Abstract
Hepatocelluar carcinoma (HCC) has rarely been associated with familial adenomatosis polyposis (FAP). Between 1950 and 2011, only a few cases of HCC associated with classic FAP have been reported in the medical literature. Here, we report the first case to our knowledge of HCC associated with attenuated FAP (aFAP). The patient possessed a single nucleotide mutation in the noncoding region after exon 4, which is rarely observed in attenuated FAP, and not previously reported in classic FAP–associated HCC. Our patient underwent liver transplantation for a 22-cm-large HCC (in China), however, her HCC recurred 1.5 years after the transplantation. Here we review the medical literature on FAP and HCC, with a particular focus on the role of the Wnt/APC/β-catenin pathway toward a better understanding of HCC pathogenesis.
Keywords: Familial adenomatosis polyposis, Hepatocelluar carcinoma
Introduction
In 1859, Charelaigue1 first described adenomatous polyposis in a 16-year-old girl and a 21-year-old man. Later, familial adenomatosis polyposis (FAP) was recognized to be the most common inherited polyposis syndrome. Cumulative evidence supports that, under the umbrella of FAP, classic FAP (cFAP), and attenuated FAP (aFAP), might be very different identities both clinically and molecularly. Patients with FAP can have extracolonic manifestations of their diseases, including thyroid carcinomas and central nerve system neoplasms.2 It is rare for patients with FAP to have hepatic neoplasms, especially HCC. Here, we report the first case to our knowledge of HCC associated with aFAP.
Case Report
A 42-year-old Caucasian woman presented with a 1-month history of early satiety and discomfort in the right upper quadrant. Her Italian-descendant paternal family history was notable for familial adenomatosis polyposis (FAP), with her father and his 5 siblings having developed multiple colon polyps while in their 40s. Her maternal family is mixed-European and had no malignancy except that her mother had head-neck cancer. By using direct DNA sequencing, she was found to have a nucleotide substitution in a noncoding intervening sequence (IVS) that occurs 5 base pairs from the end of exon 4 (IVS4+5G>A), which resulted in a truncated APC protein. Periodic screening colonoscopies (beginning at age 18 years) had been negative until she was 41 years old. At that time, she had both upper esophagogastroduodenoscopy and colonoscopy. The esophagogastroduodenoscopy revealed polyps too numerous to count in the body and fundus of the stomach. Colonoscopy showed about 2 dozen tiny adenomatous polyps (all <5 mm) in the cecum and ascending colon, few polyps in transverse colon, and no polyps in the descending colon, sigmoid colon, or rectum. She was diagnosed with attenuated FAP (aFAP) and underwent a total abdominal colectomy with ileoproctostomy 1 year before her presentation with abdominal pain. Pathology from her colectomy showed no evidence of high-grade dysplasia or malignancy.
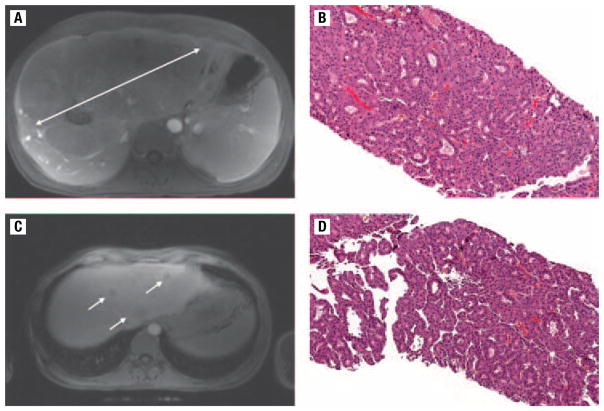
At the time of her presentation with abdominal pain, results of a physical examination revealed palpable hepatomegaly but no scleral icterus or ascites. Blood tests showed a total bilirubin, 1.0 mg/dL; aspartate aminotransferase, 46 U/L; alanine aminotransferase, 55 U/L; alkaline phosphatase, 422 U/L; gamma-glutamyl transpeptidase, 282 U/L; and alpha-fetoprotein, 0.95 ng/mL. Unfortunately, she was found to have a large liver mass (22.5 ×12.6 cm) (Figure 1A) on magnetic resonance imaging. Biopsy of the mass revealed mature hepatocytes arranged in rosettes and thickened groups, and with mild cytologic atypia (Figure 1B). A reticulin stain showed an absence of the normal reticulin framework, which supports a diagnosis of well-differentiated hepatocellular carcinoma (HCC). She was determined not to be a candidate for liver transplantation due to disease outside the Milan criteria but subsequently obtained an orthotopic liver transplantation in Beijing, China. After liver transplantation, she received adjuvant intravenous doxorubicin every 3 weeks for 5 cycles.
