Summary
Type I Interferons (IFNs) are critical for controlling pathogenic virus infections and can enhance immune responses. Hence their impact on the effectiveness of live-attenuated vaccines involves a balance between limiting viral antigen expression and enhancing the development of adaptive immune responses. We examined the influence of type I IFNs on these parameters following immunization with RepliVAX WN, a single-cycle flavivirus vaccine (SCFV) against West Nile virus (WNV) disease. RepliVAX WN-immunized mice produced IFN-α and displayed increased IFN-stimulated gene transcription in draining lymph nodes (LN). SCFV gene expression was over 100 fold-higher on days 1–3 post-infection in type I IFN receptor knockout mice (IFNAR−/−) compared to wild-type (wt) mice indicating a profound IFN-mediated suppression of SCFV gene expression in the wt animals. IFNAR−/− mice produced nearly equivalent levels of WNV-specific serum IgG and WNV-specific CD4+ T cell responses compared to wt mice. However, significantly higher numbers of WNV-specific CD8+ T cells were produced by IFNAR−/− mice and a significantly greater percentage of these T cells from IFNAR−/− mice produced only IFN-γ following antigen-specific re-stimulation. This altered cytokine expression was not associated with increased antigen load suggesting the loss of type I IFN receptor signaling was responsible for the altered quality of the CD8+ effector T cell response. Together, these results indicate that although type I IFN is not essential for the intrinsic adjuvanting of RepliVAX WN, it plays a role in shaping the cytokine secretion profiles of CD8+ effector T cells elicited by this SCFV.
Keywords: West Nile Virus, Single-cycle virus, CD8+ T cell, type I IFN
1. Introduction
Medically important flaviviruses include West Nile virus (WNV), Japanese encephalitis virus, dengue virus, and yellow fever virus which collectively infect more than 100 million individuals each year. Although the long-standing effectiveness of vaccines for yellow fever and Japanese encephalitis demonstrates that vaccines can prevent flavivirus diseases, no licensed human vaccines are available to prevent dengue or WNV-disease, although a number of candidates are in various stages of development [1–4].
Recently we developed a single-cycle flavivirus (SCFV) vaccine candidate, RepliVAX WN, which can be produced in cell lines engineered to express the WNV capsid (C) protein [5, 6]. The RepliVAX WN genome contains a large internal deletion in the gene coding for C, while retaining the rest of the WNV genome including the prM and E genes. Thus, in normal cells, RepliVAX WN can initiate the viral infection cycle and produce prM and E-containing sub-viral particles (SVPs) but does not produce infectious progeny. A single immunization with RepliVAX WN in mice and hamsters provided protection from lethal WNV challenge [5–7] and RepliVAX WN elicited a protective immune response in non-human primates [8].
IFN systems are known to play important roles in innate and adaptive immune responses to microbial infection [9]. Type I IFNs are induced when cells recognize viral components such as double-stranded RNA through pattern recognition receptors (PRRs) including TLRs, retinoic acid-inducible gene I (RIG-I), melanoma-differentiation-associated gene 5 (MDA5) and double-stranded RNA-activated protein kinase R (PKR) [10]. Type I IFNs initiate signaling cascades that result in the activation of transcription factors that regulate expression of IFN-stimulated genes (ISGs) which are important in the control and elimination of viral infection. The recognition of viral components via PRRs and the subsequent induction of immune response proteins including proinflammatory cytokines and type I IFNs are also responsible for the intrinsic adjuvanting of the immune responses to live-attenuated vaccines. Type I IFNs have been shown to act as adjuvants when given in combination with antigen resulting in the enhancement of antibody responses [11]. In addition, type I IFNs mediate the immunological effects of potent adjuvants such as complete Freund’s adjuvant [12, 13].
The interplay between replication of attenuated viral vaccines and the effectiveness of the IFN response is expected to be critical in determining the outcome of vaccination. Specifically, the intensity of the IFN response would be predicted to help adjuvant the adaptive immune response ensuring development of protective immunity. Conversely, IFN responses leading to strong acute antiviral activity could prematurely eliminate attenuated virus infections reducing vaccine potency and efficacy. Therefore, successful live-attenuated vaccines need to induce a balanced, but not overwhelming IFN response. In the studies presented here, we examined the influence of type I IFNs on SCFV gene expression and development of adaptive immunity following vaccination with an SCFV vaccine candidate, RepliVAX WN, to determine the role of type I IFNs in the intrinsic adjuvanticity of the RepliVAX WN vaccine.
2. Materials and Methods
2.1 Viruses, SCFVs, and WNV Antigen (Ag)
RepliVAX WN (previously referred to as RepliVAX WN.2 SP) was produced in BHK (VEErep/Pac-Ubi-C*) cells as previously described [5]. Firefly luciferase (FLUC)-expressing SCFV particles (FLUC-SCFV) containing a WNV replicon genome expressing a humanized FLUC gene fused to the foot-and-mouth disease virus 2A gene in place of the WNV prM and E genes (previously referred to as WNV hFLuc VRP) were produced in BHK(VEErep/C*-prM-E-Pac) cells as previously described [14]. Titration of RepliVAX WN and FLUC-SCFV particles on Vero cells was performed as previously described [15].
WNV SVPs used for ELISPOT and ELISA were produced by infection of Vero cells with RepliVAX WN under conditions analogous to those used to infect BHK (VEErep/Pac-Ubi-C*) [5]. Clarified cell culture supernatant containing WNV SVPs was concentrated using centrifugal filtration, and SVPs were purified on a sucrose gradient prior to use. WNV truncated E and WNV NS1 antigens were obtained from clarified culture fluids harvested from cultures of VEErep-bearing BHK cells as previously described [5].
2.2 Mice
C57BL/6J (B6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). B6 mice lacking the type I IFN receptor (IFNAR−/−), were bred from founders obtained from Dr. Anthony French (Washington University, St. Louis) [16]. All animal work was approved by the Institutional Animal Care and Use Committees of the University of Texas Medical Branch and North Carolina State University with oversight of staff veterinarians.
2.3 Immunization of mice with RepliVAX WN
To assess cytokine levels, and WNV-specific Ab or T cell responses, mice were immunized by the intraperitoneal (i.p.) route. Mice were immunized in the footpads for in vivo imaging and for assessment of gene expression in draining lymph nodes. RepliVAX WN inocula were delivered in L-15 medium containing 10mM HEPES and 0.5% FBS.
2.4 WNV-specific Ab detection
Virus neutralization assays were performed as described previously [5, 7]. Determination of WNV-specific IgG levels was performed by a modification of the ELISA method described previously [5]. Briefly, serial 2-fold dilutions of sera from individual mice were added to ELISA plates coated with recombinant soluble WNV E (trE), NS1 proteins, or with purified WNV SVPs. Plate-bound IgG was developed with HRP-IgG (Southern Biotech) or with biotinylated anti-mouse IgG1 or IgG2c (BD Pharmingen, San Diego, CA) followed by incubation with streptavidin peroxidase (Sigma-Aldrich, St. Louis, MO). Normalized OD readings at 450nm (OD450) obtained from the serial dilution studies were subjected to non-linear regression analyses to calculate the serum dilution equivalent to three standard deviations above OD450 values from sera obtained from mock-vaccinated animals.
