and lorazepam is not.10,11 Increased duration of action, increased effectiveness, and a lower incidence of respiratory depression have been cited as potential advantages of lorazepam over diazepam.12,13
The limited pediatric data available indicate that lorazepam metabolism in children may differ from adult patients16,17. In addition, SE itself may alter pharmacokinetics and children with SE may be receiving other anticonvulsants that may affect lorazepam metabolism. Data are needed to determine the unique pharmacology of lorazepam in children with SE.
The Best Pharmaceuticals for Children Act (BPCA)18 was passed in 2002 and provides a federal mechanism for the study of off-patent drugs in children. This study was performed under the auspices of the BPCA. The primary objective of this study was to evaluate the single dose pharmacokinetics of an intravenous dose of lorazepam in pediatric patients treated for status epilepticus (SE) or with a history of SE. Secondary objectives of the study were to evaluate the population pharmacokinetics of lorazepam and its glucuronide metabolite, determine the impact of age, weight, height, active SE, and concomitant medications on lorazepam pharmacokinetics, and compare pharmacokinetic parameters in children to those seen in adults.
METHODS
We conducted a prospective multi-center study to evaluate the pharmacokinetics of a single intravenous dose of lorazepam in children. Patients were enrolled at ten sites of the Pediatric Emergency Care Applied Research Network (PECARN), which was founded in 2001 by the Emergency Medical Services for Children program to help overcome barriers to pediatric emergency medicine research.19 The study was approved by the institutional review boards of the participating hospitals.
The Status Cohort included patients who presented to the emergency department (ED) in status epilepticus (SE) and received lorazepam as part of standard medical care. The Status Cohort patients were further categorized into patients who had pre-consented to participate in the study prior to their presentation in SE (Cohort 1a), and patients who presented to the ED in SE and consented to participate in the study after they had received lorazepam (Cohort 1b). Pre-consented patients were identified from Neurology practices and EDs and had a history of recurrent ED visits for SE.
The Elective Cohort included patients with a prior history of seizures who electively received one dose of lorazepam in a scheduled pharmacokinetic study in a clinical research center.
Patients between the ages of 3 months to 17 years were eligible. We excluded patients with pregnancy, sustained hypotension, significant dysrrhythmia, known lorazepam allergy, anemia, history of using lorazepam within 4 days of dosing, weight less than 8 kg, because of the volume of blood sampling required, and patients for whom we could not obtain blood samples.
Patients in the Status Cohort received 0.05 to 0.1 mg/kg, depending on treating physician preference, to a maximum of 4 mg, and Elective Cohort patients received 0.05mg/kg to a maximum dose of 2 mg. For both cohorts, lorazepam was withdrawn from the manufacturer’s refrigerated vial into a syringe just prior to slow intravenous push over 1 minute. For the Status Cohort, additional medications for the treatment of status epilepticus were administered at the discretion of the treating physician, including additional doses of lorazepam. Vital signs were recorded every 15 minutes for one hour after dosing and less frequently thereafter. At each vital sign interval, notation was made regarding the need for assisted ventilation during the previous time period. The Riker sedation-agitation scale20 was recorded at specified intervals because lorazepam may cause either sedation or agitation. A modified Riker score was created for preverbal subjects and was validated in a separate study (Brown KM, unpublished data). Adverse events were reported in accordance with federal regulations.
Status Cohort subjects had up to 5 pharmacokinetic samples for total lorazepam lorazepam-glucuronide concentrations collected between 0–48 hours after administration of study drug. Elective Cohort subjects had up to 13 PK samples collected from 0–48 hours. Larger sample volumes were required for free (unbound) drug concentration determination and therefore were measured at only 2 to 3 time points. Blood was sampled from a second intravenous site located on a different extremity from the site where lorazepam was administered. Blood was collected in 2 mL sodium heparin tubes;21 serum was separated and immediately frozen, then shipped in monthly batches on dry ice for analysis.
