Abstract
Bone is a connective tissue containing cells, fibers and ground substance. There are many functions in the body in which the bone participates, such as storing minerals, providing internal support, protecting vital organs, enabling movement, and providing attachment sites for muscles and tendons. Bone is unique because its collagen framework absorbs energy, while the mineral encased within the matrix allows bone to resist deformation. This article provides an overview of the structure and function of bone tissue from a macroscopic to microscopic level and discusses the physiological processes contributing to upper extremity bone health. It concludes by discussing common conditions influencing upper extremity bone health.
Introduction
Bone is a specialized connective tissue consisting of cells, fibers and ground substance. Unlike other connective tissues, its extracellular components are mineralized giving it substantial strength and rigidity. This makes bone ideally suited to fulfilling its most recognized role within the body, that of mechanical support. In the upper extremity, bone provides a structural framework allowing weight to be born when the hand is functioning in a closed-kinetic chain and provides attachment sites for muscles to produce motion at specialized bone-to-bone linkages. The later allows the hand to be moved in space against gravity and other external forces. To fulfill its mechanical role, bone needs to be stiff to resist deformation, yet flexible to absorb energy. The current article provides an overview of the anatomy and physiology of bone tissue before discussing common conditions and factors influencing upper extremity bone health.
Upper extremity bone anatomy
The upper extremity skeleton comprises all bones distal to the scapula and clavicle, and includes an assortment of long (i.e. humerus, radius and ulna), short (i.e. carpals), flat (i.e. scapula), and sesamoid (i.e. pisiform) bones. The anatomy or morphology of these bones can be viewed hierarchically starting at the gross, macroscopic level and progressing microscopically down to the nanoscale level. Macroscopically, bones of the upper extremity can be divided into two distinct types of bone tissue—cortical and trabecular (Figures 1 and 2A). These two tissue types have the same matrix composition; however, they differ substantially in terms of their structure and function, and relative distribution both between and within bones.
Figure 1.
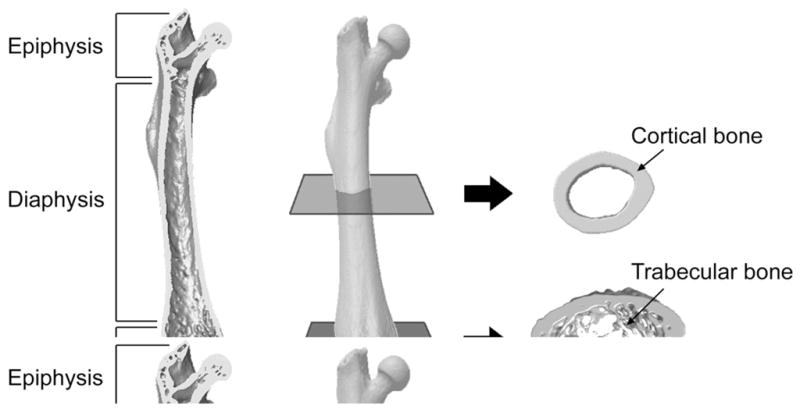
Macroscopic anatomy of a long bone. The relatively cylindrical shaft or diaphysis consists predominantly of cortical bone, whereas the expanded epiphyses have a greater proportion of trabecular bone enclosed within a relatively thinner cortical shell. Images are of a mouse femur acquired using micro-computed tomography.
Figure 2.