2.5 Interferon and cytokine detection
IFN-α and IFN-β ELISAs (PBL Biomedical Laboratories, Piscataway, NJ) were performed using manufacturer’s protocols. Briefly, experimental sera were diluted, plated and incubated for 1 hour (h) at 37°C. Samples were washed and bound IFN was detected by addition of mouse IFN-specific Ab followed by an HRP-conjugated secondary Ab and measurement of the OD450. IFN levels were calculated using a standard curve generated from serial dilutions of an IFN standard in dilution buffer containing normal mouse sera as previously described [17]. The limit of detection was 12.5 pg/ml for IFN-α and 15.6 pg/ml for IFN-β. Additional cytokine levels in the pooled sera of inoculated mice were determined using a luminescence-based multiplex bead assay (Bio-Rad, Hercules, CA) from a panel of 23 cytokines following the manufacturer’s protocols.
2.6 RT-PCR analyses
Popliteal LN were harvested from vaccinated mice and stored overnight at 4°C in RNALater (Ambion, Austin, TX). Total LN RNA was isolated and DNase treated using the RNAqueous 4 PCR Kit (Ambion) and then used to synthesize cDNA with the RT2 First Strand Kit (SABiosciences, Frederick, MD). Cytokine mRNA levels were determined at the indicated times by real-time PCR using a SYBR Green-based custom PCR array (SABiosciences) and thermocycler settings recommended for use with a BioRad iCycler. Cytokine levels of RepliVAX WN treated mice (3 mice/group) were represented as fold-change over mock-infected animals (2 mice/group).
2.7 Enzyme-linked immunospot assay (ELISPOT)
ELISPOT assays for antibody secreting cells (ASC) were performed as described previously [18] using microtiter filter plates coated with WNV NS1 or SVP Ag. Ag-specific ASC were quantified using an ImmunoSpot reader and analyzed with ImmunoSpot software (Cellular Technology Ltd, Cleveland, OH).
For IFN-γ ELISPOT assays, splenocytes from RepliVAX WN-immunized or mock-infected mice were plated on filter plates coated with purified anti-mouse IFN-γ (BD Pharmingen) and stimulated with immunogenic peptides representing WNV CD4+ and CD8+ T cell epitopes as described previously [19]. Immunogenic peptides representing the CD8+ T cell epitopes located at NS4B protein residues 2488–2496 (NS4B2488), E protein residues 347–354 (E347) and the CD4+ epitopes located at NS3 protein residues 2066–2080 (NS32066), and E protein residues 641–655 (E641) have been described previously [20–22]. Plates were developed with biotinylated anti-mouse IFN-γ (BD Pharmingen) and streptavidin-peroxidase (Sigma-Aldrich) and were quantified as described above for ASC ELISPOTs.
2.8 In vivo cytotoxic T lymphocyte assay
WNV NS4B-specific cytotoxic T lymphocyte activity in RepliVAX WN-immunized mice was quantified by injection of CFSE-labeled target cells as described previously [19]. Four h after injection, NS4B2488–pulsed and mock-pulsed CFSE-labeled target populations were quantified using a BD FACSCanto (BD Biosciences, San Jose, CA) and data were analyzed using FlowJo software (Tree Star, Ashland, OR). The percent specific lysis was calculated as described previously [19].
2.9 Intracellular cytokine staining
Whole spleens from B6 or IFNAR−/− mice taken on day 6 after RepliVAX WN vaccination were re-stimulated with 1 μM NS4B2488 peptide for 2 h followed by 6 h incubation with brefeldin A. Cells were blocked with anti-Fc RII/III mAb and surface stained with anti-CD8α (APC) and CD3 (PerCPCy5.5) then permeabilized using a Cytofix/Cytoperm kit and subsequently stained intracellularly with fluorochrome-conjugated mAb for IFN-γ (FITC), IL-2 (PE) and TNF-α (PE-Cy7). All reagents were purchased from BD Pharmingen. CD8+, CD3+ T cells were segregated into distinct populations based on the production of IFN-γ, IL-2 or TNF-α either individually or in combination. No cytokines were detected in cultures re-stimulated with an irrelevant peptide (gB498–505), in the absence of NS4B2488 peptide, or in T cells from naïve mice. Data were acquired on a BD LSRII Fortessa (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star). The total number of splenocytes secreting IFN-γ, IFN-γ + TNF-α, or IFN-γ + TNF-α + IL-2 was derived by multiplying the % of cells secreting a particular cytokine combination by the total number of viable splenocytes.
2.10 In vivo imaging (IVIS)
The posterior half of all animals was shaven prior to immunization with FLUC-SCFV. At 14, 24, 48, 72, 96 h and 168 h following immunization, animals were injected i.p. with D-luciferin (Caliper LS) in a solution of PBS corresponding to a dose of 0.15 mg/g body weight. After allowing 20 min for dissemination of D-luciferin, animals were anesthetized with ketamine and xylazine, and real-time in vivo imaging was performed using a Xenogen IVIS 200 (Caliper LS, Hopkinton, MA) at medium binning with exposure times ranging from 1–90 sec. Images were analyzed by drawing regions of interest around visible sites of FLUC activity and measuring total flux (photons per second; p/sec) and data were acquired using Living Image 4.0 software (Caliper LS). Reported FP averages are the average total flux from FP from all animals in a treatment group.
2.11 Statistical analyses
Statistical differences for T and B lymphocyte assays and IFN levels were determined using Student t test (unpaired) or ANOVA with the Tukey or Bonferoni post test as appropriate. Values for p < 0.05 were considered significant. All calculations were performed using GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA).
3. Results
3.1 Type I IFN and cytokine responses to RepliVAX WN
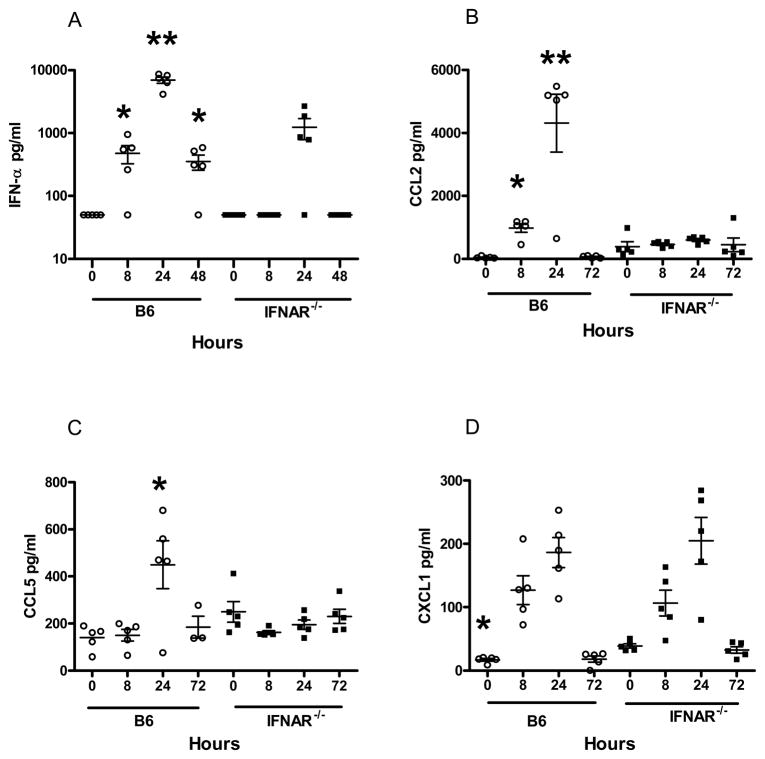
To test if immunization with the SCFV particle RepliVAX WN resulted in production of type I IFNs and IFN-dependent proinflammatory molecules, groups of B6 and IFNAR−/− mice were immunized with RepliVAX WN and serum was collected at 8, 24, and 48 h for detection and quantification of IFN-α and IFN-β. IFN-α was detected at 8 h post-immunization (hpi) in RepliVAX WN-inoculated B6 mice but not IFNAR−/− mice (Fig. 1A). Peak IFN-α levels were detected at 24 h in both mouse strains and fell to low levels by 48 hpi. IFN-α levels observed in RepliVAX WN-immunized B6 mice were significantly higher than in IFNAR−/− mice (p < 0.05, Student t test) at these three time points. IFN-β was not detected in sera obtained from either strain of mice at any time following immunization (data not shown), consistent with previous studies using a similar SCFV [17].