Separate analytical methods were developed for the analysis of total lorazepam, unconjugated lorazepam (protein bound + unbound) and unbound (free) lorazepam. The unconjugated lorazepam plasma concentrations were initially determined by a High Performance Liquid Chromatographic (HPLC) with UV detection method. This assay was linear between 20–2,000 ng/mL. After the initial samples, we determined that a more sensitive method was needed. The liquid chromatography/mass spectroscopy (LC/MS/MS) method we developed and used to measure the remaining unconjugated lorazepam samples gave lower limits of quantitation at 1 ng/mL for both the unconjugated and the unbound drug, and 2 ng/mL for total drug. Results generated by each methodology were reported separately for data analysis. Assay performance characteristics are available from the authors upon request. Unbound lorazepam concentrations were determined by ultrafiltration. Total lorazepam concentrations (molar normalized) were determined by enzymatic cleavage of the glucuronide from the lorazepam metabolite with beta glucuronidase prior to extraction and analysis. The lorazepam glucuronide concentrations were determined by subtracting the concentration of unconjugated lorazepam from the total lorazepam concentration.
Analyses
The analyses included both intensive non-compartmental (NC) and population pharmacokinetic evaluations. Non-compartmental analyses are used to describe pharmacokinetics without making assumptions about how the drug is distributed and eliminated. The NC analyses were performed on data from the Elective Cohort, and served to nest the compartmental population pharmacokinetic analysis for both cohorts.
In the NC analysis the area under the plasma drug concentration versus time curve (AUC0–∞) was determined using the linear trapezoidal rule up to the final measurable concentration point and the AUC after the final measurable concentration (Clast) estimated as Clast/λz, where λz is the negative slope of the natural log concentration vs. time profile.. Total body clearance of lorazepam was determined using the formula: Dose/AUC0–∞. The elimination half-life (T1/2) was determined from the post-distributive terminal portion of the lorazepam plasma concentration versus time curve. The volume of distribution was calculated from the equation: Vdss = Dose*AUMC/AUC2 where AUMC is the area under the first moment curve.
The population pharmacokinetic analysis was performed with data from both Cohorts using the computer software NONMEM (Version VI, ICON, Ellicott City, MD). Structurally, a two-compartment model with first order elimination (ADVAN3) was evaluated. Pharmacokinetic parameters were scaled by subject size before evaluation of other potential covariates. An allometric approach to size was used scaling subject weight to the 0.75 power (weight0.75) for clearance and weight to 1.0 power (weight1.0) for volume of distribution.22 Allometric scaling is a common approach to account for size in PK (and biologic) modeling. It is more robust than linear weight in accounting for size (i.e. liver and renal function) but does not account for maturational changes and other developmental differences. The potential impact of clinical covariates on pharmacokinetic parameters was evaluated in a two-stage approach. Potential covariates that were evaluated included age, sex, albumin level, liver enzymes, ethnicity, study cohort, and concomitant medications.
Covariates were retained in the final pharmacokinetic model if they improved the goodness of fit statistically (decrease in objective function, MOF, by > 8, p <~0.0501). The lorazepam population pharmacokinetic model was further expanded to include modeling of lorazepam glucuronide disposition to improve the PK model. The appropriateness of the final models with and without metabolites was evaluated using a bootstrap method (Wings for NONMEM http://wfn.sourceforge.net) with 1000 iterations.
Empiric Bayesian estimates of individual lorazepam pharmacokinetic parameters were generated from the final model using the POSTHOC subroutine. Group comparison from the individual Bayesian parameters were performed using Pearson correlation coefficients and Wilcoxon-Rank sum tests. A p value < 0.05 was considered significant.
The free fraction of lorazepam was determined in each subject as the free lorazepam concentration divided by total lorazepam concentration. The impact of lorazepam concentration, age, albumin and concomitant medications on free fraction was assessed using a general linear models approach. We were also specifically interested in whether recent seizure activity altered the proportion of free lorazepam compared with the proportion in the Elective cohort.
We used the model to predict peak serum levels and the duration of maintenance of therapeutic serum levels. In the absence of pharmacodynamic data, we used PK data from the literature to estimate that serum levels between 30 and 100 ng/ml are expected to provide anticonvulsant effects without producing levels associated with excessive sedation.23,24,25
Results
Sixty nine patients received study medication, of whom 6 discontinued participation because of withdrawal of consent or technical difficulties with intravenous access. Thus 63 patients had samples for pharmacokinetic analysis (Table I); 15 patients were enrolled in the Elective Cohort and 48 patients in the Status Cohort. Patients ranged in age from 5 months to 17 years with a median age of 7 years, 2 months.
Table 1.
