Abstract
Glutamate (Glu) exhibits a pH and concentration dependent chemical exchange saturation transfer effect (CEST) between its -amine group and bulk water, here termed GluCEST. GluCEST asymmetry is observed at ~3 parts per million downfield from bulk water. Following middle cerebral artery occlusion in the rat brain, an approximately 100% elevation of GluCEST in the ipsilateral side compared to the contralateral side was observed, and is predominantly due to pH changes. In a rat brain tumor model with blood brain barrier disruption, intravenous Glu injection resulted in a clear elevation of GluCEST and a comparable increase in the proton magnetic resonance spectroscopy signal of Glu. GluCEST maps from healthy human brain at 7T were also obtained. These results demonstrate the feasibility and potential of GluCEST for mapping relative changes in Glu concentration as well as pH in vivo. Potential contributions from other brain metabolites to the GluCEST effect are also discussed.
INTRODUCTION
Glutamate (Glu) and gamma-aminobutyric acid (GABA) are the major excitatory and inhibitory neurotransmitters in the brain, respectively, and are likely involved in nearly all signal processing functions of the central nervous system (CNS) as well as being altered in many CNS diseases1–4. Magnetic resonance imaging (MRI) is a noninvasive imaging technique that provides exquisite structural detail, but current MRI methods are not capable of imaging the distribution of neurotransmitters in brain.
Proton Magnetic Resonance Spectroscopy (1HMRS) can detect several neurotransmitter signature groups using a variety of techniques 5–10.However, 1HMRS techniques require long acquisition times and have poor spatial resolution. Here we describe a novel MRI technique for imaging Glu (Unpublished results; R.R., K.C., M.H., A.S., F.K., H.H., Invention disclosure, University of Pennsylvania, Philadelphia, PA, September 3, 2009) based on its chemical exchange saturation transfer (CEST)11–15 effect (GluCEST) that provides markedly increased spatial and temporal resolution than 1HMRS. The CEST effect from amide and hydroxyl protons from different amino acids, proteins and other molecules12,16,17 have previously been exploited to measure pH in vivo18,19, glycogen in the liver20, glycosaminoglycans in cartilage21, gene expression in vivo22, and myo-inositol in brain,23 but no prior studies have used the CEST effect of amineprotons to image Glu in vivo.
In the current study, the pH and concentration dependence of GluCEST is first demonstrated in vitro at 37 °C. Second, the feasibility of measuring GluCEST in vivo from rat brain with and without focal ischemia is shown. Third, changes in GluCEST and Glu 1HMRS in response to exogenous injection of Glu in a rat brain tumor model are compared. Finally, the feasibility of mapping the Glu signal from healthy human brain in vivo is demonstrated at 7T. The potential contributions to GluCEST from other brain metabolites as well as the advantages and shortcomings of this approach are also discussed.
RESULTS
Phantom Studies
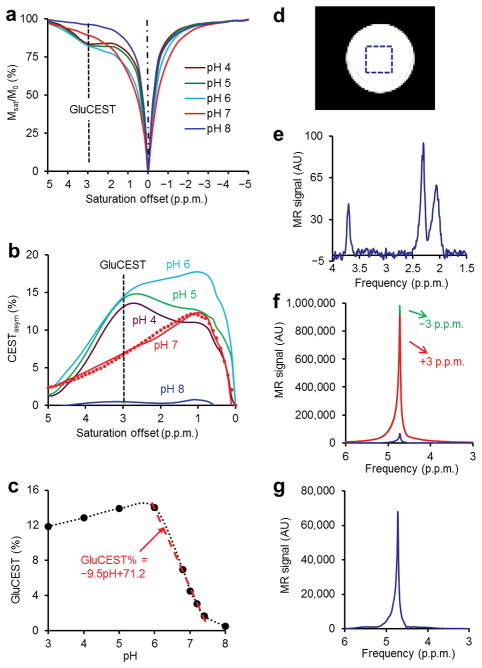
The z-spectra of 10 mM Glu at varying pH demonstrate that at lower pH (< pH 6.0), the CEST peak of Glu is sharper and centered around 3 parts per million (p.p.m.) downfield to the bulk water resonance (absolute chemical shift 7.7 p.p.m.) (Fig.1a). The z-spectral asymmetry plots (Fig.1b) exhibit a broad CEST effect ranging from 1 to 4 p.p.m. from bulk water resonance. While the GluCEST asymmetry plots from low pH solutions demonstrate clear peaks centered at ~3.00 p.p.m., the peak position of asymmetry plots from higher pH solutions (~6.0) gradually moved higher field towards water resonance. At pH 7, the maximum of asymmetry plot occurred at ~1.2 p.p.m. and its amplitude is ~100% higher than at 3 p.p.m.. This is a well-known phenomenon of intermediate to fast exchange (k ≤ Δω) mediated chemical shift averaging. Despite the shift in peak position at pH 7, all studies reported in this work still use 3 p.p.m. as the GluCEST resonance saturation frequency to maintain consistency.
