Abstract
GD12 is a murine monoclonal IgG1 (mAb) that recognizes an immunodominant linear neutralizing epitope (163-TLARSFIICIQM-174) on the A subunit (RTA) of ricin toxin. With the long-term goal of using GD12 as a potential countermeasure against ricin intoxication, we have produced a chimeric derivative of GD12 (cGD12) in which the murine heavy and light chain variable regions were fused to a human IgG1 framework. The chimeric mAb, expressed and purified using a Nicotiana-based system retained epitope specificity and ricin neutralizing activity similar to the parental murine mAb. Passive administration of cGD12 (10 μg) to mice by intraperitoneal injection protected the animals against a systemic 5 LD50 ricin challenge. In a post-exposure setting, the murine and chimeric mAbs administered as much as 6 h after toxin challenge were each capable of rescuing mice from toxin-induced death, revealing the potential of GD12 to serve as both a prophylactic and therapeutic for ricin intoxication.
Keywords: antibody, toxin, biodefense, therapeutics
1. Introduction
Ricin is a member of the type II ribosome-inactivating protein (RIP) family of toxins that includes abrin, Shiga toxin from Shigella dysenteriae, and Shiga-like toxins from Escherichia coli [1, 2]. Because the toxin can be lethal to humans following injection, inhalation and possibly ingestion, ricin has been classified by the Centers for Disease Control and Prevention as a Category B biothreat agent. The mature form of ricin consists of two subunits. The A-subunit (RTA,267 amino acids) is a RNA N-glycosidase, which mediates the selective depurination of a conserved adenosine residue in the so-called sarcin/ricin loop (SRL) of 28S ribosomal RNA [3]. The B-subunit (RTB, 262 amino acids) is a lectin specific for β1,3-linked galactose and N-acetylgalactosamine (Gal/GalNac) residues on both glycolipids and glycoproteins on the surface of cells [4].
We are interested in the development of an antibody-based therapeutic for treatment of individuals following exposure to ricin via the systemic or mucosal routes. Towards achieving this long term goal, we recently produced and characterized a collection of ricin-neutralizing monoclonal antibodies (mAbs) against both RTA and RTB [5-8]. One of these mAbs, GD12, is of particular interest because it recognizes a linear (continuous) epitope (TL163-I174) on RTA that is known to be immunodominant in humans [6, 9]. GD12 is also sufficient to passively protect mice against lethal doses of ricin administered by systemic (intraperitoneal) or mucosal (intragastric) routes [6]. Furthermore, GD12 is one of the most potent anti-RTA mAbs identified to date [10-13].
In this communication, we describe the production and characterization of a chimeric derivative of GD12 (cGD12) in which the murine heavy (VH) and light chain (VL) variable regions were fused to a human IgG1 framework. The chimeric mAb was expressed in a Nicotiana-based system, which results in the rapid (days) production of extremely high amounts of mAbs. We report that cGD12 is capable of rescuing mice from a 10 LD50 challenge with ricin, when the mAb was administered within 6 h following toxin challenge. To our knowledge, GD12 is the first partially humanized anti-ricin mAb with demonstrated capacity to serve as an immunotherapeutic in response to a high dose ricin challenge.
2. Materials and Methods
2.1 Construction of chimeric GD12 expression vectors
The VH and VL coding sequences were amplified by PCR using murine specific primers [14]. The VH and VL, GD12 deduced amino acid sequences were fused to a unique N-terminal murine signal peptide (SP) sequence (MGWSWIFLFLLSGAAGVHC) known to function in the Nicotiana expression system. The SP+VH and SP+VL sequences were then grafted on to human constant γ1(γ1CR) and κ (κCR) regions. Complete HC (SP+VH +γ1CR) and LC (SP+VL+κCR) sequences were cloned into Nicotiana expression vectors and transformed into Agrobacterium tumefaciens [15].
