Abstract
Despite rising incidence, the molecular events that support clear cell renal cell carcinoma (ccRCC) development and progression remain unclear. Herein, we evaluate the association of endothelin-2 (ET-2) expression with both ccRCC development and progression-free survival (PFS). We conducted real time PCR (rt-PCR) to determine ET-2 expression levels on 238 patients who underwent nephrectomy for localized ccRCC; 161 of whom also had adjacent normal kidney samples available. To evaluate associations with ccRCC development, linear mixed models were used to compare differential expression between tumor and normal kidney as well as to explore interactions with clinicopathologic features. To evaluate associations with prognosis, Cox proportional hazard models were used to assess the association of PFS and ET-2 expression in tumor tissue. Overall, ET-2 expression was higher in tumor samples versus patient-matched normal kidney samples with an average fold change (FC) of 1.99 (95% CI 1.48–2.60; p-value<0.0001). This over-expression in tumor versus normal was more pronounced in low- compared to high-grade tumors (interaction p-value=0.0002), in early-compared to late-stage tumors (interaction p-value=0.001), and in tumors without compared to those with necrosis (interaction p-value=0.001). Moreover, increasing ET-2 expression in tumors was associated with longer PFS (HR=0.89; 95% CI 0.80–0.99; p=0.03); however, after controlling for known clinicopathologic factors this association was attenuated (HR=0.99; 95% CI 0.89–1.09; p=0.7). Up-regulation of ET-2 is a common and early event in localized ccRCC. Higher tumor expression of ET-2 is associated with longer PFS but not after adjustment for well-known pathologic indices. Thus, although ET-2 does not appear to be an independent prognostic marker, there is evidence of a putative role in ccRCC progression. If supportive mechanistic data can be produced, ET-2 could represent a potential target for chemopreventive or neo-adjuvant therapeutics for ccRCC.
Keywords: endothelin 2, kidney cancer, biomarker, over expression, early event
INTRODUCTION
Incidence rates for renal cell carcinoma (RCC) have risen steadily over the past three decades, and this increase is not explained by enhanced detection via increased abdominal imaging [1–3]. Hallmark features of RCC include a predominance of clear cell subtype (ccRCC), well-reported associations with a history of cigarette smoking and obesity, a variable clinical course, limited treatment options beyond surgical excision and a high average years of life lost (15.7 years). Related to this, improving our understanding of the specific molecular events associated with the transformation of normal kidney tissue to neoplastic RCC tumor remains a key issue in the field of RCC research [4]. Indeed, advancing our knowledge of the molecular underpinnings of RCC development has the potential to translate into new insights into the targeted prevention and treatment of this increasingly common human malignancy.
In a pilot investigation, we employed Affymetrix-based microarray technology (GeneChip Human Genome U133 Plus 2.0 Arrays) to explore novel gene expression alterations in patient-matched sets of normal kidney and primary ccRCC tissues. In this pilot study, we observed over-expression of endothelin 2 (ET-2) in ccRCC tumor tissue when compared to patient-matched normal kidney (data not shown). Endothelins are small (21 amino acid) vasoactive peptides that bind to G-protein-linked transmembrane receptors and elicit a variety of diverse autocrine/paracrine actions [5, 6]. There are currently three known ET isoforms (ET-1, ET-2, and ET-3), which are encoded by three distinct, independently expressed genes. ET-2 specifically has been shown to play a role in regulating growth in several cancer cell types, including breast and ccRCC cell lines [7–9]. To date, investigations that evaluate expression levels of ET-2 in human ccRCC tissue samples are extremely limited. Prompted by this gap in the literature and our pilot data, we utilized a large cohort of patients treated surgically for ccRCC at our institution to compare ET-2 expression levels in patient-matched sets of ccRCC tumor and normal kidney tissue. We explored whether the expression differences between these paired sets of normal and tumor tissue vary across relevant clinicopathologic features of ccRCC. Furthermore, we examined the association of ET-2 expression levels in ccRCC tumors and risk of cancer progression (i.e. distant metastasis) following surgery.