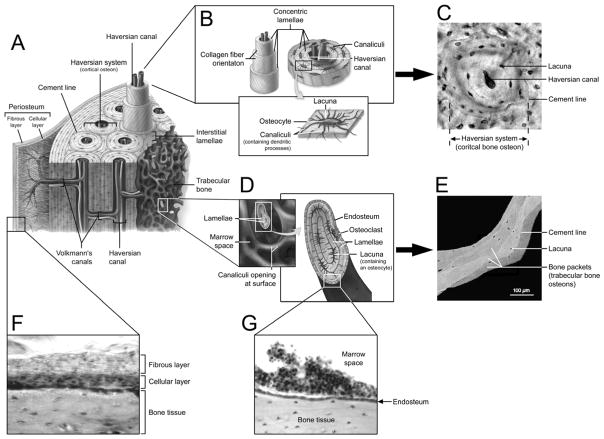
Microscopic anatomy of bone. A) Schematic diagram of bone microstructure. B) Cortical bone osteon or Haversian system with central Haversian canal, concentric lamellae and uniformly spaced lacunae. C) Cortical bone osteon as viewed in cross section via light microscopy. D) Trabeculae shown in cross section with lamellae of bone, lacunae and outer covering of endosteum. E) Trabecular bone osteons or ‘packets’ as viewed via backscattered electron imaging. F) Light microscope image of the fibrous and cellular layers of the periosteum covering the outer surface of cortical bone. G) Light microscope image of the endosteum lining the medullary cavity and trabecular bone (Panels A, B, D reproduced with permission of the McGraw-Hill Companies from McKinley M and O’Loughlin VD: Human Anatomy (2nd ed.). New York, NY: McGraw-Hill Higher Education, 2008. Panel E reproduced with permission of Elsevier Inc. from Roschger P, et al.: Bone mineralization density distribution in health and disease. Bone 2008;42:456–466).
Cortical bone
Cortical (or compact) bone makes up approximately 80% of skeletal tissue mass. It has a high matrix mass per unit volume and low porosity (microscopic pores constitute approximately 10% of total cortical bone volume). These features endow cortical bone with great compressive strength enabling it to prominently contribute to the mechanical role of bone. This is reflected in its distribution primarily within the cylindrical shaft (diaphysis) of upper extremity long bones where it forms a thick shell (cortex) surrounding a medullary canal (Figure 1). The tube-like design distributes bone mineral away from bending axes resulting in a substantial increase in bending resistance without a concomitant increase in bone mass. The net result is long bones with the strength and rigidity required for muscle action and weight bearing, yet lightness required for energy efficient motion. Cortical bone thins towards the expanded ends (epiphyses) and interposed developing region (metaphysis) of long bones where it plays a lesser, yet clinically significant mechanical role. The best example of the later is at the distal radius where cortical thickness is an important discriminator between those with and without osteoporotic fracture.1
Trabecular bone
Trabecular bone has high porosity relative to cortical bone, with pores making up 50–90% of trabecular bone volume. The pores are interspersed among an orderly network of vertical and horizontal structural elements called trabeculae, which give trabecular bone a sponge-like appearance (Figure 1). The reduced matrix mass per unit volume and high porosity of trabecular bone reduces its compressive strength to approximately one-tenth that of cortical bone;2 however, trabecular bone contributes to the mechanical role of bone by providing internal support. This supportive role facilitates the ability of bone to evenly distribute load and absorb energy, particularly in the vicinity of joints. It is also important during aging as trabecular bone is lost earlier and at a greater rate than cortical bone, which ultimately contributes to osteoporosis at trabecular rich sites such as the distal radius.3
Bone coverings
Bone surfaces are covered by specialized connective tissues. The periosteum covers external surfaces of most bones and is divided into two distinct layers—an outer fibrous and inner cellular layer (Figure 2F). The cellular or ‘cambium’ layer is positioned in direct contact with the bone surface and is of particular interest as it contains mesenchymal stem cells (MSCs) which have the potential to differentiate into osteoblasts and chondrocytes, and differentiated osteogenic progenitor cells. The localization of these cell types has made the cellular layer a target for drug therapies and cell harvesting for tissue engineering purposes.