2.2 Expression and purification of cGD12
Two unique cultures of Agrobacterium tumefaciens, each transfected with either the HC or LC expression vectors, were grown as described [15] and co-infiltrated into 4-6 week old transgenic Nicotiana benthamiana plants lacking plant-specific N-glycan residues [16]. Eight days post-infiltration, the leaf tissue was extracted in a juicer (Model GS-1000, Green Star, Tribest Corp., Anaheim, CA), using 25 ml of chilled extraction buffer (100mM Tris, 40 mM ascorbic acid, 1mM EDTA) per 100 g of green leaf tissue. The plant-derived extract was clarified by lowering the pH of the extract to pH 4.8 with 1 M phosphoric acid then re-adjusting it to pH 7.5 with 2 M Tris base to insolubilize plant debris. The mixture was then subjected to centrifugation at 16,000 × g for 30 min. The resulting supernatant was then subjected to a second round of centrifugation under the same conditions. The clarified extract was filtered through a 0.2 μm filter prior to concentration via Minim Tangential Flow Filtration System (Pall, Port Washington, NY) then 0.2 μm filtered again before loading onto a 5 ml HiTrap MabSelect SuRe Protein A column (GE Healthcare, Piscataway, NJ) at 2 ml/min. The column then was washed with running buffer (50 mM HEPES/100 mM NaCl, pH 7.5) and eluted with 0.1 M acetic acid, pH 3.0. The resulting eluate was neutralized to pH 7 using 2 M Tris, pH 8.0 and supplemented with Tween 80 to 0.01%. The mAb solution was then polished via Q filtration (Mustang Acrodisc Q membrane; Pall), aliquoted and stored at −80 °C.
2.3 ELISA, pepscan analysis and SPR
Ricin, RTA, and RTB were purchased from Vector Laboratories (Burlingame, CA). ELISA and peptide array analysis were performed exactly as described previously [6, 7], except that detection of cGD12 was achieved using horseradish peroxidase (HRP)-labeled goat polyclonal anti-human IgG-specific secondary antibodies (Southern Biotech, Birmingham, AL). The affinity of cGD12 for ricin toxin was determined by surface Plasmon resonance (SPR) using a Biacore 3000 (GE Healthcare) with ricin attached to a CM5 chip surface, as described [7].
2.3 Ricin cytotoxicity assays
Vero cell cytotoxicity assays were performed as previously described [6, 7]. All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only.
2.4 Passive protection studies
Murine GD12 (mGD12) or cGD12 (0.2 or 0.4 ml final volume) was administered to 8-12 week old female BALB/c mice (Taconic Labs, Hudson, NY) by intraperitoneal (i.p) injection, at specific time points before or after the animals had received a single i.p. injection of ricin toxin (50 μg/kg or 100 ug/kg). Blood glucose levels, used as a surrogate makers of intoxication, and survival were measured daily over a 3-day period [7, 17]. Mice were euthanized when they became overtly moribund and/or blood glucose levels fell below 25 mg/dl. Animals were housed under conventional, specific pathogen-free conditions and were treated in compliance with the Wadsworth Center‘s Institutional Animal Care and Use Committee (IACUC) guidelines. Statistical analysis and graphic representation of the data was performed using GraphPad Prism version 5.00 for Macintosh (GraphPad Software, San Diego California).
3. Results
3.1 In vitro characterization of cGD12
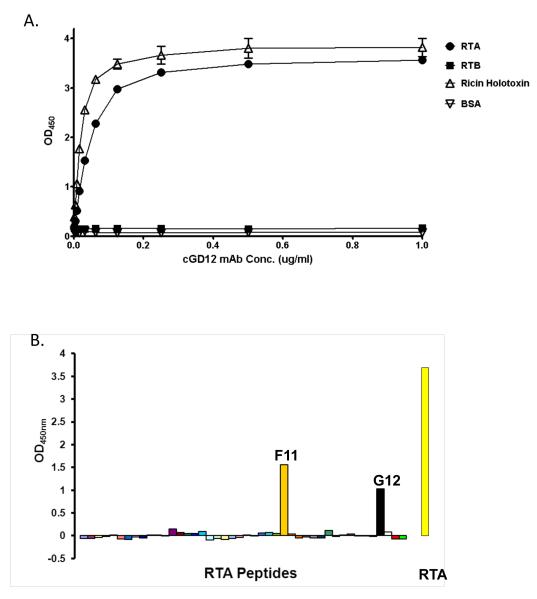
We produced a chimeric derivative of GD12 in which the murine heavy and light chain variable regions were fused to a human IgG1 framework. The chimeric mAb was expressed and purified using a Nicotiana-based system, as described in the Materials and Methods. To validate the specificity of cGD12, we examined the reactivity of the chimeric mAb with ricin holotoxin, RTA, and RTB by ELISA. As expected, cGD12 bound to ricin holotoxin and RTA, but not to RTB (Fig. 1A). The actual affinity of cGD12 for ricin was determined by Biacore analysis, which revealed that cGD12 had an association constant (KA) of 1.3 × 107 M−1 and a dissociation constant (KD) of 7.8 × 10−8 (Table 1). Thus, the affinity of cGD12 for ricin was slightly less than that of its murine counterpart. As confirmation of the mAb‘s epitope specificity, cGD12 was used to probe a peptide array consisting of 44 overlapping 12-mers that collectively span the length of the RTA sequence. cGD12 preferentially bound peptide “F11”, corresponding to residues T163-M174 (Fig. 1B) [6]. Like it‘s murine counterpart, cGD12 also has demonstrable reactivity with an isoleucine-rich peptide towards the C-terminus of RTA.