MATERIALS AND METHODS
Patient Selection
After receiving approval Institutional Review Board approval, we used our Nephrectomy Registry [10, 11] to identify 289 patients treated with open radical nephrectomy or nephron-sparing surgery for localized, unilateral, sporadic, non-cystic ccRCC between 2000 and 2003. Of these, 238 (82%) had fresh-frozen primary ccRCC tumor tissue samples available. Of those with fresh-tissue available, 161 (68%) also had paired adjacent normal kidney tissue available. We did not observe any significant differences in demographic or pathologic characteristics between those patients with and without fresh-frozen primary ccRCC tissue available for analysis (data not shown). For each of the 238 patients in our cohort, we abstracted data on the following clinical and pathologic variables from the Nephrectomy Registry: age at surgery, gender, 2002 TNM stage groupings, nuclear grade, and presence of coagulative tumor necrosis [12,13]. Information regarding clinical outcome was also abstracted, specifically, date of metastasis or death. Of note, the pathology features recorded in the Nephrectomy Registry are the result of a centralized review conducted by an experienced urologic pathologist (J.C.C.).
Real Time PCR for ET-2 Expression
We requested fresh-frozen tissue samples for the 238 primary ccRCC tumors and, where available, adjacent normal tissues (n=161) from the biorepository maintained by our Nephrectomy Registry. An experienced technician (S.N.L.) extracted mRNA from each sample, reversed transcribed the mRNA to cDNA and conducted rt-PCR to determine relative expression levels of ET-2. Briefly, frozen normal renal and renal mass tissues were homogenized using an electronic rotor-stator homogenizer and RNA was extracted using the Totally RNA kit (Ambion, part number AM1910). Pellets were re-suspended in DEPC water, and contaminant DNA was removed using DNA-free kit (Applied Biosystems, part number AM1906). Samples were then further purified using Chroma-Spin Columns (Clontech part number 636090). UV-vis. Spectrophotometry was used for RNA quantification, and samples were converted to cDNA immediately using the High Capacity cDNA archive kit (applied biosystems, part number 4368813). Real time PCR was performed using custom TaqMan low density arrays (Applied Biosystems) and the AB 7900HT Fast RealTtime System. All protocols were performed per manufacturer’s instructions.
Data Normalization
Four control genes (GUSB, HPRT1, POLR2A, and ACTB) were run on each PCR plate to provide information to carry out normalization techniques for multiple tissue types as described by Szabo et al [14]. Negative CT (−CT) values for all four of the control genes were averaged on a per sample basis and the average was subtracted from the −CT value for each sample. This normalization technique takes into account both plate-to-plate differences as well as any possible sample aliquot differences.
Statistical Methods
The Chi-square test was used to assess associations between categorical variables and availability of an adjacent normal kidney tissue sample, while the Wilcoxon rank sum test was used to assess for differences in continuous variables and availability of an adjacent normal kidney tissue sample. Linear mixed models were fit to the normalized −CT values in order to evaluate differential expression (average fold change estimates and p-values) between patient-matched tumor and normal samples; sample type (tumor/normal) was included as a fixed effect while a random intercept was fit on a per patient basis. In addition, we explored potential effect modifiers including gender, age, tumor stage, nuclear grade, and presence of necrosis. These were tested by the interaction of sample type (tumor/normal) and the relevant clinicopathologic characteristic with main effects included in the model as well. This sample type by characteristic interaction tested whether the difference between tumor and normal expression was consistent across the levels of each clinicopathologic characteristic. Cox proportional hazard models were used to test univariate and multivariable associations of ET-2 tumor expression (modeled as a continuous variable on a logarithmic scale) as well as clinicopathologic characteristics with progression-free survival (PFS). PFS was defined as the time from surgery to the first occurrence of disease progression to metastasis or death. If no event was observed, censoring occurred at the date a patient was last known to be alive with no metastases. All factors that were univariately significant at a 0.05 level were included in the multivariable model. Lastly, the Student’s t-statistic was used to evaluate associations between ET-2 tumor expression and clinicopathologic characteristics. All statistical tests were performed using the Linux release of R version 2.11.0.