Figure 1.
pH dependence and sensitivity advantage of GluCEST. (a) CEST z-spectra of 10 mM Glu at varying pH and 37°C at 7T show the CEST effect at 3 p.p.m. downfield to bulk water resonance. (b) GluCEST asymmetry curves from different pH solutions corresponding to (a). The overlay of simulated GluCEST (dotted line) on the curve corresponding to pH 7 is also shown. (c) This plot shows dependence of GluCEST on pH (3–8). The fitted line shows linear dependence of GluCEST in physiological pH range (6.0–7.4). (d) Image from a phantom of 10 mM glutamate (pH = 7.0, 37°C) prepared in PBS with the localization voxel indicated. (e) 1HMRS PRESS water suppressed spectrum (TR = 20 s, TE = 16 ms, 4 averages) from the single voxel shown in the image (d). The three resonances correspond to two –CH2 groups and one –CH group of glutamate. (f) Water 1H resonance spectra obtained while saturating at ± 3 p.p.m. as well as their difference spectrum. (g) The rescaled difference spectrum from (f). The difference spectrum represents the 3 p.p.m. point on the CEST asymmetry spectrum. The ratio of the CEST difference spectrum to Glu –CH2 resonance at 2.3 p.p.m. is ~700.
Due to non steady-state experimental conditions, the sensitivity of GluCEST was analyzed by solving numerical simulations of full Bloch-McConnell equations24 using the experimental parameters from these studies. We found an excellent agreement between the experimental z-spectral asymmetry curves and numerically simulated ones. Since the intracellular pH (pHi) in the brain of normal healthy volunteers is reported to be around 7.025, the GluCEST contrast at this pH is the most relevant for in vivo imaging. Based on the fitted z-spectral asymmetry curve from simulations (Fig. 1b), the amine proton exchange rate (k) in a 10 mM Glu (pH 7.0) solution at 37°C was estimated to be in the range of 5,500 ± 500 s−1. Since this is of the order of the chemical shift difference (Δω) at 7T (Δω= 5,700rad s−1), GluCEST imaging is feasible at 7T as well as at higher fields. For the experimental saturation parameters used in this study, GluCEST increased gradually from pH 3 to pH 6 and then sharply decreased from pH 6 to 8. The largest GluCEST observed at pH 6 is likely due to the estimated reduced exchange rate of amine protons with the bulk water. GluCEST varies linearly with pH in the physiological range (6.0–7.4) (Fig. 1c).
There is a ~700 fold sensitivity advantage of GluCEST over conventional 1HMRS at physiological concentration and temperature (Fig.1d–g). This sensitivity amplification should make it feasible to detect relatively small changes in Glu levels at high resolution (voxel size of 0.02 cc).
The GluCEST effect is linearly proportional to the Glu concentration ([Glu]) in the physiological range at pH 7 (Fig. 2a). With the imaging parameters used here, a ~0.6% CEST effect increase mM−1 [Glu] was observed (Fig. 2b). The GluCEST effect increased with peak B1 amplitude and saturation pulse duration demonstrating that it is possible to obtain higher GluCEST effects (> 1% mM−1) (Fig. 2c).
Figure 2.
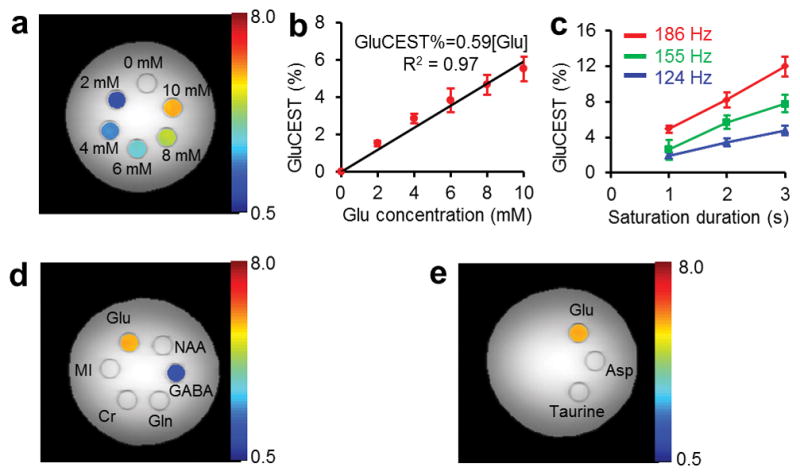
GluCEST images at 7T of a phantom consisting of test tubes with different concentrations of Glu solutions (pH 7.0) immersed in a beaker containing PBS. All the experiments were performed at 37 °C (a) Shows the GluCEST contrast color-coded on the original CEST image (3 p.p.m.), acquired with application of saturation pulse train with B1rms = 155 Hz (3.6 μT) for 2 s. Color bar represents GluCEST contrast in percentage. (b) Approximately linear dependence of GluCEST effect on Glu concentration with a slope of 0.6% mM−1 Glu. (c) GluCEST dependence on B1rms and duration of the saturation pulse. (d,e) CEST images of a phantom consisting of test tubes with solution of different metabolites at their physiological concentrations and pH 7 [Glu (10 mM), GABA (2 mM), NAA (10 mM), Gln (2 mM), Asp (2 mM), taurine (2 mM), Cr (6 mM) and MI (10 mM)] immersed in a beaker containing PBS. The CEST contrast color-coded on the original CEST images (3 p.p.m.), which show the substantial CEST contrast from Glu (~6%), and ~1% from GABA, < 0.5% from Cr and no contrast from other metabolites. Color bar represents CEST contrast in percentage.