Figure 1.
(A) Magnetic Resonance Image (MRI) of the Liver, Showing a Large Primary Hepatocellular Carcinoma About 22.5 cm in Size. (B) Microscopic View of a Core Biopsy, Revealing Hepatocellular Carcinoma With Mature Hepatocytes Arranged in Rosettes and Thickened Groups, and With Mild Cytologic Atypia. Hematoxylin and Eosin Stain at ×100 Magnification. (C) MRI of the Liver Showing 3 Recurrent Lesions in the Transplanted Liver. (D) Microscopic View of a Core Biopsy, Again Confirming Hepatocellular Carcinoma at ×100 Magnification
Our patient had hepatitis B virus (HBV) and hepatitis C virus (HCV) serologic tests before her liver transplantation, which were negative. Shortly after liver transplantation with a Chinese liver donor, she remained seronegative for HCV but became seropositive for HBV infection, with HBV core antibodies total (anti-HBc total) positive, but hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb) negative. She had no liver enzyme abnormality from 2 months to 1.5 years after the liver transplantation. At that time, the serologic test results showed that anti-HBc total changed from positive to negative, HBe antigen (HBeAg) became positive, and that hepatitis B e antibody (HBeAb), HBsAg, and HBsAb remained negative. HBV DNA viral load became detectable, at 1,090,000 IU/mL. The HBV was genotype B, and no polymerase, precore, and BCP mutations.
Routine abdominal imaging was performed after the transplantation. One and a half years after transplantation, an magnetic resonance imaging of the abdomen revealed multiple rim-enhancing masses about 3 cm in size throughout both lobes of the liver (Figure 1C) along with an extrahepatic mass in the pararenal soft tissue. Ultrasound-guided percutaneous core liver biopsies of one mass confirmed HCC (Figure 1D). She was treated with chemoembolization and subsequently with sorafenib, but her disease pursued a relatively aggressive course, and she died of progressive systemic disease approximately 1 year from the time of the documented recurrence (Figure 2).
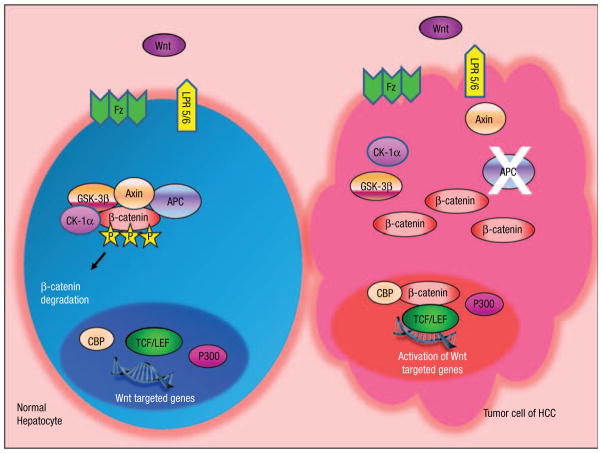
Figure 2.