Study enrollment.
| Age Group | ||||
|---|---|---|---|---|
| 3 mos to <3 yrs | 3 yrs to <13 yrs | 13 yrs to <18 yrs | Total | |
| Patients screened | 52 | 72 | 44 | 168 |
| Ineligible or refused consent | 32 | 40 | 27 | 99 |
| Enrolled | 20 | 32 | 17 | 69 |
| Status cohort | 19 | 26 | 8 | 53 |
| Elective cohort | 1 | 6 | 9 | 16 |
| PK data obtained | 18 | 29 | 16 | 63 |
| Completed 30-day follow-up | 16 | 27 | 13 | 56 |
In the Status Cohort, 36 patients received a single dose with a mean dose of 1.7 mg (0.08 mg/kg). 8 patients (16.7%) required a second dose of lorazepam within 30 minutes, with a mean total dose of 2.4 mg (0.13 mg/kg), and four patients (8.3%) received 3 or more doses (total mean dose 5.3 mg, 0.16 mg/kg) within 30 minutes. Six patients (12.5%) required crossover to another medication within 30 minutes of initial lorazepam dosing. Three patients (6.3%) required endotracheal intubation. One patient required assisted ventilation without intubation and one patient required insertion of a nasal airway. Other adverse reactions within one hour of dosing included hypotension (n = 13), extreme sedation (8), vomiting (5), tachycardia (n = 7), and agitation (n = 3). Two patients (4.2%) had suspected aspiration. We could not determine whether these adverse events were caused by lorazepam or by the underlying SE. The total mean lorazepam dose was higher in the patients with adverse events than in those without (0.12 ± 0.06 vs. 0.08 ± 0.04, p = 0.03). In the Elective Cohort, the only adverse event was agitation in one patient.
Pharmacokinetics
Table II summarizes the noncompartmental PK evaluation for the Elective Cohort. Eight pharmacokinetic samples (0.3%) were excluded, seven due to suspected contamination from the infused drug and one due to collection during the lorazepam infusion. Overall, the mean area-under-the-curve (AUC0–∞) was 822.5 ng*hr/mL and the median AUC0–∞ was 601.5 ng*hr/mL with an average dose of 0.04 mg/kg. The overall fit of the population PK model was good over the wide range of individuals in the population. There were no covariates meeting criteria for inclusion into the model. Thirty three subjects (23 Status Cohort, 10 Elective Cohort) had received, at baseline, at least one agent that can induce drug metabolizing enzymes. The calculated value for terminal (beta) half-life was 16 hours for a typical 24 kg child. The empiric Bayesian estimated parameters from the post-hoc analysis are summarized in Table III. The model demonstrated good model prediction of observed concentrations even when applied to the patients exhibiting the highest and lowest individual clearances, and in a patient who received a total of 9 doses of lorazepam during the PK sampling interval.
Table 2.
Non-compartmental pharmacokinetics parameters from Elective Cohort patients. Cmax is maximum concentration. AUC0−∞ is area-under-the-curve to infinity. CL is clearance. Vdz is apparent volume of distribution. T1/2 is half-life.
| Cmax (ng/mL) |
AUC0−∞ | CL (mL/min/kg) |
CL (mL/min/m2) |
Vdz (L/kg) |
T1/2 (hr) |
|
|---|---|---|---|---|---|---|
| N | 15 | 15 | 15 | 15 | 15 | 15 |
| Range | 29.3–209.6 | 253.3–3202.5 | 3.33–131.50 | 5.5–67.5 | 0.33–4.05 | 9.5–47.0 |
| Mean ± s.d. | 56.1 ± 44.9 | 822.5 ± 706.1 | 49.33 ± 30.83 | 31.95 ± 13.99 | 1.92 ± 0.84 | 20.5 ± 10.2 |
| Median | 42.2 | 601.5 | 41.50 | 32.34 | 1.94 | 18.1 |
Table 3.
Bayesian pharmacokinetics parameters (all subjects). CL is clearance. Vdss is volume of distribution at steady state. Beta is the terminal slope of the log concentration versus time profile. T½ Beta is the elimination half-life.