While it is possible to achieve a higher GluCEST sensitivity, in this study, the imaging parameters chosen were constrained by the exchange rate differences in phantoms and in vivo tissue as well as radio frequency (RF) specific absorption rate (SAR) issues dealing with human studies at 7T.
Potential Contributions From Other Brain Metabolites
The major metabolites in the brain visible with 1HMRS at physiological concentrations are: N-acetyl aspartate (NAA), choline (Cho), myo-inositol (MI), creatine (Cr), glutamine (Gln), taurine, aspartate (Asp), Glu and GABA. After investigating the CEST properties each of these metabolites in phantoms, it was found that, with the experimental parameters used in this study, except for a small contribution from GABA and Cr, all other metabolites contribute negligible CEST effects to GluCEST (Fig. 2d,e). Of these metabolites, NAA has an amide proton and at neutral pH it does not exhibit any CEST effect. Cho does not have any exchangeable protons. MI has exchangeable –OH protons and exhibits a CEST effect at ≤ 1 p.p.m., which does not extend beyond 2 p.p.m. at neutral pH. Cr has amine protons that exhibit a CEST effect around 1.8 p.p.m. at physiological pH. However, its contribution to the GluCEST effect at 3 p.p.m. is also not appreciable (< 0.5%). No contributions to GluCEST were observed from Asp, Gln and taurine at their physiological concentrations under the experimental conditions used, possibly due to unfavorable exchange rates and differences in the chemical shifts of their amine protons.
Animal Studies
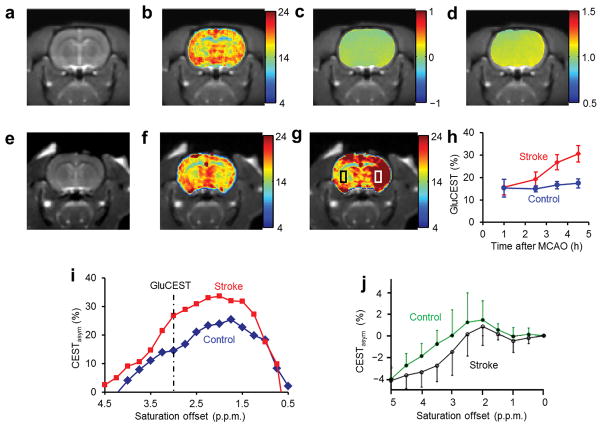
In the healthy rat brain, the gray matter (GM) and white matter (WM) are clearly separated in the GluCEST with a contrast ratio of ~1.6 (Fig. 3a–d). The Glu amine exchange rate measured in healthy rat brain was about 2,000 ± 500 s−1 (Supplementary Notes), which is well within the slow to intermediate exchange regime at 7T.
Figure 3.
GluCEST maps of healthy and ischemic rat brain. (a) Rat brain anatomic images, (b) GluCEST maps, (c) and (d) show B0, and B1 maps of the corresponding brain slices, respectively. Clear differences of GluCEST contrast in GM and WM regions can be seen. (e) Rat brain anatomic proton image. (f,g) The GluCEST maps of the rat brain acquired at 1 h and 4.5 h following the induction of stroke. (h) The GluCEST contrast vs. time after MCAO at regions of interest within the rectangular areas shown in (g). In the ipsilateral side GluCEST is almost doubled at 4.5 h after occlusion. (i) The GluCEST asymmetry plots from the contralateral side (blue curve) and ipsilateral side (red curve) at 4.5 h after occlusion. At 3.00 p.p.m., it is evident that there is almost 100% increase in GluCEST. (j) The contralateral and ipsilateral sides of MCAO rat brain data acquired at 4.7T after 4 h post occlusion and 1 h post mortem (Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine 18, Figure 4, copyright (2003)).