The Potential Role of Wnt/APC/β-Catenin Pathway in APC Gene-Mutated Hepatocarcinogenesis. The Wnt-Ligand is a Secreted Glycoprotein That Binds to Frizzled Receptors (Fz), Which Trigger a Cascade and Result in the Release of the Multifunctional Kinase GSK-3β Form the APC/Axin/GSK-3β Complex. In Normal Hepatocytes, in the Absence of Wnt-Signal (Off State), β-Catenin, is Targeted for Degradation by the APC/Axin/GSK-3β Complex. β-Catenin Undergoes Proteasomal Degradation After Phosphorylation by CK1α and GSK-3β. In Tumor Cells, Mutated or Truncated APC Protein Cannot Form the APC/Axin/GSK-3β Complex, Resulting in Stabilization of the β-Catenin Level. β-Catenin Translocates into the Nucleus and is Recruited to the LEF/TCF DNA-Binding Factors and Subsequently Leads to the Activation of Wnt-Targeted Genes
Discussion
FAP is an autosomal dominant hereditary disease that is characterized by the presence of numerous adenomatous polyps throughout the colon and rectum. The classic FAP phenotype is characterized by the early onset of polyps beginning in the teens, adenocarcinoma by age approximately 40 years old, and hundreds to thousands of polyps in the gastrointestinal tract. The aFAP phenotype is different from the “classic” FAP in the age of onset, number and location of polyps. Patients with aFAP start to develop polyps in their 40s, and normally have fewer than 100 polyps that are right-side dominant. The clinical presentation of the patient in our case was not typical of cFAP but was consistent with aFAP, with onset at older age, larger numbers of gastric polyps, and relatively few colonic polyps.
Patients with FAP can have extracolonic manifestations of their disease: stomach and duodenal adenomas; periampullary carcinomas and carcinomas of the billiary tract; jejunal and ileal adenomas; osteomas of the mandible, skull, and long bones; soft-tissue lesions, such as epidermoid cysts and desmoids tumors; thyroid carcinomas; and central nerve system neoplasms.2 It is rare for patients with FAP to have hepatic neoplasms.3 Hepatic tumors reported in patients with FAP include adenoma, hepatoblastoma, fibrolamellar carcinoma, and HCC.3,4
FAP is caused by mutations in the APC (adenomatous polyposis coli) gene. The human APC gene is located on the long (q) arm of chromosome 5 and is considered a tumor suppressor gene. More than 800 germline APC gene mutations have been identified in families with classic and attenuated FAP,5 most of which involve exon 3 to codon 1700 (exon 15), which result in truncated proteins or the absence of protein. Truncating mutations between codons 169 and 1600 are associated with the cFAP phenotype. Mutations at the 5′ and 3′ extremes of the APC gene are more commonly associated with aFAP. The IVS4+5 G>A mutation that our patient possessed is located at the 5′ end of APC and occurs in a highly conserved region that is usually necessary for proper messenger RNA processing.6 The same mutation was seen among Greek aFAP kindred.7 A similar mutation (IVS4+5 G>C) at this location has been shown to result in deletion of exon 4, frameshift, and a stop after the first 6 codons in exon 5, which generates a truncated APC protein, which is either unable to form the APC complex or to generate a dominant negative effect on the APC complex. It is intriguing that, in Japanese patients, gastric lesions occur in patients with aFAP with an exon 4 mutation8; our patient with a deletion that started from exon 4 also had numerous gastric adenomatous polyps.
With recent improvements in routine mutation detection techniques, germline APC mutations can be detected in 81% of patients with cFAP and in up to 30% of patients with aFAP.9 Analysis of recent data indicates that a significant portion of patients who have APC-mutation-negative cFAP and aFAP have mutations of MYH (MutY human homology, 7% of cFAP and 40% of aFAP, respectively). MYH encodes a critical member of the DNA base-excision repair system.9 It seems that APC mutation and MYH mutation are mutually exclusive, therefore, a MYH mutation test is unwarranted in a patient who has APC-mutated aFAP.
Between 1950 and 2011, only 10 cases of HCC associated with FAP/Gardner syndrome have been reported in the literature,3,4,10–15 including our case (Table 1). Our report is the first to our knowledge to describe HCC associated with aFAP, and the first to document recurrence of HCC after liver transplantation in a patient with aFAP. We reviewed all cases of HCC related to FAP. Unfortunately, only 2 patients had genetic testing done. Both had germline APC mutations, one that occurred between codons 1099 and 1693,4 and the other that occurred at codon 208.3 The patient with the codon 208 germline APC mutation also had a somatic mutation at codon 568. Our patient has a different germline mutation pattern from the above patients. It, therefore, seems that not just a single but rather a group of genetic alterations can breed HCC in FAP and aFAP. Unfortunately, our patient’s liver transplantation was performed in China; we do not have sufficient HCC specimen from the biopsy for somatic mutation testing.