Fig. 1.
IFN-α and chemokine responses to RepliVAX WN immunization. Groups of 5 B6 and IFNAR−/− mice were immunized i.p. with 3.0 × 107 IU of RepliVAX WN and serum was collected at the indicted times. The IFN-α concentration (A) was determined by ELISA. (* p < 0.03, ** p < 0.0003 compared to same time points for B6-IFNAR−/− mice) The limit of detection for IFNα was 12.5 pg/ml. The concentration of CCL2 (B) (* p < 0.007, ** p < 0.0005 compared to same time points for IFNAR−/− mice), CCL5 (C) (* p < 0.05 compared to same time point for IFNAR−/− mice), and CXCL1 (D) (* p < 0.006 compared to same time point for IFNAR−/− mice) were measured by a luminescence-based multiplex bead assay. Results are representative of 2 separate experiments. The limits of detection for CCL2, CCL5, and CXCL1 were 14.0, 5.0, and 3.0 pg/ml, respectively.
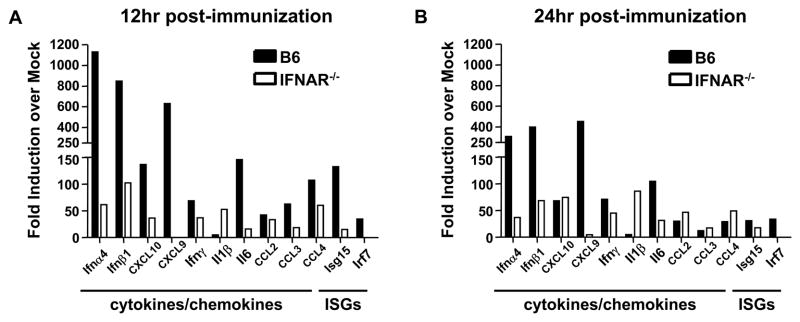
Signaling through the type I IFN receptor culminates in the transcription of a number of ISGs and in the expression of a number of immunologically active proteins such as chemokines and pro-inflammatory cytokines. Following immunization with RepliVAX WN, we detected increased expression of the chemokines CCL2 (MCP-1) (Fig. 1B) and CCL5 (RANTES) (Fig. 1C) in the serum of B6 but not in IFNAR−/− mice. These results are consistent with previous reports that type I IFNs induce expression of these chemokines [23, 24]. As with IFN-α, these chemokines were only transiently detected, with peak levels observed at 24 h. By contrast, CXCL1 (KC) was induced by RepliVAX WN immunization independently of type I IFN receptor status, since both B6 and IFNAR−/− mice showed similar kinetics of induction over a 72 h period (Fig. 1D). To further examine the results of type I IFN receptor signaling following RepliVAX WN inoculation, we examined the transcription of a number of cytokine and chemokine genes in the draining LN following FP inoculation. As expected due to the well-known autocrine signal amplification through the IFN receptor [25], B6 mice exhibited a much higher level of induction of type I IFN, cytokines, and ISGs than did IFNAR−/− mice at both 12 h and 24 h post-infection (Fig. 2). An interesting exception was the induction of the IL-1β gene which exhibited a greater than 50-fold increase over baseline at both time points in IFNAR−/− mice but was unchanged in B6 controls. These data are consistent with the recent report that type I IFN signaling inhibits both pro-IL-1β transcription and the activity of the cellular machinery (NLRP1 and NLRP3 inflammasomes) necessary to process pro-IL-1β into mature IL-1β [26]. Taken together, these results demonstrate that immunization with SCFVs results in type I IFN production, initiation of downstream IFN-stimulated gene pathways, and induction of specific cytokines and chemokines.
Fig. 2.
Relative cytokine expression in draining LN following RepliVAX WN immunization. B6 and IFNAR−/− were immunized subcutaneously (s.c.) in the rear FP with 2.0 × 107 IU RepliVAX WN and popliteal LN were removed for PCR analysis. Cytokine gene expression profiles from cDNA from draining LN harvested at 12 h (left) and 24 h (right) post-immunization. Values represent fold increases over mock-immunized animals. For immunized animals n = 3, for mock-immunized animals n = 2.
3.2 Type I IFN responses limit SCFV gene expression
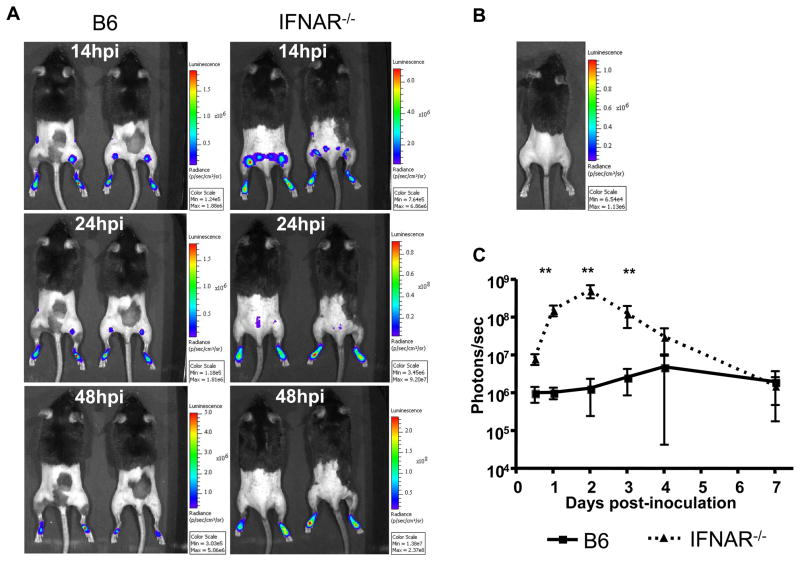
The binding of type I IFNs to the type I IFN receptor results in induction of several antiviral gene programs that inhibit viral gene expression and limit virus spread. While these responses are important for protection against pathogenic organisms, it is possible that they may also limit the development of protective adaptive immune responses following immunization with live-attenuated viral vaccines. We used a firefly luciferase-expressing SCFV, FLUC-SCFV, to test if the type I IFN responses elicited by SCFV inoculation inhibited SCFV gene expression or limited the tissue distribution of FLUC-SCFV-infected cells. Groups of B6 or IFNAR−/− mice were inoculated in the FP with FLUC-SCFV and imaged at intervals between 14 and 168 hpi. SCFV-encoded gene expression was readily detected in FP tissue of FLUC-SCFV-infected mice (Fig. 3A), but not uninfected mice (Fig. 3B) as early as 14 hpi. Additionally, transient FLUC bioluminescence was frequently observed in draining popliteal LN at 14 and 24 hpi (Fig. 3A), but rarely at 48 or 72 hpi and never after 96 hpi (data not shown). In contrast to gene expression in the LN, FLUC-SCFV gene expression was sustained at the inoculation site with FLUC bioluminescence detectable for over 4 d after SCFV inoculation (Fig. 3C). FLUC intensity was over 100-fold higher in the FP of IFNAR−/− mice compared to B6 mice (p < 0.001, Student t test) between days one and three post-inoculation (please note different scales in the various photographs in Fig 3A and B). The FLUC signal then rapidly decreased in IFNAR−/− mice and from day 4 FLUC bioluminescence levels decayed in a manner similar to the luminescence in wild type (wt) animals (Fig. 3C).