GluCEST obtained from a rat brain following middle cerebral artery occlusion (MCAO) stroke shows significant differences between ipsilateral and contralateral sides (Fig. 3e–h). The ipsilateral side demonstrated a ~100% increase in GluCEST at 4.5 h after MCAO (Fig. 3h). The z-spectral asymmetry plots from the MCAO brain in the current study exhibit an elevation of CEST, whereas the z-spectra from a previously reported18 MCAO study in the rat brain showed decreased amide proton CEST (APT) effects from the ipsilateral side compared to the contralateral side (Fig. 3i,j). Also, APT asymmetry plots show negative values at 3 p.p.m. whereas GluCEST shows positive values. This is due to key differences in the APT and GluCEST experimental conditions. In APT, due to low exchange rates (~30 s−1), a lower saturation pulse amplitude (B1rms = 50 Hz (1.2 μT)) and longer duration (~6 s) were used compared to those used in the present study (B1rms = 155 Hz (3.6 μT) and 1 s).
Literature studies reveal that in this model of ischemia, extracellular Glu levels (~1–2 μM basal value) 26 increase substantially to ~0.2 mM27. However, the total Glu concentration (extra- and intra cellular) may remain unaltered. In addition, although extracellular concentrations (in μM) of other molecules such as GABA, Asp, adenosine and taurine also increase28, their concentration changes are too small to account for the observed GluCEST effects.
In the MCAO model, it is also well-established that the magnitude of pH decrease expected over a four hour period is ~0.5 units18. In addition to pH, other factors that potentially affect GluCEST during stroke are changes to the water content, water T2, and magnetization transfer ratio (MTR). It has been reported that in the MCAO model, the T2 of water is elevated by ~20–40%, MTR is reduced by ~20%, and total water content may be changed by ~2–4%18,29. These changes in T2, MTR and water content are expected to account for only minor changes in GluCEST.
Previous studies of polypeptide solutions in PBS have shown that the CEST effect from amine and amide protons decreased with decreasing pH in the range 7.3–5.030. In contrast, GluCEST increased by almost 100% during MCAO ischemia in the present study. This increase in GluCEST is consistent with the pH dependent GluCEST behavior observed in Glu phantoms at physiological concentrations and temperature and suggests that the elevated GluCEST in the ipsilateral side of MCAO model is predominantly due to decreased pH.
In healthy brain, Glu does not cross the blood brain barrier (BBB), however the compromised BBB in a tumor model provides a means of demonstrating Glu modulation, where GluCEST should change approximately linearly with [Glu]. To demonstrate that the GluCEST measured in vivo is specific to Glu, GluCEST and 1HMRS studies were performed in a rat brain with a tumor following the intravenous injection of Glu solution (Fig. 4a–f). GluCEST maps obtained at three time points after injection show that there is a significant increase in GluCEST in the tumor with a concomitant comparable elevation in the 1HMRS of Glu.
Figure 4.
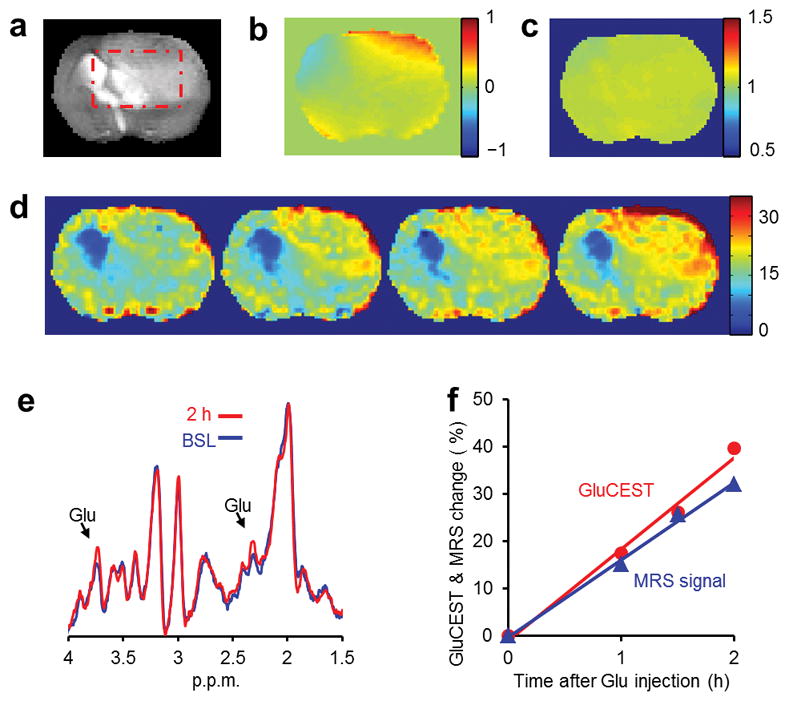
GluCEST images and 1HMRS of rat brain with tumor before and after injection of Glu. (a) Rat brain anatomic proton image demonstrating the tumor and a rectangular region of interest. (b) B0 map of the rat brain, which shows about ± 0.5 p.p.m. variation. (c) B1 map of the rat brain showing fairly uniform B1 field. (d) GluCEST maps (color bar represents GluCEST contrast in percentage) of the rat brain acquired pre-injection (BSL) and at 1, 1.5 and 2.0 h following the injection of Glu solution. Gradual elevation in the GluCEST is evident over a period of 2 h. (e) Stacked stimulated echo acquisition mode (STEAM) localized 1HMRS data acquired at baseline and at 2 h following the injection. Clear elevation of Glu –CH2 (2.3 p.p.m.) and –CH peak (3.75 p.p.m.) amplitude can be seen in the spectra. (f). Time course of GluCEST and the 2.3 p.p.m. Glu peak integral normalized with the values of pre-injection from regions of interest within the rectangular areas shown in (a). A strong correlation between GluCEST and 1HMRS data can be seen.