| Free Fraction | CL (mL/min/kg) |
CL mL/min/m2) |
Vdss (L/kg) |
Beta (hr−1) |
T½ Beta (hr) |
|
|---|---|---|---|---|---|---|
| Overall | ||||||
| N | 61 | 63 | 63 | 63 | 63 | 63 |
| Range | 0.07–0.48 | 0.3–7.75 | 6.50–147.17 | 0.49–3.40 | 0.017–0.118 | 5.9–42.0 |
| Mean ± s.d. | 0.10 ± 0.05 | 1.2 ± 0.93 | 33.33 ± 19.33 | 1.48 ± 0.54 | 0.048 ± 0.020 | 16.8 ± 7.1 |
| Median | 0.09 | 1.08 | 29.00 | 1.37 | 0.046 | 15.1 |
| 3 Month to < 3 Years | ||||||
| N | 17 | 18 | 18 | 18 | 18 | 18 |
| Range | 0.07–0.48 | 0.63–7.75 | 12.83–147.17 | 0.67–3.40 | 0.024–0.118 | 5.9–28.4 |
| Mean ± s.d. | 0.11 ± 0.10 | 1.57 ± 1.62 | 32.83 ± 30.17 | 1.62 ± 0.59 | 0.053 ± 0.027 | 15.8 ± 6.5 |
| 3 to < 13 Years | ||||||
| N | 28 | 29 | 29 | 29 | 29 | 29 |
| Range | 0.07–0.17 | 0.30–1.82 | 6.50–69.17 | 0.49–3.00 | 0.017–0.092 | 7.5–40.6 |
| Mean ± s.d. | 0.10 ± 0.02 | 1.12 ± 0.40 | 31.83 ± 13.83 | 1.50 ± 0.61 | 0.048 ± 0.017 | 16.9 ± 7.4 |
| 13 to < 18 Years | ||||||
| N | 16 | 16 | 16 | 16 | 16 | 16 |
| Range | 0.07–0.15 | 0.43–1.58 | 16.33–60.00 | 1.00–1.54 | 0.017–0.084 | 8.2–42.0 |
| Mean ± s.d. | 0.09 ± 0.02 | 0.95 ± 0.32 | 36.67 ± 12.00 | 1.27 ± 0.17 | 0.044 ± 0.016 | 17.8 ± 7.7 |
The typical population PK parameters estimates were as follows: clearance (CL) = 0.14 L/hr/kg0.75; Vc = 0.91 (L/kg); Vdss = 1.37 L/kg; and Q = 1.05 L/hr//kg0.75. Overall lorazepam PK post-hoc parameters from the population analysis were as follows: CL = 1.2 mL/min/kg); Vdss = 1.48 L/kg; and half-life = 16.8 hr (Table III). The free-fraction was determined in 109 of 343 samples and averaged 10.2 ± 5.8%. Free fraction was independent of lorazepam concentration and cohort and there was no apparent age effect. The ratio of lorazepam-glucuronide/lorazepam concentrations averaged 0.99 and increased throughout the sampling time (r=0.59, p<0.001). The non-compartmental analysis generated consistent findings to the population-based approach with a slightly lower estimate for CL (0.057 L/hr/kg) because of the older average age of patients in the Elective Cohort. The median CL and Vdss from the bootstrap analysis were identical to the values generated from the original data set with 95% confidence intervals of 0.12 – 0.16 L/hr//kg0.75 for CL and 1.25–1.58 L/kg for Vdss.
There were potentially modest age effects seen in CL and Vdss when normalized to weight; however, age-associated changes in lorazepam clearance did not meet criteria for inclusion in the final pharmacokinetic model with allometric scaling, nor when scaled by body surface area. The impact of age on volume of distribution was the most powerful covariate in univariate analyses; however, this also failed to meet criteria for inclusion in the final pharmacokinetic model. Additionally, because both CL and Vdss trended towards larger values in younger patients, the combined potential age-effect impact on CL and Vdss countered each other, with the net result of no age effect on half-life.