RESULTS
Table 1 provides an overview of demographic, clinical and pathologic characteristics for all 238 patients evaluated for ET-2 expression as well as broken down by the availability of an adjacent normal kidney tissue sample. The patients in the full cohort were predominantly male (64%) and over 60 years of age (62%). There were slightly more patients with high grade disease (nuclear grade of 3 or 4; 52%) than with low grade disease (nuclear grade 1 or 2; 48%). The majority of patients presented with early stage tumors (pT1; 64%), with fewer patients presenting with pT2 (12%) and pT3 (24%) tumors. Approximately one-fifth of patients (21%) had tumors showing presence of histological necrosis on centralized pathology review. Patients who had an adjacent normal kidney tissue available were slightly older (median age of 65 years versus 61; p=0.05), more likely to be later stage (pT2 or pT3; 42% versus 22%; p=0.004) and more likely to have had a radical nephrectomy (70% versus 53%; p=0.02) than those who did not have an adjacent normal kidney tissue available (Table 1).
Table 1.
Demographic and clinicopathologic characteristics for the full cohort and broken down by the availability of an adjacent normal kidney tissue sample. P-value reflects strength of association between variables and availability of an adjacent normal tissue sample.
| Characteristic | All Patients (N=238) | Matched Tumor and Normal (N=161) | Tumor Only (N=77) | P-value |
|---|---|---|---|---|
| Nephrectomy | 0.02 | |||
| Radical | 154 (65%) | 113 (70%) | 41 (53%) | |
| Partial | 84 (35%) | 48 (30%) | 36 (47%) | |
| Age at Surgery, n (%) | 0.05* | |||
| <50 | 41 (17%) | 27 (17%) | 14 (18%) | |
| 50–59 | 49 (21%) | 29 (18%) | 20 (26%) | |
| 60–69 | 74 (31%) | 48 (30%) | 26 (34%) | |
| 70–79 | 58 (24%) | 43 (27%) | 15 (19%) | |
| 80+ | 16 (7%) | 14 (9%) | 2 (3%) | |
| Gender, n (%) | 1.00 | |||
| Male | 153 (64%) | 104 (65%) | 49 (64%) | |
| Female | 85 (36%) | 57 (35%) | 28 (36%) | |
| Nuclear grade, n (%) | 0.07** | |||
| 1 | 22 (9%) | 13 (8%) | 9 (12%) | |
| 2 | 92 (39%) | 57 (35%) | 35 (45%) | |
| 3 | 112 (47%) | 81 (50%) | 31 (40%) | |
| 4 | 12 (5%) | 10 (6%) | 2 (3%) | |
| Pathologic Tumor Stage, n (%) | 0.004*** | |||
| pT1 | 153 (64%) | 93 (58%) | 60 (78%) | |
| pT2 | 29 (12%) | 24 (15%) | 5 (6%) | |
| pT3 | 56 (24%) | 44 (27%) | 12 (16%) | |
| Presence of Necrosis, n (%) | 0.21 | |||
| Yes | 50 (21%) | 38 (24%) | 12 (16%) | |