Figure 1. Epitope specificity of cGD12.
(A) Reactivity of cGD12 with ricin holotoxin, RTA, RTB, or BSA, as determined by ELISA. Microtiter plates were coated with 0.1 μg/well of each of the target antigens. Each datum point represents the average value of two replicate wells, with error bars representing the standard deviation from the mean. (B) Reactivity of cGD12 with an RTA peptide array. ELISA plates were coated with 44 overlapping 12-mer peptides spanning the length of RTA. cGD12 bound preferentially to peptide “F11” (gold), encompassing residues T163-M174 (TLARSFIICIQM), but showed additional reactivity with “G12” (black), a particularly isoleucine rich peptide (SVYDVSILIPII).
Table 1.
Affinities of murine and chimeric GD12 for ricin holotoxin
| mAb | KD (M) | KA (M−1) |
|---|---|---|
| mGD12 | 2.9 × 10−9 | 3.5 × 108 |
| cGD12 | 7.8 × 10−8 | 1.3 × 107 |
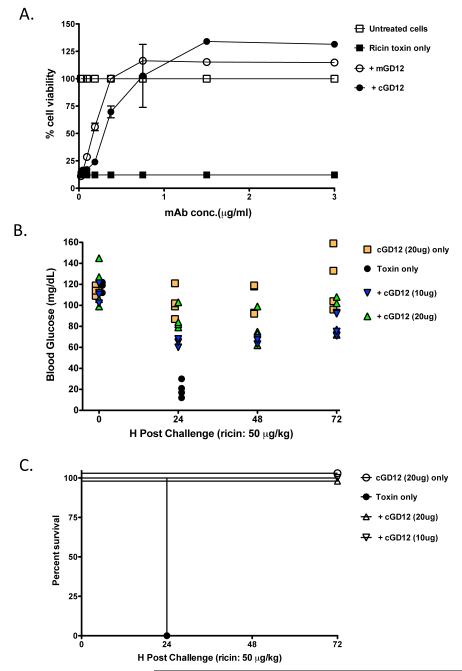
To assess the capacity of the chimeric mAb to neutralize ricin in vitro, we combined ricin (10 ng/ml) with cGD12 at a range of concentrations, and then applied this mixture in triplicate to Vero cells grown in 96-well microtiter plates. Murine GD12 protected Vero cells from the cytotoxic effects of ricin in a dose-dependent manner, and had an estimated IC50 of ~0.25 μg/ml (Fig. 2A), in accordance with what has been reported previously [6]. The chimeric mAb was marginally less effective than the murine counterpart with an estimated IC50 of ~0.3 μg /ml, possibly because of slight differences in relative affinities for ricin holotoxin. Nonetheless, cGD12‘s capacity to neutralize ricin is still considered among one of the best described to date.
Figure 2. Neutralizing and protective capacity of cGD12.
(A) Mouse or chimeric GD12, at the indicated concentrations, were mixed with ricin (10 ng/ml) for 1 h, and then applied in triplicate to Vero cells grown in 96-well microtiter plates. Cell viability was assessed 48 h later. Each data point represents the average of three replicate wells. The error bars represent the standard deviation. (B, C) Female BALB/c mice (n=4 per group) were passively immunized with cGD12 (20 or 10 μg/ animal) and then challenged 24 h later with 5 LD50s of ricin (50 μg/kg). (B) Blood glucose levels in the groups of mice were determined at time zero and 24 h intervals following ricin challenge. (C) The percent survival of mice described in the experiment in panel B was assessed at 24 hr intervals.