The endocortical surface of a bone faces the medullary canal and is lined by the endosteum, a single thin layer of bone lining cells (mature osteoblasts) and osteoblasts which form a membrane over endocortical and trabecular bone surfaces to enclose the bone marrow (Figure 2G). The endosteum contains osteoprogenitor cells, but does not appear to contain either MSCs or hematopoietic stem cells (HSCs). However, a portion of HSCs can be found next to the endosteum suggesting reciprocal communication between cells within the endosteum and multipotent HSCs.4 The close relationship between the cells forms a so called ‘stem cell niche’ whereby the cells of the endosteum physically support and influence stem cell activity.5
Microscopic bone structure
Microscopic visualization of both cortical and trabecular bone reveals tissue that is either woven or lamellar in structure. Woven bone has a disorganized collagen fibril arrangement and is not typical in the adult skeleton, except in pathological conditions (such as Paget’s disease and osteosarcoma) or following injury (such as fracture). The disorganization of woven bone results from the speed at which it forms, which precludes the orderly deposition of collagen fibrils. The net result is bone tissue that possesses enhanced flexibility at the cost of stiffness.6 This is valuable following injury as rapid, early formation of woven bone enhances early restoration of skeletal mechanical integrity prior to its replacement by lamellar bone.7
Lamellar bone is characterized by the organized arrangement of collagen fibers into layers or lamellae. This arrangement gives lamellar bone greater stiffness compared to the disorganized structure of woven bone. Lamellae in cortical bone form osteons or bone structural units (BSUs) which consist of a central canal enveloped in concentric lamellae of bone tissue. Outer lamellae form first along the boundary of the osteon known as the cement line, with each successive lamella being laid concentrically inside the preceding one (Figure 2A–C). In trabecular bone, lamellae are stacked into saucer-shaped bone packets that are separated by cement lines. The first lamellae are formed towards the center of the trabeculae with each successive lamella being stacked in parallel layers towards the bone surface (Figure 2D,E). Uniformly spaced throughout lamellae are lenticular cavities called lacunae from which branching canaliculae radiate in all directions. The canaliculae penetrate the lamellae of the interstitial substance to anastomose with canaliculae of neighboring lacunae to form a continuous network of interconnecting cavities.
Bone matrix
Bone matrix is a composite consisting of organic and inorganic components. The organic matrix makes up ~20% of bone wet weight and is comprised primarily of type I collagen which gives bone its flexibility.8 The inorganic matrix contributes approximately ~65–70% of bone wet weight and serves as an ion reservoir. The ions form crystalline structures predominantly in the form of calcium hydroxyapatite [Ca10PO4OH2] that surround and impregnate collagen fibers to give bone the majority of its stiffness.9–10 Without the addition of mineral to collagen, bone tissue would have properties similar to a rubber band, while without collagen, bone is brittle like chalk. Thus, varying the amounts and distribution of collagen and mineral provides bone with its ability to balance its flexibility and stiffness requirements. Alterations in the structure of collagen and/or its mineralization that occur from aging or genetic abnormalities such as osteogenesis imperfecta can compromise the structural integrity of bone tissue resulting in a weaker structure and a greater than normal susceptibility to fracture.
Cellular elements
Bone cells are derived either from hematopoietic stem cells (HSCs) or mesenchymal stem cells (MSCs). HSCs and MSCs give rise to the principal cells that mediate bone resorption (osteoclasts) and formation (including osteoprogenitor cells, osteoblasts, osteocytes and bone lining cells), respectively.
Osteoclasts are large, multinucleate cells that exclusively mediate the process of bone resorption. Osteoclastogenesis begins when a HSC is stimulated to generate mononuclear cells, which then become committed preosteoclasts and are introduced into the blood stream. The circulating precursors exit the peripheral circulation at or near the site to be resorbed, and fuse with one another to form a multinucleated immature osteoclast. Mature osteoclasts establish a microenvironment between themselves and the underlying bone by peripherally attaching to the matrix using integrins.11 The attachment creates a compartment between the ruffled basal border of the osteoclast and the bone surface that is isolated from the general extracellular space.12 An electrogenic proton pump transports in H+ ions to acidify the compartment which acts to mobilize the mineralized component of bone. This exposes the organic matrix which is subsequently degraded using proteases. The end result is the removal of bone matrix and the development of characteristic shallow cavities known as Howship’s lacunae.
Osteoblasts are bone forming cells and develop locally following proliferation of MSCs residing in the bone marrow stroma and periosteum. Mature osteoblasts express the matrix proteins type I collagen and osteocalcin, and alkaline phosphatase—a key enzyme in the mineralization process. Rows of active osteoblasts secrete unmineralized matrix (osteoid) before becoming either bone lining cells or incorporated into the bone matrix. Cells that become incorporated into the matrix gradually develop long cytoplasmic processes to remain in communication with surrounding cells and are considered immature osteocytes. As the matrix matures and mineralizes, and the osteoid seam moves further away, the osteocyte becomes entombed in a bony matrix.