| No | 188 (79%) | 123 (76%) | 65 (84%) | |
Wilcoxon rank sum test
Low grade (1 and 2) versus high grade (3 and 4)
Early stage (1) versus later stage (2 and 3)
Association of ET-2 Expression with ccRCC Development
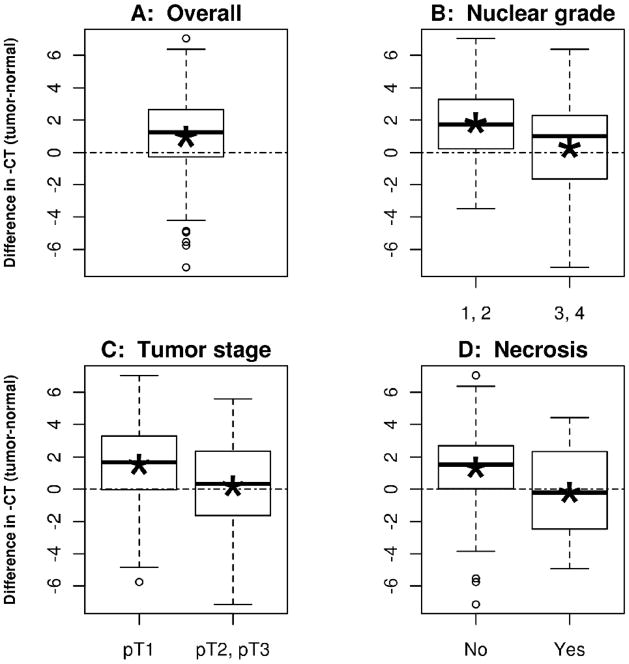
In the cohort of patients with both tumor and patient-matched adjacent normal kidney tissue samples available for analysis (n=161), ET-2 expression was higher in tumor compared to normal with an average fold change (FC) of 1.99 (95% CI 1.48–2.60; p-value < 0.0001; Figure 1a). The over-expression of ET-2 in tumor versus normal tissue was consistent across age and gender (Table 2). In contrast, we observed differential up-regulation of ET-2 across pathologic measures commonly used to evaluate aggressiveness of localized ccRCC. Specifically, up-regulation of ET-2 in ccRCC tumor versus normal kidney was more pronounced in those patients with less aggressive pathologic features. For example, up-regulation of ET-2 in tumor tissue compared to normal kidney tissue was more pronounced in low grade (nuclear grade 1 or 2) versus high grade (nuclear grade 3 or 4) tumors (FC of 3.53 vs. 1.25; tissue-by-grade interaction p-value = 0.0002; Table 2 and Figure 1B). Similarly, patients with early stage (pT1) ccRCC tumors had a higher tumor versus normal fold change when compared to those with later stage (pT2 and pT3) tumors (FC of 2.91 vs. 1.15; tissue-by-stage interaction p-value = 0.001; Table 2 and Figure 1C). Keeping with this trend, over expression of ET-2 was more pronounced in those patients with ccRCC tumors without necrosis when compared to those with necrosis (FC of 2.53 vs. 0.86; tissue-by-necrosis interaction p-value = 0.001; Table 2 and Figure 1D).
Figure 1.
Boxplots illustrating the distribution of the difference between negative CT values for paired tumor and normal samples (equivalent to the log2 of the fold change of ET-2 expression). Asterisks indicate mean negative CT values. Panel A is across all samples (n=161), panel B is split by low (n=70) and high (n=91) nuclear grade, panel C is split by early (n=93) and late (n=68) tumor stage, and panel D is split by tumor with (n=38) and without (n=123) presence of necrosis.
Table 2.
Differential expression of ET-2 between primary ccRCC and patient-matched adjacent normal samples (N=161) by demographic and clinicopathologic characteristics. Negative CT (−CT) denotes the normalized −CT values.