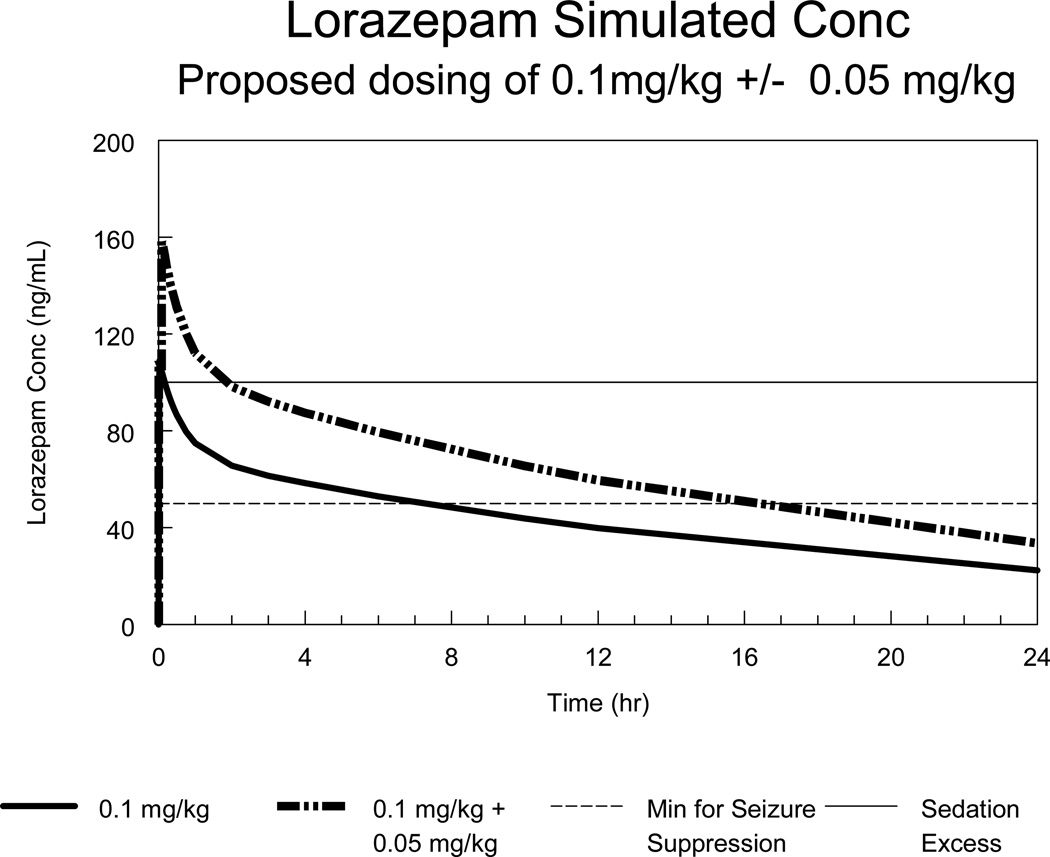
Based on the final pharmacokinetic model derived from this study, a dose of 0.1 mg/kg with a maximum 4 mg is expected to achieve near-peak concentrations of approximately 100 ng/mL and maintain concentrations above 30 to 50 ng/mL for 6 to 12 hours in the typical pediatric patient. A second dose of 0.1 mg/kg would potentially achieve maximal concentrations that are too high for the desired clinical effects, whereas a second dose of 0.05 mg/kg (maximum 2 mg) would achieve desired therapeutic serum levels for approximately 12 hours (Figure). Although some age effects were seen in this model, it is not anticipated that this would necessitate age-dependent dosing beyond the truncation of dosing at 40 kg (i.e. maximum single dose of 4 mg). If repeated doses of lorazepam are required for maintenance therapy as an inpatient, for example, then dosing should occur at least every 12 hours to prevent fluctuations in concentration more than two-fold.
Figure.
Predicted serum levels following a lorazepam dose of 0.1 mg/kg (maximum 4 mg) with and without a second dose of 0.05 mg/kg (maximum 2 mg). The lower dotted line represents estimated serum levels for effective seizure suppression.23 The upper dotted line represents estimated serum levels associated in previous studies with excessive sedation.21,22
Discussion
This study evaluated lorazepam pharmacokinetics in children with status epilepticus. One of the challenges to gaining FDA approval for this indication has been the inability to study lorazepam pharmacokinetics and efficacy in the emergency setting. We were able to measure population pharmacokinetics using sparse sampling and compartmental modeling in pediatric patients with status, augmented by intensive non-compartmental modeling in an elective cohort of subjects.
The use of a population-based approach in this study provided flexibility in sample collection time and dosing necessary to determine pharmacokinetics during active status epilepticus. For example, early PK samples were not possible in most Status patients. This approach also allowed utilization of lorazepam-glucuronide concentrations to provide additional information on lorazepam metabolism, thus improving the precision of the clearance estimates. Although there was more than a 20-fold range in lorazepam clearance, we did not find any significant differences in lorazepam elimination from patients with active seizures compared to those with an elective PK evaluation. In addition, protein binding was not different between the two cohorts. Importantly for clinical practice, none of the common anticonvulsants were associated with alterations in lorazepam elimination.