In vivo Human Brain Study
A GluCEST map obtained from a healthy human brain (Fig. 5b) demonstrates a similar GM and WM distribution pattern as compared to a published Positron Emission Tomography (PET) map of the metabotropic Glu receptor subtype 5 (mGluR5) (Fig. 5c)31. Measured [Glu] from the 1HMRS data [WM = 5.9 ± 0.5 mM and GM = 9.4 ± 1.4 mM] are also consistent with the GluCEST from the same regions of interest (ROI) (Fig. 5a,b,f).
Figure 5.
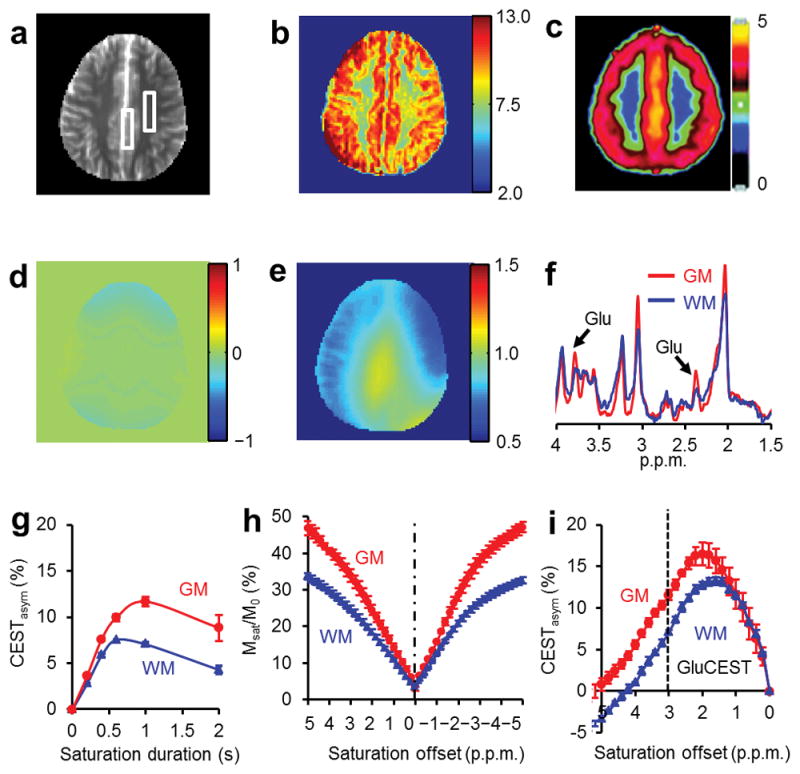
GluCEST imaging and 1HMRS from a healthy human brain acquired at 7T. (a) Anatomic proton image of the axial slice. (b) B1 and B0 corrected GluCEST contrast map (Color bar represents GluCEST contrast in percentage). (c) Map of distribution volumes (DVs) of metabotropic Glu receptor subtype 5 from a PET image (Reprinted by permission of the Society of Nuclear Medicine: J Nucl Med 31, Figure 2, copyright (2007)). The GluCEST map and the PET image show similar distribution pattern of Glu in brain, which is higher in GM compared to WM. (d) B0 map and (e) B1 map corresponding to the slice in (a). (f) 1HMRS spectra obtained from regions of interest of GM and WM as shown in (a). These spectra show higher amplitude of Glu –CH2 resonance (2.3 p.p.m.) and –CH resonance (3.75 p.p.m.) in GM than that in WM. (g) Saturation pulse duration dependence of GluCEST data from human brain. The GluCEST reaches a maximum at a saturation duration of ~1 s and decreases with further increase in duration. (h,i) z-spectra and corresponding asymmetry plots from human GM and WM regions. The GluCEST at 3 p.p.m. (dotted line in figure i) is ~11% from GM and ~7% from WM.