| Characteristic | Average Difference in −CT (tumor-normal) | Average Fold Change Estimate (2(difference in −CT)) | Tissue Type by Characteristic Interaction p-value |
|---|---|---|---|
| Gender | |||
| Female | 1.01 | 2.02 | |
| Male | 0.95 | 1.94 | 0.89 |
| Age at Surgery | |||
| <60 years | 1.15 | 2.22 | |
| ≥60 years | 0.88 | 1.84 | 0.53 |
| Presence of Necrosis | |||
| No | 1.34 | 2.53 | |
| Yes | −0.21 | 0.86 | 0.001 |
| Pathologic Tumor Stage | |||
| pT1 | 1.54 | 2.91 | |
| pT2, pT3 | 0.20 | 1.15 | 0.001 |
| Nuclear Grade | |||
| 1 or 2 | 1.82 | 3.53 | |
| 3 or 4 | 0.32 | 1.25 | 0.0002 |
Association of ET-2 Tumor Expression with Prognosis
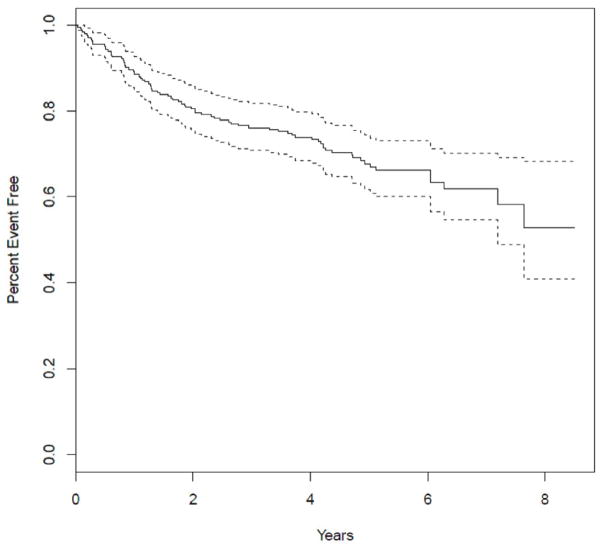
In addition to evaluating the relationship between ET-2 expression and development of ccRCC, we also explored the relationship between ET-2 tumor expression and PFS. In the full cohort of primary ccRCC tumors (n=238) 50 patients had disease progression to metastasis and 30 died prior to progression (Figure 2). Higher expression of ET-2 was associated with better PFS. Specifically, for every 2-fold increase of ET-2 expression, the hazard ratio for PFS was 0.89 (95% CI: 0.80–0.99; p=0.03; Table 3). Clinicopathologic characteristics that were univariately associated with PFS included lower grade (1 and 2 versus 3 and 4), earlier stage (pT1 versus pT2 and pT3) and patients with ccRCC tumors without necrosis (all significant at p≤0.0001; Table 3). After adjusting for these clinicopathologic characteristics in a multivariable model, the association of ET-2 expression with PFS was no longer present (HR=0.99; 95% CI: 0.89–1.09; p=0.70). In order to explore explanations for this attenuation, we compared tumor ET-2 expression values across these three commonly-used pathologic indicators of ccRCC outcome and noted consistent evidence of an inverse association. That is, lower ET-2 tumor expression is significantly associated with higher grade, later stage and presence of necrosis (Table 4).
Figure 2.
Progression free survival (PFS) curve.
Table 3.
Association of PFS with clinicopathologic characteristics and ET-2 tumor expression; HR denotes hazard rate.
| Progression-free Survival | ||
|---|---|---|
| Univariate HR (95% CI; p-value) |
Multivariable HR (95% CI; p-value) |
|
| ET-2 Tumor Expression | 0.89 (0.80–0.99; p=0.03) | 0.99 (0.89–1.09; p=0.7) |
| Gender | ||
| Female | --- | ---------- |
| Male | 1.43 (0.89–2.31; p=0.13) | |
| Age at Surgery | ||
| <60 years | --- | ---------- |
| ≥60 years | 1.52 (0.94–2.47; p=0.08) | |
| Presence of Necrosis | ||
| No | --- | --- |
| Yes | 5.75 (3.67–8.99; p<0.0001) | 3.02 (1.75–5.21; p<0.0001) |
| Pathologic Tumor Stage | ||
| pT1 | --- | --- |
| pT2, pT3 | 4.68 (2.96–7.42; p<0.0001) | 2.86 (1.70–4.79; p<0.0001) |
| Nuclear Grade | ||
| 1 or 2 | --- | --- |
| 3 or 4 | 2.90 (1.79–4.70; p<0.0001) | 1.35 (0.76–2.39; p=0.31) |
Table 4.