Fig. 3.
Magnitude of SCFV gene expression in vivo in B6 and IFNAR−/− mice inoculated with FLUC-SCFV. Groups of B6 or IFNAR−/− mice were inoculated s.c. in both rear FP with 1×107 IU FLUC-SCFV and imaged at the indicated time points as described in Materials and Methods. Bioluminescence was observed using up to 1.5 min exposures at medium binning, was analyzed by measuring total flux emanating from each FP, and is reported as the average over time of all FP from each mouse genotype. Please note different scales in the various photographs in Fig 3A and B. N=4 mice per genotype. Error bars represent standard deviation. *** p<0.001.
3.3 Type I IFN receptor signaling influences development of adaptive immunity to RepliVAX WN
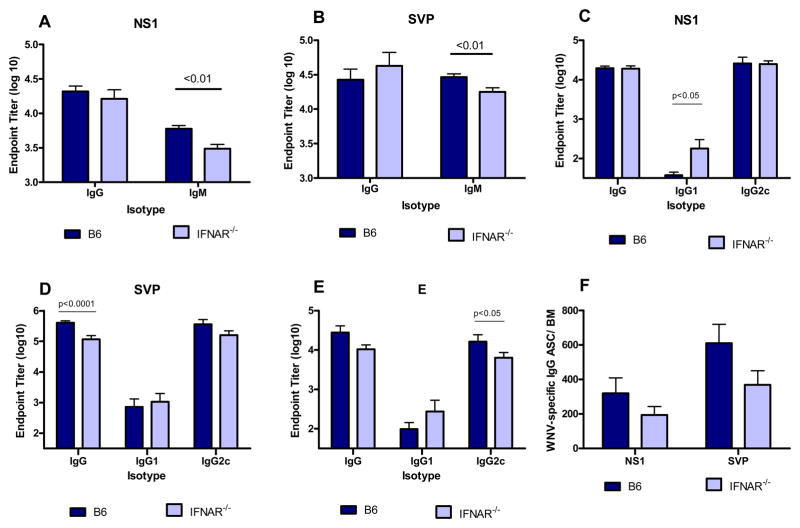
Type I IFN has been shown to influence the magnitude and effector function of developing adaptive immune responses [11, 27–29]. To determine if signaling via the type I IFN receptor played a critical role in the intrinsic adjuvanting of the SCFV vaccine, groups of B6 and IFNAR−/− mice were immunized with RepliVAX WN. Mice were bled at 7 dpi to test for early influences of type I IFNs on antibody production. Both strains of mice produced NS1-specific (Fig. 4A) and SVP-reactive antibodies (Fig. 4B) in response to immunization. B6 mice produced significantly greater titers of serum IgM but similar levels of serum IgG specific for both WNV antigens compared to IFNAR−/− mice (p < 0.01, Student t test). Type I IFN has been reported to influence class switching and IgG subclass expression of developing antibody responses [30]. We examined the expression of IgG1 and IgG2c by WNV-specific serum antibodies in RepliVAX WN-immunized B6 and IFNAR−/− mice (Fig 4C–E). Strong serum IgG responses reactive with 3 different WNV antigens were induced in both mouse strains. RepliVAX WN-immune IFNAR−/− mice generally produced more IgG1 antibody than B6 mice but the difference only reached significance for the NS1 antigen (p < 0.05, ANOVA). Both mouse strains produced predominantly IgG2c subclass antibody. However, B6 mice produced significantly more WNV E antigen-specific IgG2c antibody than IFNAR−/− mice (p< 0.05, ANOVA, Fig. 4E). The functional activity of serum antibodies also did not differ between the strains of mice. The 90% neutralization titers for B6 and IFNAR−/− mice were 1:160 and 1:80, respectively, on day 21 dpi.
Fig. 4.
Effect of type I IFN receptor signaling on WNV-specific Ab responses following immunization with RepliVAX WN. Groups of IFNAR−/− and B6 mice were immunized with 106 IU RepliVAX WN i.p. and the endpoint titers on day 7 post immunization for serum IgG and IgM antibody specific for WNV NS1 (A) or WNV SVP (B) were determined for individual mice. Results are pooled from 2 experiments (n= 15mice/group for IgG, n= 20 mice/group for IgM). Endpoint titers of IgG, IgG1, and IgG2c Ab specific for WNV NS1 (C), SVP (D), or E proteins (E) were determined by ELISA from 21 and 28 day samples. Results are pooled from 2 experiments (n= 10 mice/group). (F) WNV NS1 and SVP-specific antibody secreting cell response in the bone marrow of RepliVAX WN immunized mice. Groups of 5 IFNAR−/− and B6 mice were immunized with 106 IU RepliVAX WN i.p. On day 28, cells were harvested from the femurs of immunized mice and analyzed by ELISPOT for IgG ASC specific for WNV NS1 or SVPs as described in Materials and Methods. Data are presented as the mean ± standard error of the mean (SEM) of WNV-specific IgG-producing cells per bone marrow from individual mice. The results shown are from a representative experiment of 2 performed.
Long-lived plasma cells in the bone marrow are thought to serve as the major, long-term source of antigen-specific IgG Ab in serum [31]. To test if the absence of type I IFN signaling would alter development of this cell subset, we harvested bone marrow cells from femurs 28 d after RepliVAX WN immunization and quantified the total NS1- and SVP-specific IgG ASC by ELISPOT (Fig. 4F). The number of NS1-specific and SVP-specific IgG ASC was lower in IFNAR−/− mice compared to B6 mice; however, in neither case did the difference reach significance.
3.4 Cell-mediated immune responses to RepliVAX WN in the absence of type I IFN receptor signaling
Type I IFNs have been reported to play an important role in determining the magnitude and function of the T cell responses to many different pathogens [28, 32, 33]. To determine if type I IFN receptor signaling was required for development of the vaccine-induced T lymphocyte response, we immunized groups of B6 and IFNAR−/− mice with RepliVAX WN and assessed the magnitude and effector function of the WNV-specific T cell responses.
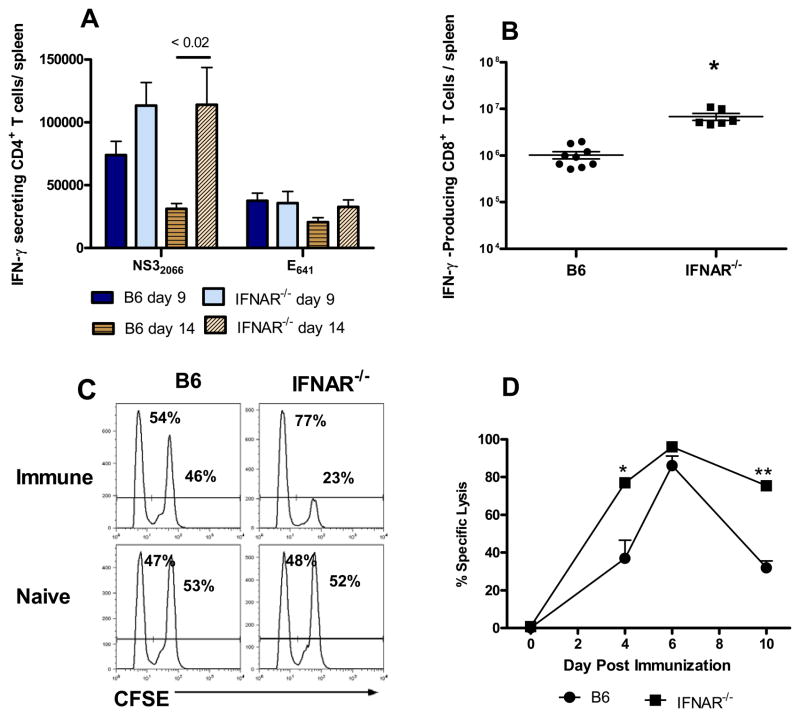
Vigorous CD4+ T cell responses to 2 immunodominant CD4+ T cell epitopes [34] were observed in both RepliVAX WN-immunized IFNAR−/− and B6 mice (Fig. 5A). Consistent with previous results [19], the IFN-γ secreting cell response of splenocytes from immunized B6 mice to the NS32066 peptide was of greater magnitude than the response to the E641 epitope on both days 9 and 14. A similar response pattern to these epitopes was observed in RepliVAX-immunized IFNAR−/− mice. Interestingly, the NS32066-specific response in IFNAR−/− mice was significantly higher than the response of B6 mice on day 14 post-immunization (p < 0.02, Student t test) suggesting a more prolonged response to this epitope whereas the response to the E641 epitope in B6 and IFNAR−/− mice was of similar magnitude on both days 9 and 14.