In human brain, the GM and WM GluCEST contrast is the highest at a saturation pulse length of ~1 s (Fig. 5g). The z-spectra and CEST asymmetry curves from GM and WM regions of the brain are rather broad and show a maximum CEST contrast at around 2 p.p.m. (Fig. 5i,j). The broadness in asymmetry plots may be partly due to the (i) broad asymmetry of Glu and the chemical shift averaging effect that shifts the line to higher field towards water resonance, and (ii) an additional potential contribution from creatine32. Similar results were observed from three volunteers. The intra-subject coefficient of variation of measured GluCEST was less than 5%.
The GluCEST maps obtained in healthy human brain reflect the known regional variation of physiological concentrations of glutamate33,34. Since 1HMRS data is highly specific to [Glu], the observed strong correlation between GluCEST ratio from GM and WM ROIs (1.6 ± 0.2) with the measured [Glu] ratio from 1H MRS data from the same ROIs (1.6 ± 0.1) provides additional confirmation that the observed GluCEST in the human brain is predominantly due to Glu. We estimate that ~70–75% of the observed GluCEST is from Glu (Supplementary Table 1 and Supplementary notes).
DISCUSSION
These findings provide the first evidence that GluCEST can be used to provide noninvasive images of [Glu] with excellent spatial and temporal resolution. In addition to its role as the predominant excitatory neurotransmitter in the brain, glutamate also serves as a metabolic intermediate, and it is likely that GluCEST detects both of these pools.
Although the GM to WM GluCEST contrast ratio in humans as well as in healthy rat brains is very similar (~1.6 ± 0.2), the GluCEST magnitude in the rat brain is higher than that in the human brain due to: (1) Higher field (9.4T vs. 7T), 2) continuous saturation pulses (constrained by the scanner software) and 3) lower direct water saturation at 9.4T compared to 7T. Due to the fast exchange rate of Glu amine protons, the slow to intermediate exchange rate condition is not fulfilled at 3T and hence these studies are expected to have poor sensitivity at 3T.
Potential contributions of MTR asymmetry to GluCEST, due to bound pool of water from rigid macromolecules in biological tissues, cannot be excluded. MTR asymmetry effect35, if any, would compete with the GluCEST effect, and would lead to an underestimation of actual GluCEST levels. Indeed, the simulated MTR asymmetry (−0.14% in GM and −0.22% WM) (Supplementary Table 1) indicates that its contribution to the observed GluCEST is likely negligibly small.
In this study, SAR issues at 7T limit the observed GluCEST sensitivity in humans. However saturation pulse power, duration, pulse shapes and readout sequences in conjunction with multi-channel RF coils could be further optimized to enhance GluCEST effects within FDA guidelines for SAR deposition. The Glu contribution to GluCEST may have slight variations per site depending on the RF coil or static field strength used, and future work should evaluate these contributions for specific experimental conditions.
Studies at ultrahigh fields beyond 7T would further enhance the sensitivity of GluCEST, albeit with additional challenges in dealing with SAR deposition. For proof of principle purposes, the current study used only a single slice with a two-dimensional imaging sequence. However, it would be relatively straightforward to implement a three dimensional acquisition of GluCEST maps.
In summary, GluCEST detection is feasible in the human brain at ultra high field (7T) without exceeding the allowed limits on SAR. About 70–75% of the observed CEST is from Glu with the remaining 25–30% coming from Cr, GABA and other macromolecules. Despite small contamination from other molecules in vivo, the GluCEST method can be used to study relative changes in Glu via endogenous or exogenous Glu modulation in vivo. This method provides noninvasive, nonradioactive and high spatial and temporal resolution imaging of relative changes in Glu for use in both human subjects and preclinical models. Further, the exquisite sensitivity of GluCEST to changes in pH could potentially be used as a pH marker in evolving stroke and tumors. Future studies using this approach may provide new insights into Glu function and demonstrate its potential as a biomarker for the diagnosis and treatment of CNS disorders.
ONLINE METHODS
All the phantom and human MRI studies were performed in a 7 T Siemens whole body MRI scanner (Siemens Medical Systems, Malvern, PA, USA) using a circularly polarized RF coil. All phantoms were prepared in PBS and all the experiments were performed at 37°C. Animal experiments were performed in a 9.4 T Varian scanner (Agilent Technologies, Palo Alto, CA, USA).
Phantom Imaging
A new pulse sequence was written to use a frequency selective saturation pulse followed by a segmented RF spoiled gradient echo (GRE) readout sequence. The imaging parameters were: slice thickness = 10 mm, flip angle = 10 °, TR = 5.6 ms, TE = 2.7 ms, field of view (FOV) = 120 × 120 mm2, matrix size = 192 × 192, with one saturation pulse and 64 segments acquired every 15 s. CEST images were collected using a saturation pulse at a B1rms of 155 Hz (3.6 μT) and 2 s duration and frequencies from − 5 to + 5 p.p.m. in steps of 0.2 p.p.m..