Osteocytes are the most numerous bone cells and are dispersed throughout the matrix where they occupy lacunae (Figure 2B,C). Lacunae are interconnected by an elaborate network of thin tunnels called canaliculi through which osteocytes pass cytoplasmic or dendritic processes.13 These processes connect individual osteocytes with neighboring cells via gap junctions to facilitate both the transport of nutrients for osteocyte viability and the conveying of intercellular messages. Intercellular communication is also facilitated by the osteocytic release of signaling molecules into the extracellular fluid which flows through the lacuna-canalicular system.14–15 Osteocyte function remains unclear; however, their principal role appears to be the sensing of mechanical stimuli.16–17 In addition, recent evidence has also found osteocytes have the capacity to regulate mineral metabolism and alter their surrounding matrix.17–19
Upper extremity bone physiology
Bone is a dynamic tissue capable of altering its structure and mass in order to adapt to changing requirements. The adaptation is achieved by different fundamental tissue-level activities, including growth, modeling, remodeling and healing.
Upper extremity bone growth
Bones of the upper extremity predominantly develop by endochondral ossification wherein condensations of mesenchymal cells differentiate into chondrocytes to form a cartilaginous template (or ‘anlage’). Exceptions are parts of the clavicles and scapulae which form via intramembranous ossification which does not involve a cartilaginous precursor. In the anlage, chondrocytes hypertrophy and an ossification center forms by neovascularization of the initially avascular cartilaginous template. Osteoblasts associated with the newly developed vasculature begin secretion and mineralization of a type-I collagen-containing extracellular matrix. As development continues, the ossification center propagates towards the epiphyseal growth plates.
Epiphyseal growth plates are responsible for longitudinal bone growth. Toward the end of a developing bone, a resting pool of chondrocytes supplies cells to a population of proliferating chondrocytes which in turn differentiate to form a pool of hypertrophic chondrocytes. Ultimately, the hypertrophic chondrocytes die by apoptosis and are replaced by trabecular bone. As long as the rate of chondrocyte proliferation within the growth plate stays ahead of the rate of hypertrophy, the growth plate remains ‘open’ and longitudinal bone growth continues. During this period, the growth plate is a site of relative weakness and susceptible to injury. This is no longer the case towards skeletal maturity when the final chondrocytes in the growth plate hypertrophy and become apoptotic resulting in cessation of longitudinal bone growth and growth plate closure.
Upper extremity bone modeling
Modeling functions to move bone tissue through space altering bone cross-sectional size and shape, as opposed to bone length. It primarily occurs during growth, but continues to some degree throughout life as evidenced by lifelong periosteal expansion.20 Modeling is accomplished by ‘modeling drifts’ whereby bone tissue is selectively added or removed from an existing surface.21 The addition or removal of bone is achieved by the temporally and spatially independent actions of bone forming osteoblasts and bone resorbing osteoclasts, respectively. As formation and resorption during modeling do not occur at the same location, the two processes are said to be ‘uncoupled.’
Modeling is influenced by stimuli including mechanical loading, administration of parathyroid hormone (PTH) and the withdrawal of estrogen, and is an important tissue-level activity as it can alter bone strength without overtly increasing the overall mass of the skeleton. It does this by strategically placing bone tissue where needed most. For instance, periosteal apposition results in a bone with a larger diameter, which is useful as the ability of a bone to resist bending and torsional forces is related to the fourth power of its diameter. By adding material to the outer surface of a bone there is a disproportionate increase in its ability to resist mechanical forces for the gain in mass.