Fig. 5.
Type I IFN receptor signaling is not required for the intrinsic adjuvanting of T cell responses against RepliVAX WN. (A) CD4+ T cell response. B6 or IFNAR−/− mice were immunized with 106 IU RepliVAX WN and the number of IFN-γ-secreting cells specific for the NS32066 or E641 CD4+ T cell epitopes were quantified by ELISPOT on days 9 and 14 as described in Materials and Methods. Results are expressed as the number of IFN-γ-secreting cells/spleen (mean ± SEM) and are compiled from 2 separate experiments (n = 10–12 mice/group). (B) CD8+ T cell responses to the NS4B2488 epitope were quantified by detection of intracellular IFN-γ by flow cytometry on day 6 as described in Methods. Results are expressed as the number of IFN-γ secreting cells/spleen (mean ± SEM) and are compiled from 2 separate experiments (n = 6–9 mice/group). (C) Representative histograms of CFSE-labeled target cells in spleens of naïve and RepliVAX WN-immunized B6 and IFNAR−/− mice 4 days after immunization. Numbers in each histogram represent the % No-peptide targets (CFSElow) and % NS4B2488 peptide-pulsed targets (CFSEhi) for an individual animal. (D) Cytotoxic CD8+ T lymphocyte activity from B6 and IFNAR−/− mice after RepliVAX WN immunization. The percentages of CFSElow and CFSEhi targets derived as shown in Fig. 5C, were used to calculate a % specific lysis as described previously [19]. Results are expressed as the % specific lysis (mean ± SEM) from 5 mice/group for NS4B2488-coated targets (* p < 0.005, ** p < 0.0001 compared to RepliVAX WN-immunized B6 mice). Naïve mice routinely exhibited < 2% specific lysis.
The magnitude of the effector CD8+ T cell response was measured by quantification of IFN-γ-producing cells using intracellular cytokine staining and flow cytometry. Strong CD8+ T cell responses to the immunodominant epitope NS4B2488 were observed on day 6 post-immunization in both B6 and IFNAR−/− mice (Fig. 5B). However, the IFN-γ producing cell response was significantly higher in IFNAR−/− mice (p < 0.0001, Student t test) representing a greater than 6-fold increase in the total number of WNV NS4B-specific, IFN-γ-producing cells. The effector function of CD8+ T cells of RepliVAX WN -immunized B6 and IFNAR−/− mice was further assessed by measuring the in vivo cytotoxicity response against the immunodominant NS4B2488 epitope (Fig. 5C, D). High levels of WNV NS4B-specific cytotoxicity were observed in both strains of mice at the peak of the response on day 6. However, consistent with the significantly greater IFN-γ response of IFNAR−/− mice (Fig. 5B), the percent specific lysis of NS4B2488-pulsed target cells in IFNAR−/− mice was significantly higher on days 4 (p < 0.005, Student t test), and 10 (p < 0.0001, Student t test) compared to B6 mice, indicating a more rapid and prolonged CD8+ T cell response.
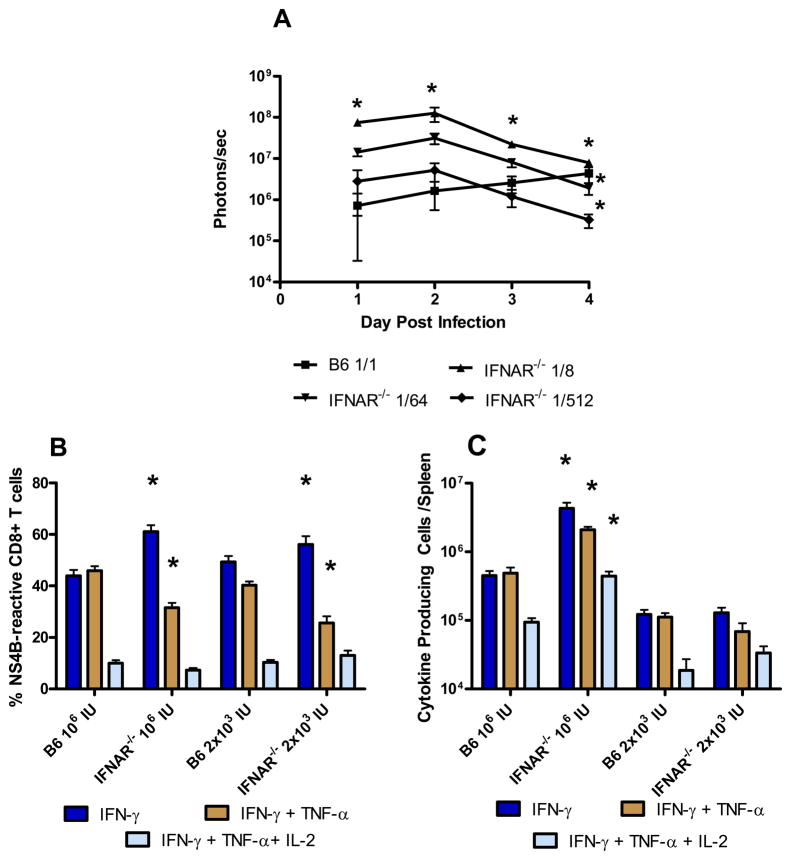
We also examined the cytokine production profile of WNV NS4B-specific T cells induced by RepliVAX WN to determine if the quality of the CD8+ effector T cell response would be altered in the absence of IFNAR signaling. Given the significantly greater increase in SCFV gene expression in IFNAR−/− mice (Fig. 3), we considered it possible that increased antigen load in these mice rather than the lack of IFNAR signaling may be responsible for any influence in cytokine production patterns. To control for this possibility, we therefore inoculated IFNAR−/− mice with SCFV at a dose either representing an equivalent number of RepliVAX WN particles used to immunize B6 mice or with a dose that would result in equivalent SCFV gene expression relative to inoculated B6 mice. To determine the dose of SCFV that would result in equivalent gene expression in IFNAR−/− mice relative to B6 mice, we inoculated B6 mice with FLUC-SCFV particles and inoculated IFNAR−/− mice with a series of dilutions (1/8, 1/64, 1/512) of the same FLUC-SCFV inoculum. FLUC-SCFV gene expression was visualized and quantified by IVIS analysis on days 1–4 (Fig. 6A). Over the first three days post inoculation, gene expression in IFNAR−/− mice which received a 1/512 dilution of FLUC-SCFV most closely approximated, and was not different from, gene expression in B6 mice which received the full FLUC-SCFV dose. Because dendritic cells isolated from SCFV inoculated mice are already loaded with SCFV antigen by day three post immunization (G. Milligan, unpublished results), we utilized a dose of 2 × 103 IU RepliVAX WN (1:500 dilution of SCFV inoculum) in IFNAR−/− mice to approximate the gene expression and antigen load resulting from inoculation of B6 mice with 106 IU RepliVAX WN in subsequent experiments. Fig. 6B shows the percentage of NS4B-specific CD8+ T cells from low- or high-dose RepliVAX WN immunized mice which produced IFN-γ alone or simultaneously produced IFN-γ + TNF-α or IFN-γ + TNF-α + IL-2. Overall, the cytokine production pattern associated best with the presence or absence of IFNAR signaling rather than with antigen load. Specifically, the percentage of cells secreting IFN-γ alone was significantly higher (p < 0.0001, ANOVA) in IFNAR−/− mice immunized with either a high or low SCFV dose compared to B6 mice while the percentage of cells secreting IFN-γ + TNF-α was significantly lower in these mice (p < 0.0001, ANOVA). A similar fraction of NS4B2488-specific CD8+ T cells from B6 and IFNAR−/− cells simultaneously produced IFN-γ, TNF-α and IL-2 regardless of immunizing SCFV dose or mouse strain. As expected from Fig 5B, the total number of CD8+ T cells secreting IFN-γ, IFN-γ + TNF-α, or IFN-γ + TNF-α + IL-2 (Fig. 6C) was significantly higher in high dose IFNAR−/− mice compared to B6 mice or IFNAR−/− mice immunized with a low dose of RepliVAX WN (p < 0.0001, ANOVA). Together, these results suggest that antigen dose affected the magnitude of the CD8+ T cell response whereas IFNAR signaling influenced the cytokine production pattern of WNV-specific CD8+ effector T cells. Interestingly, the loss of IFNAR signaling apparently did not influence all effector functions as high levels of WNV-specific cytotoxicity were detected in IFNAR−/− mice.