3.2 In vivo characterization of cGD12
We used an established mouse model of systemic ricin intoxication to assess the capacity of cGD12 to neutralize ricin in vivo [6, 17]. For these challenge studies, hypoglycemia was used as a quantitative measure of ricin intoxication [17]. Monoclonal antibody cGD12 was passively administered to female BALB/c mice (10 or 20 μg/animal) by i.p injection. Twenty-four hours later, the animals were challenged by i.p. injection with the equivalent of 5 LD50 of ricin (50 μg/kg) and blood glucose levels were subsequently assessed at 24 h intervals. Ricin-challenged control mice experienced a dramatic decline in blood glucose levels within 24 h and subsequently died or were euthanized (Fig. 2B, C). cGD12-treated animals, on the other hand, experienced only a moderate reduction in blood glucose levels and all survived toxin challenge. In fact, in the case of the group of mice given 20 μg of cGD12, blood glucose levels were not significantly different before and 24 h post ricin challenge, as determined by a paired t-test.
3.3 Post exposure therapeutic assessment of murine and cGD12
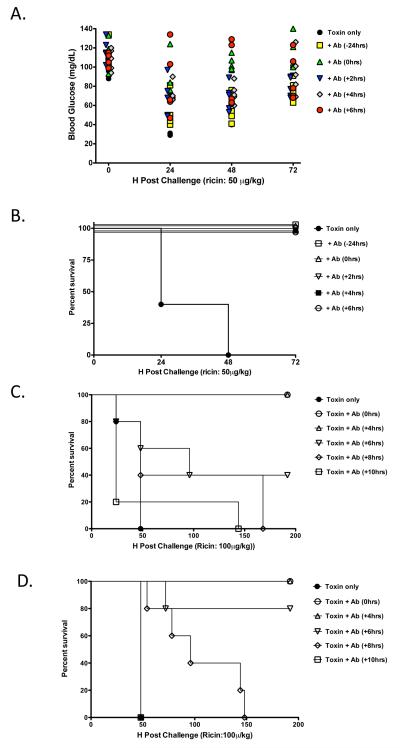
To examine the degree to which GD12 can rescue mice from ricin intoxication, groups of animals were administered the murine version of GD12 (10 μg/animal) by the i.p. route at 0, 2, 4 or 6 h post systemic challenge with 5 LD50 of ricin. Control mice succumbed to ricin intoxication within 48 h. In contrast, mice treated with mGD12 at all time points tested experienced only a minimal drop in blood glucose levels and were completely protected from toxin-induced death (Fig.3A, B). These data reveal the potential of GD12 to function as a post exposure therapeutic.
Figure 3. Capacity of murine and chimeric GD12 to rescue mice from ricin toxin-induced death.
Female BALB/c mice (n=5 per group) were administered mGD12 (A,B) or cGD12 (C,D) before, concurrently, or at intervals after ricin challenge. (A) Blood glucose levels in the groups of mice treated with mGD12 (10 μg/animal) were determined at time 0 and then at 24 h intervals following challenge with 5 LD50 of ricin. Each symbol represents an individual mouse. (B) Percent survival of mice described in Panel A. (C-D) Protection afforded by cGD12. BALB/c mice were administered cGD12 at (C) 100 μg/animal or (D) 200 μg/animal concurrently or at indicated intervals after challenge with 10 LD50 of ricin.
Having established that mGD12 was capable of rescuing mice up to 6 h post ricin exposure, we next sought to establish the degree to which cGD12 can protect mice when administered at clinically-relevant concentrations [18] and against a more stringent toxin challenge [10]. Groups of mice were administered cGD12 at doses of 100 or 200 μg/animal by i.p. injection at 0, 4, 6, 8 or 10 h after ricin (10 LD50) challenge (Fig. 3 C,D). Control mice that received ricin only had a mean survival time of 48 h. It should be noted that survival curves for toxin control mice given 5 LD50 of ricin were not significantly different from those given 10 LD50 of ricin, as determined by a Gehan-Breslow-Wilcoxon Test.