The age-related changes in lorazepam clearance, scaled allometrically, did not reach the pre-defined level needed for inclusion in the final population pharmacokinetic model. Glucuronidation by the liver increases early during infancy and may exceed adult capacity in young children due to the larger relative liver size. In the current study, there were only five subjects less than 1 year of age, all greater than 4 months of age. This modest number of young infants, the large inter-subject variability in lorazepam clearance and use of allometric scaling to approximate for liver size limited the ability to detect developmental changes in lorazepam clearance. However, when lorazepam clearance was modeled using raw weight (weight1.0), it was greater in infants and younger children than the adolescents. Our results are consistent with previous literature concerning UDP-glucuronosyltransferase (UGT) metabolism demonstrating marked increases in the neonatal period but only modest changes in UGT activity after 6 months of age.26,27
In adult studies, the average lorazepam clearance ranges from 0.75 to 1.28 mL/min/kg and elimination half-life 9–22 hours (Table IV; available at www.jpeds.com).28,29,30,31,32,33,34,35 Taking the adult experience as a whole, lorazepam clearance in our patients was approximately 20% higher than in adults, and the elimination half-life was approximately equal to that in adults. The average lorazepam clearance in this study is consistent with studies in pediatric patients with cancer between 2 to 12 years of age, although the current study had a longer half-life (15 vs 10.5 hr). This difference may be related to the longer sampling duration in the current study.38
Overall, lorazepam was successful in treating status epilepticus. Of the 48 status patients, 42 (87.5%, 95% CI 74.8–95.3%) were successfully treated with one or two doses; only 6 of 48 patients required a third dose of an anticonvulsant within 30 minutes. Four of 48 (8.3%, 95% CI 2.3–20%) status patients required assisted ventilation for respiratory depression; in one patient this was transient. Although this study was not designed to test the efficacy and safety or lorazepam for pediatric SE, our results are consistent with previous pediatric studies demonstrating successful treatment in 65–81%36,37,38 and respiratory depression in 4–27%.36,38,39
The pediatric lorazepam PK parameters are within the range previously reported in adults and clearance similar to that previously reported in pediatric patients with cancer. Weight-adjusted CL and Vdss tended to be higher in younger infants and children compared to older children; however, the magnitude of these differences was not statistically significant. Concomitant medications or enzyme-inducing anticonvulsant drugs did not affect lorazepam clearance. Status epilepticus did not affect lorazepam protein binding and clearance compared to subjects without status. These PK results, along with safety and tolerability data, indicate that a dose of 0.1 mg/kg (4 mg maximum) will achieve and maintain lorazepam concentrations in the range associated with anticonvulsant effects for 6–12 hours and would not exceed those associated with heavy sedation. If a second dose of lorazepam is required for SE, a dose of 0.05 mg/kg (2 mg maximum) will result in serum levels associated with anticonvulsant activity for approximately 12 hours without achieving levels associated with excessive sedation.
Supplementary Material
Acknowledgments
Supported by the Emergency Medical Services for Children program of the Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services (U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.[mh1]
Contributor Information
James M. Chamberlain, Division of Emergency Medicine, Children’s National Medical Center, Professor of Pediatrics and Emergency Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC.
Edmund V. Capparelli, Clinical Professor Pediatrics and Pharmacy, University of California San Diego, School of Medicine and Skaggs School of Pharmacy and Pharmaceutical Sciences, San Diego, CA.
Kathleen M. Brown, Division of Emergency Medicine, Children’s National Medical Center, Associate Professor of Pediatrics and Emergency Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC.
Cheryl W. Vance, Professor of Emergency Medicine and Pediatrics, University of California Davis, School of Medicine, Sacramento, CA.
Kathleen Lillis, Professor of Clinical Pediatrics, School of Medicine and Biomedical Sciences, State University of New York at Buffalo.
Prashant Mahajan, Associate Professor of Pediatrics and Emergency Medicine, Wayne State University School of Medicine, Children’s Hospital of Michigan, Detroit, MI.
Richard Lichenstein, Associate Professor of Pediatrics, University of Maryland School of Medicine, Division of Pediatric Emergency Medicine, University of Maryland Hospital for Children, Baltimore, MD.
Rachel M. Stanley, Assistant Professor of Emergency Medicine and Pediatrics, University of Michigan Health System Department of Emergency Medicine, Ann Arbor, MI.
Colleen O. Davis, University of Rochester Medical Center, Departments of Emergency Medicine and Pediatrics, Rochester, NY.