Fig. 6.
Cytokine production by WNV NS4B-specific CD8+ T cells following RepliVAX immunization of B6 and IFNAR−/− mice. (A) SCFV gene expression in IFNAR−/− mice compared to B6 mice. B6 mice were inoculated in the FP with 107 IU FLUC-SCFV. IFNAR−/− mice received a 1:8 (1.25 × 106 IU), 1/64 (1.56 × 105 IU), or 1/512 (1.95 × 104 IU) dilution of the same inoculum. Mice were imaged at the indicated time points as described in Materials and Methods. Bioluminescence was observed using up to 1.5 min exposures at medium binning followed by measuring total flux emanating from each FP, and is reported as the average over time of all FP from each group (n= 4 mice/group). (* p < 0.01, ANOVA). (B) Whole splenocyte populations were harvested from B6 or IFNAR−/− mice on day 6 after primary RepliVAX WN vaccination and re-stimulated with NS4B2488 peptide prior to characterization by multiparameter flow cytometry as described in Materials and Methods. The results shown are compiled from 4 separate experiments (n= 3–9 mice/group). (C) The number of total cells per spleen secreting IFN-γ, IFN-γ + TNF-α, or IFN-γ + TNF-α + IL-2 from the mice described in 6B was derived by multiplying the percent of cells secreting a particular cytokine combination by the total number of viable splenocytes.
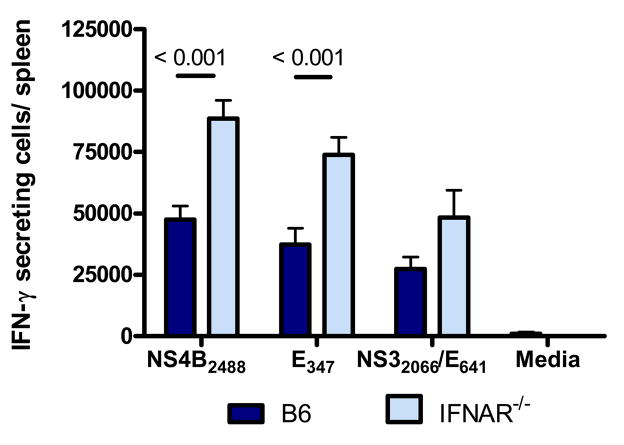
Type I IFNs have been reported to play an important role in development of memory T cell responses to several pathogens [32, 33]. To test if the development of WNV-specific memory T cell responses to a SCFV particle required type I IFN activities, we immunized groups of B6 or IFNAR−/− mice with RepliVAX WN and quantified WNV-specific memory T cells in the spleen 56 days post-immunization. As shown in Figure 7, CD8+ memory T cells specific for the NS4B2488 and E347 epitopes were present at significantly higher levels in IFNAR−/− mice compared to B6 mice (P < 0.001, Student t test). Similarly, development of the RepliVAX WN-induced CD4+ memory T cell did not require signals generated through the type I IFN receptor as comparable numbers of memory T cells specific for the CD4+ T cell epitopes NS32066 and E641 were detected in both strains of mice. Although greater numbers of CD4+ memory T cells were detected in IFNAR−/− mice compared to B6 controls, the difference did not reach significance.
Fig. 7.
CD8+ and CD4+ memory T cell response in B6 and IFNAR−/− mice 8 weeks following RepliVAX WN immunization. Groups of mice were immunized with RepliVAX WN and splenocytes removed after 8 weeks for quantification of WNV-epitope-specific T cells by ELISPOT as described in Materials and Methods. Results are from a representative experiment of 2 performed (n= 5/group/experiment).
4.0 Discussion
The limited infections established by SCFVs make them ideal tools for examining the relative influence of the antiviral and adjuvanting aspects of the type I IFN response following immunization. Specifically, SCFVs can efficiently replicate their RNA genomes within cells thereby activating the intrinsic IFN induction system from these initially infected cells, yet their inability to produce progeny virions prevent them from producing a lethal systemic disease in immunocompromised animals. RepliVAX WN infection is expected to produce PRR ligands that bind to multiple PRRs including TLR3 [35, 36], TLR7 [37, 38], the cytoplasmic helicases RIG I and MDA5 [38], or PKR [10] leading to the production of type I IFNs. In addition to establishing an antiviral environment in virus-infected and surrounding cells, type I IFNs have also been shown to influence development of the adaptive immune response [11, 27–29, 39] and have been shown to act as an adjuvant when injected in combination with protein antigens or commercial influenza vaccines [11, 13]. Moreover, type I IFNs have been shown to be responsible for the adjuvanting activity of both complete and incomplete Freund’s adjuvants and for the TLR9-dependent adjuvant, IC31 [12, 13]. Thus, following immunization with live-attenuated viral vaccines such as RepliVAX WN, type I IFNs may potentially negatively influence the development of adaptive immune responses by diminishing the availability of antigen through inhibition of viral gene expression in infected cells. Alternatively, they may positively influence the adaptive immune response by directly mediating the intrinsic adjuvanting of the vaccine. The current studies examined the influence of the host type I IFN system on RepliVAX WN infection and gene expression, and ultimately how this interaction between host response and viral infection shaped the adaptive immune response to RepliVAX WN immunization.