Upper extremity bone remodeling
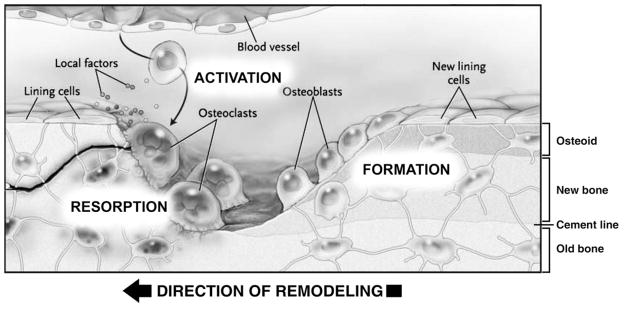
Remodeling represents bone reconstruction wherein discrete, measureable ‘packets’ of bone are removed and replaced by new bone. It occurs continuously throughout life in response to stimuli including mechanical forces, microscopic bone damage (microdamage) and systemic hormones,22 and involves the temporally and spatially coordinated actions of osteoclasts and osteoblasts. These cells form teams collectively known as basic multicellular units (BMUs) (Figure 3). As osteoblasts always trail behind osteoclasts in BMUs and the entire structure moves as a unit, the resorption and formation processes are said to be coupled to one another. Coupling in remodeling is a strictly controlled process which ensures that where bone is removed new bone is deposited.23 The net amount of old bone removed and new bone restored in the remodeling cycle is a quantity called the bone balance.22
Figure 3.
Bone remodeling by a basic multicellular unit (BMU). A stimulus activates osteoclast precursors to differentiate and form an advancing front of actively resorbing osteoclasts. The resorptive bay created by osteoclastic bone resorption is lined by mononuclear cells (not shown) prior to the formation of osteoid (unmineralized bone matrix) by osteoblasts (Reproduced with permission of the Massachusetts Medical Society [©2007; all rights reserved] from Canalis E, et al.: Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 2007;357:905–916)
Although coupling is rarely affected, bone balance varies in disease states. For instance, in osteoporosis, prolonged best rest, or hemi-, para- or quadri-plegia, resorption and formation are coupled but there is a negative bone balance such that more bone is resorbed than is replaced by the typical BMU. The result is a net loss of bone mineral which can be assessed clinically by performing non-invasive bone mass assessments. Logically, many pharmaceutical agents for the treatment of conditions wherein there is a net bone loss attempt to create a positive bone balance whereby bone formation exceeds resorption in a typical BMU. The result is a net gain of bone mineral, which can be achieved either by inhibiting bone resorption (such as occurs with the administration of bisphosphonates therapies) or by stimulating osteoblasts to produce greater quantities of bone (such as occurs with the administration of PTH).24
Upper extremity bone healing
Bone is a frequent site for injury with the most common injury being a fracture. Bone heals in response to injury by regeneration, as opposed to repair. This is an important distinction as regeneration restores the native tissue and mechanical properties at the injured site enabling bone to meet its continuing mechanical demands.
The two major types of bone healing are primary and secondary. Primary or direct healing occurs when the fracture ends are rigidly fixed via early surgical intervention such that there is very little motion between the bone fragments. In this scenario, the need for an early stabilizing external cartilage callus is bypassed with healing occurring by direct synthesis of lamellar bone parallel to the bone’s long axis.25 In contrast, when macro- and micro-motion is permitted between the bone fragments, secondary or indirect healing occurs which involves varying amounts of intramembranous and endochondral bone formation.
Secondary healing of bone fractures is more frequent and follows three overlapping phases: 1) inflammation, 2) reparation, and 3) remodeling. Inflammation begins at the time of injury and initiates the complex cascade of events resulting in appropriate cellular recruitment, timed genetic expression, and the sequenced synthesis of numerous compounds. The subsequent reparative phase combines chondrogenesis and osteogenesis to initially form a bridge (or primary callus) which spans and surrounds the fracture site. The phase involves intramembranous woven bone being laid down underneath the periosteum slightly distant from the fracture gap, and the formation of a large cartilaginous mass both outside (external callus) and within (internal callus) the cortices. The cartilaginous callus serves to stabilize the fracture site which favors subsequent bone formation. The completion of this process results in clinical union. Following union, osteogenesis predominates with the cartilage formed during primary callus formation being replaced with new bone in a process of endochondral ossification that recapitulates bone development. The result is the formation of the secondary or definitive callus and consolidation of the fracture clinically. The final stage of the repair process involves transforming the woven bone to lamellar bone, resorbing the no longer required external callus, and remodeling the bone to form native tissue complete with osteons.25
Factors influencing upper extremity bone health
The preceding sections demonstrate the processes endowing bone with the ability to modify its mass and structure in response to its prevailing environment. Since bone mass and structure determine bone strength, the clinical consequence of alterations in these properties is altered fracture risk. Common conditions altering upper extremity bone health and fracture risk include osteoporosis, motor paralysis and complex regional pain syndrome (CRPS).