Association of ET-2 tumor expression and clinicopathologic characteristics.
| T-Test | |
|---|---|
| Fold Change (p-value) | |
| Gender | |
| Female | --- |
| Male | 1.09 (0.68) |
| Age at Surgery | |
| <60 years | --- |
| ≥60 years | 0.86 (0.43) |
| Presence of Necrosis | |
| No | --- |
| Yes | 0.45 (0.002) |
| Pathologic Tumor Stage | |
| pT1 | --- |
| pT2, pT3 | 0.58 (0.009) |
| Nuclear Grade | |
| 1 or 2 | --- |
| 3 or 4 | 0.53 (0.0006) |
DISCUSSION
In parallel with the steady rise in ccRCC incidence over the past three decades, there continues to be considerable interest in identifying and exploring common molecular events that support the development and progression of ccRCC. In particular, much effort is currently focused on identifying molecular events within ccRCC tissues that correlate with well-known pathologic features of aggressiveness and patient outcome. Motivating these efforts is the understanding that identifying frequent molecular events within tumor tissue, especially those that correlate with known pathologic features of aggressiveness and disease progression, has the potential to provide insight into novel approaches to cancer prevention, prognosis and treatment. In the current study, we report that up-regulation of ET-2 in tumor tissue compared to patient-matched normal kidney samples is a common event in patients with ccRCC. In particular, we demonstrate that the up-regulation of ET-2 in ccRCC tumor tissue compared to adjacent normal tissue is more pronounced in tumors with less aggressive pathologic features. Moreover, we report that expression of ET-2 is also inversely associated with risk of ccRCC progression following surgery. That is, higher levels of ET-2 expression are associated with lower risk of development of distant metastasis after surgery. This inverse association is attenuated after adjustment for well known clinicopathologic features of ccRCC aggressiveness.
Of the three known ET peptides, to date only expression of ET-1 has been convincingly implicated in the development and progression of human cancer [15, 16]. With regard to ccRCC specifically, previous investigators have reported that ET-1 expression in both ccRCC cell lines [17] and in human ccRCC tumors [18]. Moreover, high expression levels of ET-1 have been reported to be associated with increased ccRCC aggressiveness, tumor progression and poor survival [19, 20]. By contrast, while over expression of ET-2 has been proposed as a novel mechanism by which human tumor cells can withstand hypoxic stress and support of tumor development and progression [7], the data supporting this as a specific mechanism in ccRCC are very limited. Ohkubo et al [8] were the first to report that human renal adenocarcinoma cell lines expressed high levels of the ET-2 mRNA transcript. Following that work several years later, Jiang et al [9] used serial analysis of gene expression on renal adenocarcinoma cell lines to identify ET-2 as a gene that is inducible by hypoxia in the absence of a functioning von Hippel Lindau (VHL) gene. Given that loss of VHL is a common event in ccRCC [21], these data suggest that up-regulation of ET-2 may represent a VHL-independent mechanism for ccRCC to respond to hypoxic stress and further support tumor growth. The importance of these cell line studies notwithstanding, we are aware of only one other investigative group that has directly examined the expression of ET-2 in human RCC tissue samples. In a small case series of 22 patients with surgically-resected ccRCC, Douglas et al [22] observed no difference in mRNA expression levels of preproendothelin 2 (an intermediate precursor to ET-2) in tumor versus normal tissue; however, the small sample size and lack of centralized pathology review hampered the ability to draw meaningful conclusions or to explore evidence of differential expression across important subcategories.
Of interest, in our stratified analysis we report that over expression of ET-2 in ccRCC tumor compared to patient-matched normal kidney is more pronounced in those patients with tumors showing a less aggressive phenotype (i.e. low grade, early stage, no necrosis). One interpretation of our results is that over expression of ET-2 is an early event in the timeline of ccRCC carcinogenesis. To confirm our hypothesis, we mined in-house unpublished gene-expression microarray data where Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays were run on 15 primary RCC tumors and patient-matched lung metastases. Post frozen robust multiarray analysis normalization [23], the average fold change of metastatic expression relative to patient-matched primary expression was 0.54 (p=0.007) for the probeset (206758_at) that mapped to ET-2. As such, therapeutics targeted at ET-2 could be studied further as a potential novel method for ccRCC chemoprevention (particularly in high risk individuals) as well as a neoadjuvant treatment for patients undergoing surgical excision of a clinically localized ccRCC tumor [24, 25]. Moreover, given that ET-2 encodes a secretory protein [5, 26], circulating levels of the ET-2 protein could also be explored as a marker of early disease in high risk populations as well.