Table 1.
Summary of Patients With HCC Associated With FAP/Gardner Syndrome
| Reference | Family History | Polyposis Syndrome | Age of FAP Dx (y) | Age of HCC Dx (y) | Other Tumors | Germline Mutation | Somatic Mutation |
|---|---|---|---|---|---|---|---|
| Veale,10 1965 | − | FAP | — | 25 | — | — | — |
| Weinberger et al,14 1981 | + | Gardner | 20 | 3 | Epidermoid cysts | — | — |
| Zeze et al,15 1983 | + | FAP | 31 | 33 | — | — | — |
| Laferla et al,11 1988 | − | FAP | 43 | 43 | Gastric carcinoma | — | — |
| Van Steenbergen et al,13 1989 | + | FAP | 19 | 19 | — | — | — |
| Spigelman et al,12 1991 | + | FAP | — | 78 | Tumors of the bile ducts, pancreas and duodenum | — | — |
| Gruner et al,4 1998 | − | Gardner | 18 | 15. Fibrolamellar HCC. | Desmoid tumor | Between codon 1099–1693 | — |
| + | FAP | — | 9 | — | — | — | |
| Su et al,3 2001 | + | Gardner | 15 | 28 | Desmoid tumor | Codon 208 | Codon 568 |
| Li et al, 2011 | + | aFAP | 41 | 42 | — | IVS4+5G>A | — |
Abbreviations: − = negative; + = positive; — = unknown; aFAP = attenuated familial adenomatous polyposis; Dx = diagnosis; FAP = familial adenomatous polyposis; HCC = hepatocellular carcinoma; IVS = intervening sequence.
Cases of HCC in FAP highlight that the Wnt/APC/β-catenin signaling pathway could potentially be important in hepatocarcinogenesis in general. Accumulating evidence supports a crucial role of the Wnt/APC/β-catenin pathway not only in normal embryogenesis but also in carcinogenesis of various cancer types.16 Wnt protein interacts with its receptors of serpentine proteins Frizzled (Fz), which then activates the downstream effector Dishevelled (Dsh), and eventually the APC protein complex. APC is considered a tumor suppressor protein, which forms a large complex with glycogen synthase kinase 3-beta (GSK-3β) and axin. The APC complex binds to casein kinase (CK1)-phosphorylated β-catenin,17 and the GSK-3β component of complex phosphorylated β-catenin a second time, ultimately leads to its ubiquitination and degradation by proteasomes.18 Loss of APC function, therefore, leads to stabilization and accumulation of β-catenin in the cytoplasm and nucleus, where it binds to the transcription factor Tcf/Lef complex and activates the transcription of Wnt target genes. About 150 Wnt target genes have been identified (ie, MYC, MYB, CJUN, and CYCD1), which play pivotal roles in cell proliferation, differentiation, migration, and interaction with the extracellular matrix.19
Abnormal regulation of β-catenin appears to be a key event in HCC carcinogenesis. β-catenin mutations have been discovered in about 17% of HCCs; mutated sites include the GSK-3β-phosphorylation site. Approximately 50%–70% of HCCs have increased levels of β-catenin in the cytoplasm and nucleus.20 It has been estimated that 12%–26% of cases of β-catenin accumulation come from β-catenin gene mutation and another 8%–13% from β-catenin-regulating gene mutation.21
If the Wnt/APC/β-catenin pathway is important in both colorectal cancer and HCC carcinogenesis, how can we explain the exceeding rarity of HCC associated with FAP? First, there may be natural selection for APC genotypes that retain some activity in downregulating β-catenin signaling,22 and, therefore, require other genetic “hits” that are more likely to occur in colonic cells. Second, germline APC mutation alone may be insufficient in determining HCC development.3,23 Third, the Wnt/APC/β-catenin signaling pathway may not play a dominant role in HCC tumorigenesis but rather may create genetic instability that, when combined with other genetic or extraneous factors, results in HCC. It is of interest to note that β-catenin-overexpressing transgenic mice develop significant hepatomegaly but not hepatic neoplasms.21