Administration of 100 μg of cGD12 to mice at times 0 or 4 hr post ricin challenge was sufficient to completely protect the animals from toxin-induced death. Administration of cGD12 at 6 hr post ricin challenge conferred partial protection (2/5 survived) and extended the mean time to death to 96 h. Delivery of 100 μg of cGD12 at 8 or 10 h post-ricin challenge offered no measureable protection or increase in mean survival time (Fig. 3C). Doubling the amount of cGD12 that was administered to the mice afforded greater protection and increased survival time. In particular, 4/5 mice that received 200 μg of cGD12 at 6 hr post-ricin challenge survived toxin-induced death, and the mean time to death in the group of mice administered cGD12 8 hr after toxin challenge was extended to 96 h (Fig. 3D). These data demonstrate a clear dose-dependent benefit of cGD12 as a therapeutic in response to a high-dose ricin challenge. Based on these results, we estimate that cGD12 protected mice at a molar toxin:antibody ratio of ~1:43, which is almost 7 times more effective than other murine mAbs tested in a post-exposure challenge mouse model [12].
4. Discussion
There is an urgent need for the development of countermeasures against potential biothreat agents, particularly highly lethal and fast-acting toxins like ricin [19]. The murine mAb GD12 was first identified as recognizing a human immunodomainant linear B-cell epitope on RTA, defined as residues 163-TLARSFIICIQM-174. GD12 proved to be highly effective at neutralizing ricin in vitro and in conferring passive protection to mice against systemic and mucosal ricin challenge. To explore GD12 as a post exposure therapeutic, we produced and characterized a chimeric derivative of GD12 in which the murine VH and VL regions were fused to a human IgG1 framework. The chimeric mAb was successfully expressed in a Nicotiana-based system and was nearly as capable as its murine counterpart in neutralizing ricin in vitro and in vivo. More importantly, when administered as much as 4 h following challenge with 10 LD50 of ricin, cGD12 proved completely effective at protecting mice against toxin-induced death. GD12 is therefore the first partially humanized anti-ricin mAb with the potential capacity to serve as an immunotherapeutic in response to a high dose ricin challenge.
Several specific attributes of cGD12 make it an appealing candidate for further development as a therapeutic for ricin intoxication. First, both the murine and chimeric versions of GD12 were extremely effective at neutralizing ricin in vitro and in vivo. In particular, the murine version of GD12 was capable of protecting mice at a toxin:mAb molar ratio of ~1:4, as evidenced by the fact that 10 μg of mGD12 was sufficient to protect mice against a challenge of 1 μg of ricin, which was equivalent to 5 LD50 in our model. Thus, mGD12 is roughly 2 times more effective than RAC18, 15 times more effective than R70, and possibly 70 times more effective than a cocktail of RTA- and RTB-specific mAbs at inactivating ricin in a mouse model [10, 12, 13]. Although cGD12 was slightly less potent at neutralizing ricin than was its murine counterpart, it was still highly effective at rescuing mice from ricin intoxication. We are currently producing different glycoforms and subclasses of cGD12 in an effort to enhance the mAb‘s ability to neutralize ricin.
Chimeric GD12 also has the added benefit over other ricin-specific mAbs of being expressed in a Nicotiana-based expression system [15, 18]. As compared to conventional mammalian-based expression systems, expression in Nicotiana results in the production of extremely high amounts of mAbs in a matter of days, especially with the recent advent of viral-based transient expression vectors. Second, the availability of transgenic plants with altered glycosylation pathways enables the production of mAbs with mammalian glycoforms. Finally, large scale manufacturing of antibody under current Good Manufacturing Practice is now routine [20]. Thus, development of partially humanized antibody based therapeutics against biothreat agents such as ricin via this plant based method offers a rapid, versatile, low cost and large capacity system to rapidly address a bioterror event.
While we demonstrated in this report that cGD12 mAb can rescue mice from ricin intoxication when administered up to 4 h post-toxin exposure, we expect that this therapeutic window could be extended by increasing the amount of antibody and/or co-administering cGD12 with one or more other ricin-neutralizing antibodies. Of particular interest would be combining cGD12 with mAbs that target other immunodominant regions of RTA, as there is evidence that they may neutralize ricin by different mechanisms from GD12 [7]. Alternatively, cGD12 could be mixed with one or more RTB-specific mAbs [8]. There is evidence that RTB-specific mAbs can act synergistically with RTA-specific mAbs to neutralize ricin [5]. Thus, we are currently investigating the use of a partially humanized neutralizing anti-RTB mAb in combination with cGD12.