Animal Experiments
All animal experiments were performed according to an approved University of Pennsylvania institutional animal care and use committee (IACUC) protocol. For healthy and MCAO studies Sprague-Dawley male rats (265–315 g) and for tumor model studies female Fisher rats (130–150 g) were used. Both MCAO and tumor models were prepared as described in the Supplementary Notes.
Animal Imaging and Spectroscopy
Healthy (n = 2), MCAO (n = 3) and tumor bearing rats (n = 3) were imaged using 35-mm diameter quadrature RF coil (m2m Imaging Corp., Cleveland, OH, USA). Animals were kept under anesthesia (1.5% isoflurane in 1 l min−1 oxygen) and kept warm with the warm air generated from a heater. Respiration and body temperature were monitored using a MRI compatible small animal monitor system (SA Instruments, Inc., Stony Brook, NY, USA). CEST imaging was performed using a frequency selective continuous wave saturation pulse followed by a segmented GRE readout sequence. The imaging parameters were: FOV = 35 × 35 mm2, slice thickness = 2 mm, flip angle = 15°, TR = 6.2 ms, TE = 2.9 ms, matrix size = 128 × 128, number of averages = 2, with one saturation pulse and 32 segments acquired every 4 s. CEST images were collected using a 1 s long rectangular saturation pulse at B1rms of 250 Hz (5.9 μT) at multiple frequencies in the range − 5 to + 5 p.p.m. with a step size of 0.2 p.p.m.. Data for B1 and B0 maps were also acquired. In vivo exchange rate in healthy rat brain at 9.4T was measured using a previously described method14.
In tumor bearing rats, along with GluCEST data, water suppressed stimulated echo acquisition mode (STEAM) single voxel spectra were obtained with the following parameters: voxel size = 11 × 8 × 5 mm3, spectral width = 4 kHz, Number of points = 2048, average = 128, TE = 8 ms, TR = 6 s.
After collecting the baseline CEST map and 1HMRS, the animals were injected with 2.5 ml, 100 mM glutamate solution through the tail vein. CEST and 1HMRS data were gathered periodically for about 2 h post injection.
Human Studies
The study was conducted under an approved Institutional Review Board protocol of the University of Pennsylvania. GluCEST imaging and z-spectrum acquisitions on human brain at 7T were performed on three normal volunteers (male, age: 27-35 years). Informed consent from each volunteer was obtained after explaining the study protocol. Brain CEST imaging was performed with the application of the same imaging protocol as described for the phantoms except a larger FOV = 240 × 240 mm2, averages = 4 and matrix size = 128 × 128. Original CEST images and GluCEST contrast maps were corrected for B0 and B1 inhomogeneities (Supplementary Notes).
Water suppressed 1HMRS were obtained from GM and WM regions using the standard point resolved spectroscopy (PRESS)36 localization technique with the following parameters: voxel size = 10 × 40 × 10 mm3, spectral width = 4 kHz, number of points = 2048, average = 100, TE = 16 ms, and TR = 3 s.
Data Processing
All image processing and data analysis was performed using in-house written programs in MATLAB (version 7.5, R2007b). Acquired images were corrected for B0 and B1 inhomogeneities and z-spectra were obtained from these images by plotting the normalized image intensity as a function of resonance offset of saturation pulse for each sample.
CEST contrast was calculated from the Equation [1]37
| [1] |
where Msat (± Δω) are the magnetizations obtained with saturation at a ‘+’ or ‘−’ Δω offset from the water resonance.
1HMRS spectra were fitted using non-linear least squares methods with Gaussian functions. Peak integrals were calculated and normalized with a non-water suppressed proton signal for calculating Glu concentrations in vivo.
Supplementary Material
Acknowledgments
We gratefully acknowledge stimulating discussions with R. N. Bryan, M. D. Schnall, J. D. Glickson and W. S. Englander. Our thanks are due to W. Liu and S. Pickup for their technical assistance in using the 9.4 T research scanners, W. R. T. Witschey for technical support, P. Waghray for experimental help and D. Reddy, K. Nath and T. Hiraki for help with animal models. This work was performed at an US National Institutes of Health supported resource with funding from P41RR02305.
Footnotes
Author Contribution
K. C., M. H. and A. S. designed and performed experiments, analyzed data and wrote the manuscript; F. K. performed experiments and helped with manuscript editing; J. H. G. helped with animal studies and with manuscript editing; J. A. D. advised on neuroimaging aspects and contributed to the manuscript editing; H. H. provided pulse sequence design, technical guidance, and contributed to the manuscript writing; R. R. provided conception, overall experimental design and contributed to manuscript writing and editing.