Osteoporosis
Osteoporosis is the most significant condition influencing fracture risk within the upper extremity. It is a metabolic bone disease characterized by reduced bone mass and structural deterioration of the skeleton resulting in an elevated low trauma fracture risk. While most attention regarding osteoporosis has focused on fractures of the hip and spine, the upper extremity represents an important site for osteoporosis. Fractures of the humerus and forearm accounted for over one-quarter of osteoporosis-related fractures in the year 2000,26 with the lifetime risk for osteoporosis-related fracture of the humerus and forearm in 45-year-old women being 13.3% and 21.5%, respectively.27 Over the past 3 decades there has been a 3-fold increase in the incidence of osteoporotic fractures of the proximal humerus, with an additional 3-fold increase anticipated over the next 3 decades.28
Osteoporotic fractures of the upper extremity are multi-factorial, and generally result from excessive loads placed onto a mechanically compromised bone. Excessive loads typically result from falls onto an outstretched upper extremity, with recent work suggesting falls are the largest risk factor for osteoporotic fracture.29 However, for a bone to break during an otherwise unremarkable fall, the underlying mechanical properties of the bone must be compromised.
Bone strength is determined by the combination of its material composition and structure. As indicated earlier, there is negative bone balance in remodeling BMUs during aging such that there is a net loss of bone mass. However, as bone loss within single BMUs is small, the loss of bone during aging is determined more by the rate of remodeling (i.e. number of BMUs) than by the magnitude of the negative balance in individual BMUs.30 The increase in the number of remodeling sites during aging increases fracture risk independent of the bone balance within BMUs by: 1) replacing densely mineralized bone with younger, less dense bone, thereby reducing material stiffness; 2) creating regions of stress concentration susceptible to microdamage formation due to the lag time between bone resorption and subsequent formation within a BMU, and; 3) impairing of collagen isomerization and maturation which increases bone fragility.30
The combination of negative bone balance in BMUs and an increase in their frequency ultimately leads to mechanical degradation of the bone, which is particularly prominent following menopause. The estrogen deficiency associated with menopause increases the rate of remodeling, increases the volume of bone resorbed within BMUs by prolonging osteoclast lifespan, and decreases the volume of bone formed within BMUs by reducing osteoblast lifespan.31
Structural decay of the skeleton during aging presents at both trabecular and cortical rich sites. As remodeling occurs on bone surfaces and trabecular bone has more surface than cortical bone, trabecular bone has more remodeling sites per unit volume than cortical bone. Trabecular bone is consequently lost at a higher rate than cortical bone during aging. The trabecular bone loss not only decreases mass, but also reduces trabecular connectivity which produces a greater deficit in bone strength than simple trabecular thinning.32 Once trabeculae begin to disappear, remodeling continues on endocortical and intracortical surfaces resulting in trabecularization of the cortical bone. The net result is a decrease in cortical bone thickness and increase in its porosity, both of which are believed to contribute to osteoporotic fracture risk in the upper extremity.33 Concurrent bone apposition on the bone surface during aging partly offsets the cortical thinning that occurs with aging; however, there is ultimately a net loss of bone compressive and bending strength, and consequent increase in fracture risk.
Motor paralysis
The skeleton is mechanosensitive, and responds and adapts to its mechanical environment. Mechanical loading associated with exercise during growth is principally anabolic stimulating modeling on the periosteal surface. The net result is structural optimization of the skeleton whereby bone material is distributed in such a way that it is better positioned to resist external loads without excessively increasing the overall mass of the skeleton. In the mature skeleton, loading may limit bone loss by reducing negative bone balance within BMUs by decreasing the amount of bone resorbed or increasing the amount of bone formed by each team of cells.
Given the anabolic and anti-resorptive benefits of mechanical loading associated with exercise, it fits that mechanical unloading is detrimental to the skeleton. Muscles are considered a primary source of mechanical stimuli that induce bone adaptation in the upper extremity. Consequently, it is without surprise that a reduction in muscle loading due to motor paralysis presents a significant disturbance to bone.