Note that our patient had no known history of HBV infection before liver transplantation. Her posttransplantation laboratory results uncovered chronic active genotype B HBV infection, which likely came from the donor. HBV has long been known as a major risk factor for HCC. Results of several studies have suggested that infection by HBV genotypes B and C are associated with an increased risk of HCC.24 The mechanism of HBV carcinogenesis likely includes the integration of HBV DNA allows persistence of the virus and induces genetic alterations. In recent years, the X gene (HBx), the smallest open reading frame in the HBV genome that encodes a 154 amino acid protein has been recognized as being essential for productive HBV infection and replication. Interestingly, HBx activates Wnt/β-catenin signaling by upregulating the cytoplasmic β-catenin.25 Our patient was at high risk for HCC recurrence after transplantation primarily due to tumor size, but it is interesting to speculate that the HBV infection could have contributed to recurrence. However, her HBV viral load converted from nondetectable to high about the time of her HCC recurrence, which makes us doubt the significance of the HBV infection in this case. Currently, there are no data reported about the recurrence rate of HCC in patients who are HBV seronegative and who have received an HBV-infected donor liver. Data from the National Institutes of Health HBV orthotopic liver transplantation study group summarized the final, long-term outcome of patients transplanted for HBV-related HCC. With a mean follow-up of 36.5 months after orthotopic liver transplantation, 12 (12.2%) of 98 patients developed recurrence of HCC.26
This patient had been healthy before her diagnosis of HCC. She had never lived in another country or had known exposure to hepatotoxins. She received 5 cycles of doxorubicin right after liver transplantation and received immunosuppressive medications, including tacrolimus, mycophenolate mofetil, prednisone, and sirolimus after transplantation. It has been reported that immunosuppressive medication, with the exception of mTOR inhibitors, may contribute to HCC carcinogenesis.27 Yokoyama et al28 reported that the administration of combination therapy with cyclosporine and steroid, and/or doxorubicin was associated with a significant reduction in doubling-time for HCC from 273 days to 37 days. Interestingly, a recent study showed that Wnt–β-catenin signaling in dendritic cells regulates the balance between inflammatory vs. regulatory responses.29 Upregulation of β-catenin signaling may potentiate dendritic cells to a tolerogenic state, which may contribute to tumorigenesis. It is unclear whether APC gene mutation and immunosuppressive medication caused a mutually intensifying effect on β-catenin and resulted in higher immunotolerance and subsequent HCC recurrence in our patient.
Conclusion
To our knowledge, this is the first report of a case of HCC associated with aFAP. The role of this specific APC gene mutation in HCC development deserves further investigation toward a better understanding HCC pathogenesis.
Acknowledgments
The authors thank Dr John T. Woosley from the University of North Carolina for the expertise in pathology. The authors thank Millie Arnold, RN, and Dr C. Richard Boland from Baylor Health Care for information about the pedigree of the patient’s family.
References
- 1.Chargelaigue A. Thesis. Paris: 1859. Des Polypes du Rectum. [Google Scholar]
- 2.Harned RK, Buck JL, Olmsted WW, et al. Extracolonic manifestations of the familial adenomatous polyposis syndromes. AJR Am J Roentgenol. 1991;156:481–5. doi: 10.2214/ajr.156.3.1847274. [DOI] [PubMed] [Google Scholar]
- 3.Su LK, Abdalla EK, Law CH, et al. Biallelic inactivation of the APC gene is associated with hepatocellular carcinoma in familial adenomatous polyposis coli. Cancer. 2001;92:332–9. doi: 10.1002/1097-0142(20010715)92:2<332::aid-cncr1327>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Gruner BA, DeNapoli TS, Andrews W, et al. Hepatocellular carcinoma in children associated with Gardner syndrome or familial adenomatous polyposis. J Pediatr Hematol Oncol. 1998;20:274–8. doi: 10.1097/00043426-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Lagarde A, Rouleau E, Ferrari A, et al. Germline APC mutation spectrum derived from 863 genomic variations identified through a 15-year medical genetics service to French patients with FAP. J Med Genet. 2010;47:721–2. doi: 10.1136/jmg.2010.078964. [DOI] [PubMed] [Google Scholar]