Finally, the partially humanized GD12 may prove valuable as a RTA-specific human IgG standard as serum samples from clinical trials of two leading ricin vaccines are underway [21]. Assessing ricin-specific antibody titers and toxin neutralizing activities in the sera of immunized individuals will serve as the sole indicator of vaccine efficacy in humans. At the present time there is currently no accepted standardized ricin ELISA protocols or cytotoxicity assays within the ricin scientific-clinical community. In order to enable comparative analysis of vaccine efficacies, it is imperative that a single standard be adopted across institutions.
Highlights.
>GD12 is a murine monoclonal antibody that protects mice from ricin toxin.
>A partially humanized version of GD12 was produced in plants.
>Chimeric GD12 neutralizes ricin toxin in vitro and in vivo.
> Chimeric GD12 protected mice when administered 6 hr post ricin challenge.
Acknowledgements
We gratefully acknowledge Dr. Jane Kasten-Jolly of the Wadsworth Center Immunology Core facility for assistance with antibody affinity analysis by SPR, and Dr. Karen Chave of the Wadsworth Center Protein Expression Core for assistance in the purification of murine GD12. We would also like to extend our special thanks to Anastasyia Yermakova, Chrystal Chadwick, Dr. David Vance, and Dr. Sarita Ahlawat for their assistance in the ricin challenge studies. This work was supported by grant AI091078 from the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004 Sep 15;44(4):361–70. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [2].Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004 Sep 15;44(4):371–83. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [3].Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–12. [PubMed] [Google Scholar]
- [4].Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. JBiolChem. 1979;254(19):9795–9. [PubMed] [Google Scholar]
- [5].McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006 Jun;74(6):3463–70. doi: 10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Neal LM, O’Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010 Jan;78(1):552–61. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O’Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010 Aug 18; doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yermakova A, Mantis NJ. Protective immunity to ricin toxin conferred by antibodies against the toxin’s binding subunit (RTB) Vaccine. 2011 Aug 25; doi: 10.1016/j.vaccine.2011.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, et al. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin’s lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol. 2004 May;136(2):365–72. doi: 10.1111/j.1365-2249.2004.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–21. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- [11].Pratt TS, Pincus SH, Hale ML, Moreira AL, Roy CJ, Tchou-Wong KM. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A-chain for post-exposure treatment. Exp Lung Res. 2007 Oct-Nov;33(8-9):459–81. doi: 10.1080/01902140701731805. [DOI] [PubMed] [Google Scholar]
- [12].Prigent J, Panigai L, Lamourette P, Sauvaire D, Devilliers K, Plaisance M, et al. Neutralising antibodies against ricin toxin. PLoS One. 2011;6(5):e20166. doi: 10.1371/journal.pone.0020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, et al. Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab Invest. 2008 Nov;88(11):1178–91. doi: 10.1038/labinvest.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Essono S, Frobert Y, Grassi J, CrÈminon C, Boquet D. A general method allowing the design of oligonucleotide primers to amplify the variable regions from immunoglobulin cDNA. Journal of Immunological Methods. 2003;279(1-2):251–66. doi: 10.1016/s0022-1759(03)00242-4. [DOI] [PubMed] [Google Scholar]
- [15].Hiatt A, Pauly M. Monoclonal antibodies from plants: A new speed record. Proceedings of the National Academy of Sciences. 2006 October 3;103(40):14645–6. doi: 10.1073/pnas.0607089103. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnology Journal. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- [17].Pincus SH, Eng L, Cooke CL, Maddaloni M. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp Med. 2002 Dec;52(6):530–3. [PubMed] [Google Scholar]
- [18].Whaley KJ, Hiatt A, Zeitlin L. Emerging antibody products and Nicotiana manufacturing. Hum Vaccin. 2011 Mar 1;7(3) doi: 10.4161/hv.7.3.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mantis NJ, Morici LA, Roy CJ. Mucosal Vaccines for Biodefense. Curr Top Microbiol Immunol. 2011 Apr 3; doi: 10.1007/82_2011_122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J. 2010 Jun;8(5):638–54. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- [21].Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2268–73. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]