The potential value of identifying molecular events that support ccRCC carcinogenesis notwithstanding, a key clinical issue in the field remains identifying biomarkers that correlate with ccRCC patient outcome (i.e. progression to metastasis after surgery). Related to this, the lethality associated with ccRCC results from tumors gaining the ability to invade surrounding tissues and metastasize to distant sites in the body. Indeed, five year survival drops from approximately 60% to less than 10% once a patient with clinically localized ccRCC progresses to distant metastatic disease [27]. Motivated by this, there is a clear need to better understand the biologic underpinnings that support the mechanism of ccRCC metastasis and furthermore, to translate this understanding into improved patient care. The existing data indicating that ET-2 expression may mediate the development and growth of several human cancers, including ccRCC, suggests that this biomarker should be evaluated for its ability to predict ccRCC patient outcome following surgery. Moreover, our current understanding of the biologic function of ET-2 further suggests that higher expression levels of ET-2 would be predicted to be associated with less aggressive phenotype, lower risk of disease progression after surgery and improved survival. To date, however, investigations that have evaluated expression levels of ET-2 in human ccRCC tissue samples and their association with patient outcome are nonexistent. In our study, we are the first to report that higher expression levels of ET-2, a known mediator of cancer cell growth and proliferation, are associated with a lower risk of disease progression among patients undergoing surgery for clinically localized ccRCC. After adjustment for clinicopathologic predictors of ccRCC outcome, the association of ET-2 expression and PFS we observed was attenuated. While this diminishes the enthusiasm for ET-2 expression as a clinically relevant biomarker that could improve outcome prediction and enhance patient management it does not lessen the interested in ET-2 expression and its putative role in ccRCC progression. The prospect of utilizing ET-2 expression in the future to predict response to emerging therapeutics remains plausible. Moreover, if supportive data from mechanistic studies can be generated, our results do not preclude the possibility of developing adjuvant therapies that would enhance ET-2 expression signaling pathways to reduce the risk of progression in high risk patients. As such, these data represent an important advancement in our understanding of the potential role of ET-2 as a mediator of ccRCC progression.
CONCLUSION
The need for external validation notwithstanding, our data support that up-regulation of ET-2 in tumor tissue compared to patient-matched normal kidney tissue is a common event in patients with clinically localized ccRCC. Moreover, over expression of ET-2 appears to be an early event in ccRCC carcinogenesis given that this association is more pronounced in early stage, low grade disease, and those tumors without necrosis. From a clinical standpoint, higher tumor expression of ET-2 is associated with lower risk of progression to metastases after surgery; however, this association is attenuated after accounting for known clinicopathologic factors of RCC aggressiveness. Thus, although ET-2 is not an independent prognostic marker, the significant association between ET-2 expression and risk of progression implies a putative role in ccRCC progression. Therefore, if our patient-based results are confirmed in future studies and supportive mechanistic data can also be generated, ET-2 may represent a useful target for not only chemoprevention of ccRCC in high risk populations but also as a target for adjuvant therapy following surgery.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Murai M, Oya M. Renal cell carcinoma: etiology, incidence and epidemiology. Curr Opin Urol. 2004;14:229–33. doi: 10.1097/01.mou.0000135078.04721.f5. [DOI] [PubMed] [Google Scholar]