Infection of B6 mice with RepliVAX WN was sufficient to induce production of type I IFNs which ultimately influenced the quantity and composition of immunologically active molecules produced by immunized mice. Analysis of cytokine, chemokine, and ISG expression in the LN of RepliVAX WN-immunized mice revealed differences between B6 and IFNAR−/− strains, with the knockout mice displaying lower levels of gene induction for type I and II IFN, CXCL9, and IL-6. Interestingly, genes for other chemokines (CXCL10, CCL2, CCL3, CCL4) were induced to greater levels in the LN of B6 mice at 12 hpi, but by 24 hpi these levels were equivalent in wt and IFNAR−/− mice. Taken together, these results demonstrate that type I IFNs are important for defining the milieu of immune molecules induced by RepliVAX WN immunization which potentially influences the development and mobilization of innate and adaptive immune responses. The ability to initiate an innate response, including type I IFNs, and subsequent adaptive immune response in the absence of prolonged viral replication may also be important for limiting vaccine reactogenicity. Early immunization studies with low doses of live attenuated flaviviruses resulted in reactogenicity due to viremia resulting from inadequate initial innate immune responses [40]. The single-cycle nature of the RepliVAX WN immunization thus allows immunization over a range of doses to maximize vaccine efficacy while preventing reactogenicity that could result from vaccine virus replication.
To evaluate the role of type I IFNs in controlling expression of antigen by RepliVAX WN, we performed in vivo imaging experiments of animals inoculated with FLUC-SCFVs to observe the distribution and magnitude of SCFV infection in the presence or absence of IFNAR signaling. These studies revealed an approximately 100-fold increase in FLUC gene expression in IFNAR−/− mice from days 1–3 post-immunization, demonstrating that type I IFN played an important role in controlling SCFV gene expression. We further observed that FLUC-SCFV-infected cells produced a robust signal at the site of inoculation, while infected cells were observed transiently in the draining popliteal LN and occasionally in more distal LNs at later times regardless of the ability of mice to respond to type I IFNs. These results confirm our previous finding that WNV antigen and genomes were detected in the draining LN of mice after SCFV infection [17]. These studies further suggest that SCFVs infect a population of cells that migrate from the site of inoculation through the lymphatic system to the draining LN, and/or that the SCFV particles themselves are transported via lymph to the draining LN where they infect resident cells. Our IVIS studies also revealed that SCFV gene expression was maintained for several days at the inoculation site. Since soluble Ags (SVPs and NS1) released from cells at the inoculation site could also traffic to lymphoid tissues and contribute to immune responses, it is impossible to determine the relative importance of SCFV-infected cells within the draining LN or the inoculation sites in the development of the immune responses elicited by SCFV vaccines.
Type I IFNs have been shown to exert a range of actions that directly affect the magnitude and quality of the Ab response. They may act on B cells either directly or indirectly through the activation of dendritic cells early in the response to viral infection to enhance B cell expansion and induction of activation markers [30, 41, 42]. Type I IFNs have also been shown to protect B cells from apoptosis in vitro [41]. Consistent with these findings, early Ab responses to influenza [30] and VSV [43] infections in mice have been shown to be diminished in the absence of type I IFN receptor signaling. In the current studies, the lack of type I IFN receptor signaling resulted in reduced early (day 7) serum IgM responses to RepliVAX WN immunization but had little effect on the IgG antibody response at any time point measured. While type I IFNs have been reported to influence IgG isotype expression [11], the lack of type I IFN receptor signaling had only modest effect on the magnitude or subclass expression in the current studies. IgG1 titers against WNV antigens were generally higher in IFNAR−/− mice; however they were significantly higher than IgG1 titers in B6 mice only for WNV NS1-specific antibodies. The IgG Ab response to RepliVAX WN-immunization was predominated by IgG2c subclass Ab in both strains of mice. Together, these results could be interpreted to indicate that type I IFNs were not required for the development of a WNV-specific IgG antibody response following immunization with RepliVAX WN. However, this interpretation is confounded by the finding that SCFV gene activity in IFNAR−/− mice was over 100 times greater over a 3 day period compared to wt mice. It remains possible that the limited effects that loss of type I IFN signaling had on B cell responses in IFNAR−/− mice in the current studies might be a result of increased SCFV gene expression and thus high antigen loads in IFNAR−/− mice compared to B6 mice.
Induction of the RepliVAX WN-induced CD4+ and CD8+ T cell responses did not require signaling through the type I IFN receptor suggesting that type I IFNs do not mediate the intrinsic adjuvanting of RepliVAX WN. These results are in contrast to reports of deficient CD4+ T cell responses in IFNAR−/− mice due to lack of type I IFN survival signals for virus-specific CD4+ T cells [28]. Interestingly, the reported strict requirement for type I IFN for ensuring clonal expansion of CD4+ T cells has been shown to be pathogen-dependent [28], suggesting that inflammatory milieus resulting from different infections may provide the necessary factors for clonal expansion and survival. In the case of RepliVAX WN immunization, the inflammatory milieu in RepliVAX WN-immunized IFNAR−/− mice was sufficient to support development of a vigorous CD4+ T cell response suggesting type I IFN was not required for expansion of this subset. The apparent lack of requirement for type I IFN signaling for the optimal development of CD4+ and CD8+ responses in our studies compared to previous results by others may thus reflect differences in the inflammatory milieu due to background strain of IFNAR−/− mice employed or the pathogen system utilized.
Type I IFNs have been shown to have direct effects on T lymphocytes including inhibition of proliferation in vitro [44], influencing T cell survival [39] and enhancing T cell clonal expansion [28, 32]. Similarly, type I IFNs have been shown to be necessary as a third signal for development and differentiation of CD8+ T cell responses in some infection models [32] although not in others [33, 45] suggesting redundancy of function by other cytokines including IL-12. In our studies, although IL-12 was not detected in the serum of immunized mice using our luminescence-based cytokine assay, it was detected in the serum of IFNAR−/−, but not B6 mice, at 24 h post immunization using an ELISA-based assay (data not shown) and may have provided the required third signal for development of this T cell subset. Additionally, we detected a vigorous WNV-specific CD8+ T cell response in the absence of type I IFN receptor signaling that was significantly greater than the response of wt mice. Because the magnitude of the CD8+ effector T cell response in B6 and IFNAR−/− mice was dependent on the immunizing dose (Fig. 6C), the greater magnitude response in IFNAR−/− mice compared to B6 mice most likely reflected the higher expression of SCFV genes, and thus SCFV antigen, in IFNAR−/− mice compared to B6 mice in the first 72 h after immunization. Our studies demonstrate that type I IFNs did, however, play a role in the development of CD8+ T cell effector function in that the cytokine production patterns of WNV-specific CD8+ T cells, measured at the single cell level, were altered in the absence of type I IFN receptor signaling. Importantly, WNV-specific cytotoxicity appeared unaffected by loss of type I IFNR signaling. T cell responses to pathogens display functional heterogeneity and much evidence suggests that the simultaneous production of multiple cytokines and and/or expression of cytolytic function by individual T cells is important for infection control [46]. Interestingly, under conditions of prolonged antigen exposure or exposure to very high antigen loads, T cells undergo a stepwise loss of function involving progressive loss of IL-2 and TNF-α production [47]. In the current studies, while CD8+ T cell responses in IFNAR−/− mice were of significantly higher magnitude compared to wt mice and expressed cytolytic activity, a significant proportion of these cells had lost the ability to produce TNF-α and IL-2. This loss of production of these cytokines by CD8+ T cells in IFNAR−/− mice was not associated with increased viral gene expression compared to wt mice and strongly suggests that the type I IFN may directly influence some aspects of effector function such as cytokine production without affecting other effector functions such as virus-specific cytotoxicity.