Bone changes resulting from motor paralysis in children have most commonly been reported in association with cerebral palsy—a condition describing a group of permanent, non-progressive disturbances in the developing brain that contribute to disorders of movement and posture development. Cerebral palsy interferes with skeletal growth and modeling leading to the development of bones with reduced length, mass and size.34 For instance, Demir et al.35 reported children aged 4–8 years with spastic hemiplegia resulting from cerebral palsy to have up to 4.5% shorter upper extremity bones compared to controls, while Golomb et al.36 reported adolescents with severe hemiplegic cerebral palsy to have 39% less radial bone mass and 30% smaller projected bone area in their plegic arm compared with their non-plegic arm. The consequence of these bone deficits is an increase in the risk for low trauma fractures in individuals with cerebral palsy.37
Bone changes resulting from motor paralysis in adults have most commonly be associated with stroke. Stroke refers to the interruption of blood supply to any part of the brain and frequently leads to hemiplegia, which particularly afflicts the upper extremity. In the adult skeleton, motor paralysis interferes with remodeling and stimulates new modeling to reduce bone mass and alter bone structure. For instance, in the first year following stroke bone loss in the proximal humerus of the hemiplegic upper extremity averages 17%, with values as high as 27% in those with severe paralysis.38 The net result is an increase in the risk for low trauma fractures in individuals following a stroke.
Complex regional pain syndrome
CRPS is an important condition impacting upper extremity bone health. Characterized by signs and symptoms of regional pain, discoloration, edema, temperature changes, and decreased function, CRPS typically affects distal regions of the extremities such as the hand. It is classified into type I (reflex sympathetic dystrophy) and type II (causalgia) which occur following minor injuries or fractures and injury to a major peripheral nerve, respectively. The underlying pathology of the two CRPS subtypes is thought to be similar, with the main difference being the presence of nerve damage with type II CRPS.39
Localized reduced bone mass is a recognized consequence of CRPS, and is likely multifactorial resulting from both an increase in osteoclastic bone resorption and decrease in osteoblastic bone formation. Disuse contributes to the observed skeletal changes, with the extent of nerve damage in type II CRPS potentially being particularly important considering the preceding discussion on the skeletal effects of muscle paralysis. In addition to disuse, the bone changes associated with CRPS likely also result from changes within the sympathetic nervous system and sensory neurons which are both involved in the pathology of CRPS.
An intimate relationship exists between the nervous system and bone tissue, with bone being richly innervated by both sympathetic and sensory neurons.40 These nerves serve sensory and vascular functions, but may also influence bone cell activities. For instance, the sympathetic nervous system has been shown to have direct skeletal effects.41 Sympathetic nervous system function is mediated through adrenergic receptors and osteoblasts possess functional adrenergic receptors. Activation of the sympathetic nervous system, such as occurs in CRPS, and subsequent activation of osteoblastic adrenergic receptors results in a loss of bone mass. Conversely, inhibition of adrenergic receptors using beta-adrenergic antagonists enhances bone mass. Bone health in CRPS may also be influenced by unmyelinated sensory neurons which release numerous neuropeptides in the vicinity of bone cells, including vasoactive-intestinal peptide, pituitary adenylate cyclase activating peptides, neuropeptide Y, substance P, and calcitonin gene-related peptide, to name a few. Selective lesioning of unmyelinated sensory neurons negatively influences bone metabolism.42
Conclusion
Bone is a specialized connective tissue that provides the structural framework of the upper extremity. Without stiff, yet still flexible bone tissue providing a means to resist mechanical forces and provide attachment sites for muscles, the hand would not be able to perform its many essential functions. Consequently, it is important for therapists to recognize the importance of upper extremity bone health. By understanding skeletal structure and the underlying means by which bone tissue is capable of responding and adapting to its prevailing environment, therapists may be able to develop novel therapies to offset the skeletal consequences of different conditions afflicting the upper extremity.
Acknowledgments
This contribution was made possible by support from the National Institutes of Health (R01 AR057740 and R15 AR056858 [S.J.W.]; K01 AR054408 [R.K.F.])