- 3.Wallen EM, Pruthi RS, Joyce GF, Wise M. Urologic Diseases in America Project. Kidney cancer. J Urol. 2007;177:2006–18. doi: 10.1016/j.juro.2007.01.126. discussion 2018–9. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Vogelzang NJ Priorities of the Kidney/Bladder Cancers Progress Review Group. Report of the Kidney/Bladder Cancers Progress Review Group. Bethesda, MD: National Cancer Institute; [updated 2002 Aug; cited 2011 Jul 20]. Available from: http://planning.cancer.gov/library/2002kidneyreport.pdf. [Google Scholar]
- 5.Grimshaw MJ. Endothelins and hypoxia-inducible factor in cancer. Endocr Relat Cancer. 2007;14:233–44. doi: 10.1677/ERC-07-0057. [DOI] [PubMed] [Google Scholar]
- 6.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 7.Grimshaw MJ, Naylor S, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002;1:1273–81. [PubMed] [Google Scholar]
- 8.Ohkubo S, Ogi K, Hosoya M, et al. Specific expression of human endothelin-2 (ET-2) gene in a renal adenocarcinoma cell line. Molecular cloning of cDNA encoding the precursor of ET-2 and its characterization. FEBS Lett. 1990;274:136–40. doi: 10.1016/0014-5793(90)81348-r. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Zhang W, Kondo K, et al. Gene expression profiling in a renal cell carcinoma cell line: dissecting VHL and hypoxia-dependent pathways. Mol Cancer Res. 2003;1:453–62. [PubMed] [Google Scholar]
- 10.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 11.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 12.Moch H, Artibani W, Delahunt B, et al. Reassessing the current UICC/AJCC TNM staging for renal cell carcinoma. Eur Urol. 2009;56:636–43. doi: 10.1016/j.eururo.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–20. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
- 14.Szabo A, Perou CM, Karaca M, et al. Statistical modeling for selecting housekeeper genes. Genome Biol. 2004;5:R59. doi: 10.1186/gb-2004-5-8-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant K, Loizidou M, Taylor I. Endothelin-1: a multifunctional molecule in cancer. Br J Cancer. 2003;88:163–6. doi: 10.1038/sj.bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–6. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Totsune K, Kitamuro T, Sone M, Murakami O, Shibahara S. Three vasoactive peptides, endothelin-1, adrenomedullin and urotensin-II, in human tumour cell lines of different origin: expression and effects on proliferation. Clin Sci (Lond) 2002;103 (Suppl 48):35S–38S. doi: 10.1042/CS103S035S. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann E, Eltze E, Bierer S, et al. Expression of the Endothelin-axis in the different histologic subtypes of renal cell carcinoma: a tissue microarray analysis. Oncol Rep. 2007;17:275–80. [PubMed] [Google Scholar]
- 19.Pflug BR, Zheng H, Udan MS, et al. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007;246:139–48. doi: 10.1016/j.canlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Yao M, Huang Y, Shioi K, et al. A three-gene expression signature model to predict clinical outcome of clear cell renal carcinoma. Int J Cancer. 2008;123:1126–32. doi: 10.1002/ijc.23641. [DOI] [PubMed] [Google Scholar]
- 21.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–85. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas ML, Richardson MM, Nicol DL. Endothelin axis expression is markedly different in the two main subtypes of renal cell carcinoma. Cancer. 2004;100:2118–24. doi: 10.1002/cncr.20222. [DOI] [PubMed] [Google Scholar]
- 23.McCall MN, Bolstad BM, Irizarry RA. Frozen robust multiarray analysis (fRMA) Biostatistics. 2010;11:242–253. doi: 10.1093/biostatistics/kxp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smollich M, Wülfing P. The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol. 2007;5:239–48. doi: 10.2174/157016107781024082. [DOI] [PubMed] [Google Scholar]
- 25.Smollich M, Wülfing P. Targeting the endothelin system: novel therapeutic options in gynecological, urological and breast cancers. Expert Rev Anticancer Ther. 2008;8:1481–93. doi: 10.1586/14737140.8.9.1481. [DOI] [PubMed] [Google Scholar]
- 26.Battistini B, D’Orléans-Juste P, Sirois P. (Endothelins: circulating plasma levels and presence in other biologic fluids. Lab Invest. 1993;68:600–28. [PubMed] [Google Scholar]
- 27.Reese JH. Renal cell carcinoma. Curr Opin Oncol. 1991;3:537–44. doi: 10.1097/00001622-199106000-00016. [DOI] [PubMed] [Google Scholar]