References
- 1.Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PJ. Metabotropic glutamate receptor agonists for schizophrenia. Br J Psychiatry. 2008;192:86–87. doi: 10.1192/bjp.bp.107.045088. [DOI] [PubMed] [Google Scholar]
- 3.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 4.Chojnacka-Wojcik E, Klodzinska A, Pilc A. Glutamate receptor ligands as anxiolytics. Curr Opin Investig Drugs. 2001;2:1112–1119. [PubMed] [Google Scholar]
- 5.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryner LN, Sorenson JA, Thomas MA. 3D localized 2D NMR spectroscopy on an MRI scanner. J Magn Reson B. 1995;107:126–137. doi: 10.1006/jmrb.1995.1068. [DOI] [PubMed] [Google Scholar]
- 7.Ryner LN, Sorenson JA, Thomas MA. Localized 2D J-resolved 1H MR spectroscopy: strong coupling effects in vitro and in vivo. Magn Reson Imaging. 1995;13:853–869. doi: 10.1016/0730-725x(95)00031-b. [DOI] [PubMed] [Google Scholar]
- 8.Hurd R, et al. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk M, Lamalle L, Segebarth C. Short-TE localised 1H MRS of the human brain at 3 T: quantification of the metabolite signals using two approaches to account for macromolecular signal contributions. NMR Biomed. 2008;21:507–517. doi: 10.1002/nbm.1219. [DOI] [PubMed] [Google Scholar]
- 10.Petroff OA, Mattson RH, Rothman DL. Proton MRS: GABA and glutamate. Adv Neurol. 2000;83:261–271. [PubMed] [Google Scholar]
- 11.Forsen S, Hoffman RA. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- 12.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 13.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, van Zijl PC. Chemcial exchange saturation transfer imaging and spectroscopy. Prog NMR Spectrosc. 2006;48:109–136. [Google Scholar]
- 16.Jones CK, et al. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 17.Englander SW, Downer NW, Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 19.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27:1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 20.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104:4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105:2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilad AA, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 23.Haris M, Cai K, Singh A, Hariharan H, Reddy R. In vivo mapping of brain myo-inositol. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McConnell HM. Reaction rates by nuclear magnetic resonance. J Chem Phys. 1958;28:430–431. [Google Scholar]
- 25.Chu WJ, et al. Is the intracellular pH different from normal in the epileptic focus of patients with temporal lobe epilepsy? A 31P NMR study. Neurology. 1996;47:756–760. doi: 10.1212/wnl.47.3.756. [DOI] [PubMed] [Google Scholar]
- 26.Davalos A, Shuaib A, Wahlgren NG. Neurotransmitters and pathophysiology of stroke: evidence for the release of glutamate and other transmitters/mediators in animals and humans. J Stroke Cerebrovasc Dis. 2000;9:2–8. doi: 10.1053/jscd.2000.18908. [DOI] [PubMed] [Google Scholar]
- 27.Kiewert C, Mdzinarishvili A, Hartmann J, Bickel U, Klein J. Metabolic and transmitter changes in core and penumbra after middle cerebral artery occlusion in mice. Brain Res. 2010;1312:101–107. doi: 10.1016/j.brainres.2009.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melani A, et al. Striatal outflow of adenosine, excitatory amino acids, gamma-aminobutyric acid, and taurine in awake freely moving rats after middle cerebral artery occlusion: correlations with neurological deficit and histopathological damage. Stroke. 1999;30:2448–2454. doi: 10.1161/01.str.30.11.2448. discussion 2455. [DOI] [PubMed] [Google Scholar]
- 29.Tuor UI, et al. Differential progression of magnetization transfer imaging changes depending on severity of cerebral hypoxic-ischemic injury. J Cereb Blood Flow Metab. 2008;28:1613–1623. doi: 10.1038/jcbfm.2008.49. [DOI] [PubMed] [Google Scholar]
- 30.McMahon MT, et al. New “multicolor” polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn Reson Med. 2008;60:803–812. doi: 10.1002/mrm.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ametamey SM, et al. Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med. 2007;48:247–252. [PubMed] [Google Scholar]
- 32.Sun PZ, Sorensen AG. Imaging pH using the chemical exchange saturation transfer (CEST) MRI: Correction of concomitant RF irradiation effects to quantify CEST MRI for chemical exchange rate and pH. Magn Reson Med. 2008;60:390–397. doi: 10.1002/mrm.21653. [DOI] [PubMed] [Google Scholar]
- 33.Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993;187:219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- 34.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Hua J, et al. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007;58:786–793. doi: 10.1002/mrm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu G, Gilad AA, Bulte JW, van Zijl PC, McMahon MT. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol Imaging. 2010;5:162–170. doi: 10.1002/cmmi.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.