- 6.Brosens LA, Keller JJ, Offerhaus GJ, et al. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–43. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihalatos M, Apessos A, Dauwerse H, et al. Rare mutations predisposing to familial adenomatous polyposis in Greek FAP patients. BMC Cancer. 2005;5:40. doi: 10.1186/1471-2407-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao H, Shinmura K, Yamada H, et al. Identification of 5 novel germline APC mutations and characterization of clinical phenotypes in Japanese patients with classical and attenuated familial adenomatous polyposis. BMC Res Notes. 2010;3:305. doi: 10.1186/1756-0500-3-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipe B, Baltazar C, Albuquerque C, et al. APC or MUTYH mutations account for the majority of clinically well-characterized families with FAP and AFAP phenotype and patients with more than 30 adenomas. Clin Genet. 2009;76:242–55. doi: 10.1111/j.1399-0004.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 10.Veale A. Eugenics Laboratory Memoir Series. Vol. 40. New York: Cambridge University Press; 1965. Intestinal Polyposis. [Google Scholar]
- 11.Laferla G, Kaye SB, Crean GP. Hepatocellular and gastric carcinoma associated with familial polyposis coli. J Surg Oncol. 1988;38:19–21. doi: 10.1002/jso.2930380107. [DOI] [PubMed] [Google Scholar]
- 12.Spigelman AD, Farmer KC, James M, et al. Tumours of the liver, bile ducts, pancreas and duodenum in a single patient with familial adenomatous polyposis. Br J Surg. 1991;78:979–80. doi: 10.1002/bjs.1800780828. [DOI] [PubMed] [Google Scholar]
- 13.Van Steenbergen W, Fevery J, De Groote J, et al. Hepatocellular carcinoma in a case of familial polyposis coli. Am J Gastroenterol. 1989;84:1120–1. [PubMed] [Google Scholar]
- 14.Weinberger JM, Cohen Z, Berk T. Polyposis coli preceded by hepatocellular carcinoma: report of a case. Dis Colon Rectum. 1981;24:296–300. [PubMed] [Google Scholar]
- 15.Zeze F, Ohsato K, Mitani H, et al. Hepatocellular carcinoma associated with familial polyposis of the colon. Report of case. Dis Colon Rectum. 1983;26:465–8. doi: 10.1007/BF02556528. [DOI] [PubMed] [Google Scholar]
- 16.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–9. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 17.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–7. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 18.Tickenbrock L, Kossmeier K, Rehmann H, et al. Differences between the interaction of beta-catenin with non-phosphorylated and single-mimicked phosphorylated 20-amino acid residue repeats of the APC protein. J Mol Biol. 2003;327:359–67. doi: 10.1016/s0022-2836(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 19.The Wnt homepage. [Accessed June 8, 2011];Wnt Target Genes. Available at: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes.
- 20.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 22.Albuquerque C, Breukel C, van der Luijt R, et al. The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–60. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 23.Moisio AL, Jarvinen H, Peltomaki P. Genetic and clinical characterisation of familial adenomatous polyposis: a population based study. Gut. 2002;50:845–50. doi: 10.1136/gut.50.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Ye L, Wang QW, et al. Effect of a conserved peptide derived from Kunitz domain of hepatitis B virus x protein on the cell cycle and apoptosis of HepG2 cells via the proteasome pathway. Chin Med J (Engl) 2009;122:460–5. [PubMed] [Google Scholar]
- 25.Longato L, de la Monte S, Kuzushita N, et al. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935–43. doi: 10.1002/hep.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SH, Reddy KR, Keeffe EB, et al. Clinical outcomes of liver transplantation for HBV-related hepatocellular carcinoma: data from the NIH HBV OLT study. Clin Transplant. 2010 doi: 10.1111/j.1399-0012.2010.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hj SFMAS, et al. Immunosuppression and hepatocellular carcinoma. Liver Transpl. 2011 doi: 10.1002/lt.22318. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama I, Carr B, Saitsu H, et al. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095–100. doi: 10.1002/1097-0142(19911115)68:10<2095::aid-cncr2820681002>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manicassamy S, Reizis B, Ravindran R, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–53. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]