Stephen Gordon, Assistant Clinical Professor of Pediatrics, Columbia University College of Physicians and Surgeons, Division of Pediatric Emergency Medicine, New York-Presbyterian Morgan Stanley Children's Hospital, New York, NY.
Jill M. Baren, Associate Professor of Emergency Medicine and Pediatrics, Department of Emergency Medicine, Hospital of the University of Pennsylvania, Department of Pediatrics, The Children’s Hospital of Philadelphia.
John N. van den Anker, Evan and Cindy Jones Chair in Pediatric Clinical Pharmacology, Vice Chair of Pediatrics for Experimental Therapeutics, Children's National Medical Center, Professor of Pediatrics, Pharmacology & Physiology, George Washington University School of Medicine and Health Sciences.
References
- 1.Shinnar S, Berg AT, Moshe SL, O'Dell C, Alemany M, Newstein D, Kang H, Goldensohn ES, Hauser WA. The risk of seizure recurrence after a first unprovoked afebrile seizure in childhood: an extended follow-up. Pediatrics. 1996;98:216–225. [PubMed] [Google Scholar]
- 2.De Negri M, Baglietto MG. Treatment of Status Epilepticus in Children. Pediatr Drugs. 2001;3:411–420. doi: 10.2165/00128072-200103060-00002. [DOI] [PubMed] [Google Scholar]
- 3.Treiman DM. The role of benzodiazepines in the management of status epilepticus. Neurology. 1990;40(5) Suppl 2:32–42. [PubMed] [Google Scholar]
- 4.Graneto JW, Strange GR. Seizures. In: Fleisher GR, Ludwig S, editors. Textbook of Pediatric Emergency Medicine. 3rd ed. Baltimore: Williams and Wilkins; 1993. p. 468. [Google Scholar]
- 5.Gunn VL, Nechyba C, editors. A Manual for Pediatric House Officers. St Louis: Mosby; 2002. The Harriet Lane Handbook. [Google Scholar]
- 6.Chamberlain JM, Altieri MA, Futterman C, Young GM, Waisman Y. A randomized, controlled trial comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13:92–94. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brevoord JC, Joosten KF, Arts WF, Van Rooij RW, De Hoog M. Status epilepticus: clinical analysis of a treatment protocol based on midazolam and phenytoin. J Child Neurol. 2005;20:476–481. doi: 10.1177/08830738050200060201. [DOI] [PubMed] [Google Scholar]
- 8.Rivera R, Segnini M, Baltodano A, Pérez V. Midazolam in the treatment of status epilepticus in children. Crit Care Med. 1993;21:991–994. doi: 10.1097/00003246-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Igartua J, Silver P, Maytal J, Sagy M. Midazolam coma for refractory status epilepticus in children. Crit Care Med. 1999;27:1982–1985. doi: 10.1097/00003246-199909000-00043. [DOI] [PubMed] [Google Scholar]
- 10.Bleck TP. Management approaches to prolonged seizures and status epilepticus. Epilepsia. 1999;40 Suppl 1:S59–S63. doi: 10.1111/j.1528-1157.1999.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 11.Sabo-Graham T, Seay AR. Management of status epilepticus in children. Peds Rev. 1998;19:306–309. doi: 10.1542/pir.19-9-306. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt DJ, Divoll M. Diazepam versus lorazepam: relationship of drug distribution to duration of clinical action. In: Delgado-Escueta A, Wasterlain CG, Treiman DM, Porter RJ, editors. Status epilepticus: mechanisms of brain damage and treatment. New York: Raven Press; 1983. pp. 487–491. [PubMed] [Google Scholar]
- 13.Cock HR, Schapira AHV. A comparison of lorazepam and diazepam as initial therapy in convulsive status epilepticus. QJM. 2002;95:225–231. doi: 10.1093/qjmed/95.4.225. [DOI] [PubMed] [Google Scholar]
- 17.Relling MV, Mulhern RK, Dodge RK, Johnson D, Pieper JA, Rivera GK, Evans WE. Lorazepam pharmacodynamics and pharmacokinetics in children. Pediatr Pharmacol Therapeutics. 1989;114:641–646. doi: 10.1016/s0022-3476(89)80713-9. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed 5/29/2011]; Available at http://www.gpo.gov/fdsys/pkg/PLAW-107publ109/pdf/PLAW-107publ109.pdf.