Taken together, the results of these studies have interesting implications for the ability of attenuated vaccines to produce consistent immune responses in vaccinated populations. Specifically, we have shown that type I IFN controls the early genome replication and expression of viral genes after immunization with a single-cycle vaccine. Vigorous adaptive immune responses developed in the absence of type I IFN receptor signaling suggesting that type I IFN is not essential for the intrinsic adjuvanting of the RepliVAX WN vaccine. Conversely, although type I IFN decreased SCFV gene expression and presumably antigen load in B6 mice, strong WNV-specific antibody and T cell responses were elicited by RepliVAX WN immunization of B6 mice suggesting that type I IFN does not greatly impair the magnitude of the developing adaptive immune response to this single cycle vaccine. Overall, the magnitude of the WNV-specific IgG response to WNV proteins was not significantly altered in the absence of type I IFN receptor signaling; however, the quality of the CD8+ effector T cell response measured as the ability of individual cells to simultaneously produce more than one cytokine was diminished in IFNAR−/− mice suggesting that type I IFN receptor signaling plays a role in shaping cytokine production in antigen-specific effector T cell responses.
Highlights.
Type I IFN is produced following single-cycle flavivirus (SCFV) vaccine immunization.
Type I IFN severely restricts SCFV gene expression in wild type mice.
Significant increase in CD8+ T cell response in the absence of type I IFN receptor.
Altered patterns of cytokine production, but not virus-specific cytotoxicity, by WNV-specific CD8+ T cells in the absence of type I IFN receptor.
Acknowledgments
We thank Chin-Fun Chu, Yinghong Ma, and Summer Gorder for technical assistance and Dr. Anthony French for providing IFNAR−/− mice. This work was supported by National Institutes of Health Grants UO1 AI082960 and R21 AI077077. D. G. Widman, M. H. Nelson, and J. Xia were supported by a James W. McLaughlin Predoctoral Fellowship. E. R. Winkelmann and M. H. Nelson were supported by a Sealy Center for Vaccine Development Predoctoral Fellowship.
Abbreviations used in this paper
- ASC
antibody secreting cell
- B6
C57BL/6 mice
- FLUC
firefly luciferase
- FP
footpad
- hpi
hours post-immunization
- IFNAR−/−
IFN-α β receptor knockout mouse
- ISG
IFN-stimulated gene
- LN
lymph node
- PRR
pattern recognition receptor
- SCFV
single-cycle flavivirus
- SVP
sub-viral particle
- WNV
West Nile virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6694–9. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guirakhoo F, Kitchener S, Morrison D, Forrat R, McCarthy K, Nichols R, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin. 2006 Mar-Apr;2(2):60–7. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman MM, Nerurkar VR, Luo H, Cropp B, Carrion R, Jr, de la Garza M, et al. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clin Vaccine Immunol. 2009 Sep;16(9):1332–7. doi: 10.1128/CVI.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007 Dec 15;196(12):1732–40. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008 May 23;26(22):2762–71. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006 Aug 1;351(2):432–43. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widman DG, Ishikawa T, Winkelmann ER, Infante E, Bourne N, Mason PW. RepliVAX WN, a single-cycle flavivirus vaccine to prevent West Nile disease, elicits durable protective immunity in hamsters. Vaccine. 2009 Sep 18;27(41):5550–3. doi: 10.1016/j.vaccine.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Widman DG, Ishikawa T, Giavedoni LD, Hodara VL, Garza Mde L, Montalbo JA, et al. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am J Trop Med Hyg. 2010 Jun;82(6):1160–7. doi: 10.4269/ajtmh.2010.09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002 Aug;14(4):432–6. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 10.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008 Jan;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 11.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001 Apr;14(4):461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 12.Prchal M, Pilz A, Simma O, Lingnau K, von Gabain A, Strobl B, et al. Type I interferons as mediators of immune adjuvants for T- and B cell-dependent acquired immunity. Vaccine. 2009 Dec 30;27( Suppl 6):G17–20. doi: 10.1016/j.vaccine.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002 Jul 1;169(1):375–83. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 14.Gilfoy F, Fayzulin R, Mason PW. West Nile virus genome amplification requires the functional activities of the proteasome. Virology. 2009 Mar 1;385(1):74–84. doi: 10.1016/j.virol.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi SL, Zhao Q, O’Donnell VK, Mason PW. Adaptation of West Nile virus replicons to cells in culture and use of replicon-bearing cells to probe antiviral action. Virology. 2005 Jan 20;331(2):457–70. doi: 10.1016/j.virol.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, et al. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol. 2005 Jun 1;174(11):7217–25. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- 17.Bourne N, Scholle F, Silva MC, Rossi SL, Dewsbury N, Judy B, et al. Early production of type i interferon during West Nile virus infection: role for lymphoid tissues in IRF3-independent interferon production. J Virol. 2007 Sep;81(17):9100–8. doi: 10.1128/JVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson MH, Winkelmann E, Ma Y, Xia J, Mason PW, Bourne N, et al. Immunogenicity of RepliVAX WN, a novel single-cycle West Nile virus vaccine. Vaccine. Dec 16;29(2):174–82. doi: 10.1016/j.vaccine.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson MH, Winkelmann E, Ma Y, Xia J, Mason PW, Bourne N, et al. Immunogenicity of RepliVAX WN, a novel single-cycle West Nile virus vaccine. Vaccine. Nov 2; doi: 10.1016/j.vaccine.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purtha WE, Myers N, Mitaksov V, Sitati E, Connolly J, Fremont DH, et al. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007 Jul;37(7):1845–54. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 21.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007 Jul;37(7):1855–63. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 22.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008 Dec 15;181(12):8568–75. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection. J Immunol. 2009 Jul 15;183(2):1271–8. doi: 10.4049/jimmunol.0900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cremer I, Ghysdael J, Vieillard V. A non-classical ISRE/ISGF3 pathway mediates induction of RANTES gene transcription by type I IFNs. FEBS Lett. 2002 Jan 30;511(1–3):41–5. doi: 10.1016/s0014-5793(01)03276-8. [DOI] [PubMed] [Google Scholar]
- 25.Malmgaard L, Salazar-Mather TP, Lewis CA, Biron CA. Promotion of alpha/beta interferon induction during in vivo viral infection through alpha/beta interferon receptor/STAT1 system-dependent and -independent pathways. J Virol. 2002 May;76(9):4520–5. doi: 10.1128/JVI.76.9.4520-4525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. Feb 25;34(2):213–23. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003 Aug;19(2):225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 28.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006 Mar 15;176(6):3315–9. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 29.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005 Apr 15;174(8):4465–9. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 30.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006 Apr 1;176(7):4343–51. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 31.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995 Mar;69(3):1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005 Sep 5;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006 Aug 1;177(3):1746–54. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 34.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008 Dec 15;181(12):8568–75. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008 Nov;82(21):10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004 Dec;10(12):1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 37.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009 Feb 20;30(2):242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008 Jan;82(2):609–16. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999 Feb 1;189(3):521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez V, Gimenez S, Tomlinson B, Chan PK, Thomas GN, Forrat R, et al. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3) Vaccine. 2006 Jun 5;24(23):4914–26. doi: 10.1016/j.vaccine.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 41.Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. International immunology. 2002 Apr;14(4):411–9. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 42.Purtha WE, Chachu KA, Virgin HWt, Diamond MS. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J Virol. 2008 Nov;82(22):10964–74. doi: 10.1128/JVI.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fink K, Lang KS, Manjarrez-Orduno N, Junt T, Senn BM, Holdener M, et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006 Aug;36(8):2094–105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 44.Dondi E, Rogge L, Lutfalla G, Uze G, Pellegrini S. Down-modulation of responses to type I IFN upon T cell activation. J Immunol. 2003 Jan 15;170(2):749–56. doi: 10.4049/jimmunol.170.2.749. [DOI] [PubMed] [Google Scholar]
- 45.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002 Dec 15;169(12):6842–9. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 46.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. 2008 Apr;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 47.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003 Apr;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]