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melton LJ, III, Christen D, Riggs BL, et al. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int. 2010 Jul;21(7):1161–1169. doi: 10.1007/s00198-009-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mow VC, Ratcliffe A, Woo SL-Y. Biomechanics of Diarthrodial Joints. New York: Springer; 1990. [Google Scholar]
- 3.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008 Feb;23(2):205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005 Apr 1;105(7):2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 5.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006 May;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currey JD. The many adaptations of bone. J Biomech. 2003 Oct;36(10):1487–1495. doi: 10.1016/s0021-9290(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 7.Silva MJ, Touhey DC. Bone formation after damaging in vivo fatigue loading results in recovery of whole-bone monotonic strength and increased fatigue life. J Orthop Res. 2007 Feb;25(2):252–261. doi: 10.1002/jor.20320. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371–386. [PubMed] [Google Scholar]
- 9.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech. 1996 Feb 1;33(2):192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Landis WJ, Hodgens KJ, Song MJ, et al. Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol. 1996 Jul-Aug;117(1):24–35. doi: 10.1006/jsbi.1996.0066. [DOI] [PubMed] [Google Scholar]
- 11.McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000 Feb;105(4):433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000 Sep 1;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 13.Hirose S, Li M, Kojima T, et al. A histological assessment on the distribution of the osteocytic lacunar canalicular system using silver staining. J Bone Miner Metab. 2007;25(6):374–382. doi: 10.1007/s00774-007-0764-x. [DOI] [PubMed] [Google Scholar]
- 14.Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006 Oct;3(10):7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Wang Y, Han Y, et al. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11911–11916. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2007 Dec 17; doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 17.Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007 Jun;5(6):464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006 Mar;21(3):466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006 Nov;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003 Jul 24;349(4):327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 21.Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff's law: the bone modeling problem. Anatomical Record. 1990;226:403–413. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]
- 22.Dempster D. Bone modeling and remodeling. In: Dempster D, Felsenberg D, van Der Geest S, editors. The Bone Quality Book: A Guide to Factors Influencing Bone Strength. Amsterdam, Netherlands: Excerpta Medica; 2006. pp. 64–73. [Google Scholar]
- 23.Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000 Apr;26(4):319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- 24.Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005 Aug;26(5):688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro F. Bone development and its relation to fracture repair: the role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 26.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006 Dec;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 27.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11(8):669–674. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 28.Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop Relat Res. 2006 Jan;442:87–92. doi: 10.1097/01.blo.0000194672.79634.78. [DOI] [PubMed] [Google Scholar]
- 29.Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008 Jan 19;336(7636):124–126. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006 May 25;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 31.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000 Apr;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 32.van der Linden JC, Homminga J, Verhaar JA, Weinans H. Mechanical consequences of bone loss in cancellous bone. J Bone Miner Res. 2001 Mar;16(3):457–465. doi: 10.1359/jbmr.2001.16.3.457. [DOI] [PubMed] [Google Scholar]
- 33.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 34.Houlihan CM, Stevenson RD. Bone density in cerebral palsy. Phys Med Rehabil Clin N Am. 2009 Aug;20(3):493–508. doi: 10.1016/j.pmr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demir SO, Oktay F, Uysal H, Seluk B. Upper extremity shortness in children with hemiplegic cerebral palsy. J Pediatr Orthop. 2006 Nov-Dec;26(6):764–768. doi: 10.1097/01.bpo.0000235393.34289.82. [DOI] [PubMed] [Google Scholar]
- 36.Golomb MR, McDonald BC, Warden SJ, et al. In-home virtual reality videogame telerehabilitation in adolescents with hemiplegic cerebral palsy. Arch Phys Med Rehabil. 2010 Jan;91(1):1–8 e1. doi: 10.1016/j.apmr.2009.08.153. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson RD, Conaway M, Barrington JW, Cuthill SL, Worley G, Henderson RC. Fracture rate in children with cerebral palsy. Pediatr Rehabil. 2006 Oct-Dec;9(4):396–403. doi: 10.1080/13638490600668061. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000;11(5):381–387. doi: 10.1007/s001980070103. [DOI] [PubMed] [Google Scholar]
- 39.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010 Sep;113(3):713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 40.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 41.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002 Nov 1;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 42.Offley SC, Guo TZ, Wei T, et al. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005 Feb;20(2):257–267. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]