- 19.Dayan P, Chamberlain JM, Dean JM, Maio RF, Kuppermann N and the Pediatric Emergency Care Applied Research Network. The Pediatric Emergency Care Applied Research Network: Progress and Update. Clin Ped Emerg Med. 2006;7:128–135. [Google Scholar]
- 20.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Desmond PV, Roberts RK, Wood AJ, Dunn GD, Wilkinson GR, Schenker S. Effect of heparin administration on plasma binding of benzodiazepines. Br J Clin Pharmacol. 1980;9:171–175. doi: 10.1111/j.1365-2125.1980.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokin. 1996;30:329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 23.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiol. 2001;95:286–298. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Ellinwood EH, Nikaido AM, Heatherly DG. Simultaneous modeling of the pharmacokinetic and pharmacodynamic properties of benzodiazepines. I: Lorazepam. J Pharmacokinet Biopharm. 1990;18:89–102. doi: 10.1007/BF01063553. [DOI] [PubMed] [Google Scholar]
- 25.Walker JE, Homan RW, Crawford IL. Lorazepam: a controlled trial in patients with intractable partial complex seizures. Epilepsia. 1984;25:464–466. doi: 10.1111/j.1528-1157.1984.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 26.Bouwmeester NJ, Hop WJ, Dijk M, Anand KJS, Anker JN, Tibboel D. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Inten Care Med. 2003;29:2009–2015. doi: 10.1007/s00134-003-1899-4. [DOI] [PubMed] [Google Scholar]
- 27.De Wildt SN, Johnson TN, Chhonara I. The effect of age on drug metabolism. Paediatr Perinat Drug Ther. 2003;5:101–106. [Google Scholar]
- 28.Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6:89–105. doi: 10.2165/00003088-198106020-00001. [DOI] [PubMed] [Google Scholar]
- 29.Greenblatt DJ, Comer WH, Elliott HW, Shader RI, Knowles JA, Ruelius HW. Clinical pharmacokinetics of lorazepam. III. Intravenous injection. Preliminary results. J Clin Pharmacol. 1977;17:490–494. doi: 10.1002/j.1552-4604.1977.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 30.Kraus JW, Desmond PV, Marshall JP, Johnson RF, Schenker S, Wilkinson GR. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24:411–419. doi: 10.1002/cpt1978244411. [DOI] [PubMed] [Google Scholar]
- 31.Greenblatt DJ, Allen MD, Locniskar A, Harmatz JS, Shader RI. Lorazepam kinetics in the elderly. Clin Pharmacol Ther. 1979;26:103–113. doi: 10.1002/cpt1979261103. [DOI] [PubMed] [Google Scholar]
- 32.Patwardhan RV, Yarborough GW, Desmond PV, Johnson RF, Schenker S, Speeg KV., Jr Cimetidine spares the glucuronidation of lorazepam and oxazepam. Gastroenterol. 1980;79(5 Pt 1):912–916. [PubMed] [Google Scholar]
- 33.Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41:1225–1231. doi: 10.1177/00912700122012779. [DOI] [PubMed] [Google Scholar]
- 34.Crom WR, Relling MV, Christensen ML, Rivera GK, Evans WE. Age-related differences in hepatic drug clearance in children: studies with lorazepam and antipyrine. Clin Pharmacol Ther. 1991;50:132–140. doi: 10.1038/clpt.1991.117. [DOI] [PubMed] [Google Scholar]
- 35.Lorazepam package insert. [Accessed November 23, 2010]; Available at http://www.drugs.com/pro/lorazepam-injection.html. [Google Scholar]
- 36.Giang DW, McBride MC. Lorazepam versus diazepam for the treatment of status epilepticus. Pediatr Neurol. 1998;4:358–361. doi: 10.1016/0887-8994(88)90083-5. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi A, Wassmer E, Davies R, Berry K, Whitehouse WP. Comparitive Audit of Intravenous Lorazepam and Diazepam in the Emergency Treatment of Convulsive Status Epilepticus in Children. Seizure. 2002;11:141–144. doi: 10.1053/seiz.2001.0635. [DOI] [PubMed] [Google Scholar]
- 38.Appleton RE, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. 1995;37:682–688. doi: 10.1111/j.1469-8749.1995.tb15014.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiulli DA, Terndrup TE, Kanter RK. The influence of diazepam or lorazepam on the frequency of endotracheal intubation in childhood status epilepticus. J Emerg Med. 1991;9:13–17. doi: 10.1016/0736-4679(91)90525-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.