Abstract
OBJECTIVES
Replacement of the glycosaminoglycan (GAG) layer with intravesically-administered GAGs is an effective therapy for interstitial cystitis in at least some patients. Intravesically-administered chondroitin sulfate was previously shown to bind to and restore the impermeability of surface-damaged (“leaky”) urothelium to small ions. This study investigated whether a physiologic effect of “GAG replenishment therapy” altered recruitment of inflammatory cells in an acute bladder damage model.
METHODS
Rat bladders were damaged with 10mM HCl. Negative control bladders were treated with PBS. On the following day, the animal bladders were treated with 20mg/mL chondroitin sulfate in PBS, while the negative and positive controls were treated with PBS alone. Two and four days after treatment with chondroitin sulfate, animals were euthanized, and sections of their bladders were analyzed by Toluidine Blue staining for mast cells immunohistochemical labeling using antibodies against CD-45 for lymphocytes, and myeloperoxidase for neutrophils.
RESULTS
Chondroitin sulfate treatment statistically significantly reduced recruitment of inflammatory cells including neutrophils and mast cells to the suburothelial space but did not alter recruitment of CD-45-positive lymphocytes.
CONCLUSIONS
For the first time we demonstrate that intravesical GAG replenishment therapy also produces a physiological effect of decreasing recruitment of inflammatory cells in an acute model of damaged bladder. These findings support use of intravesically administered GAG for bladder disorders that result from a loss of impermeability, including interstitial, radiation and chemical cystitis, and possibly others as well.
Keywords: Interstitial Cystitis, Inflammation, Glycosaminoglycans, Chondroitin Sulfate
Introduction
Interstitial cystitis (IC) is a disorder that presents with various combinations of a triad of symptom complexes—lower abdominal pain, urgency and frequency8, 10. Although the disorder was thought to be solely a problem of the bladder, modern research investigating the prevalence of co-morbidities strongly suggests IC may be the bladder manifestations of a wider, systemic visceral problem that is being labeled chronic pelvic pain2, 17, 26 (CPP). Animal models have shown that acute colonic inflammation alters bladder smooth muscle function19 and that communication occurs through nerves via the dorsal root ganglion16 thus demonstrating that “organ cross talk” could be responsible for diffuse manifestations of IC/CPP.
Although the cause is unknown, the bladder symptoms seem to be derived from a loss of the impermeability barrier that is localized in the apical cell layer9, 21. Normally the bladder urothelium is the least permeable of mammalian membranes13, but in IC the urothelium is dysfunctional with partial loss of umbrella cells with their associated defenses that include tight junctions and glycosaminoglycan (GAG) layer7, 9, 12, 15, 24 and multiple changes in expression of differentiation and barrier-related proteins, as we showed earlier12, 24. Whether the morphologic and biochemical changes in the urothelium is due to failure to differentiate properly or to urinary cytotoxins23 that damage the urothelium similarly to the effects of acid, protamine sulfate or stress29 (or both) is not clear. Other than the cited structural studies, remarkably little direct evidence for loss of permeability has been presented. The strongest evidence is indirect; a high proportion of patients respond with pain to instillation of an 0.1 to 0.2 M solution of potassium ions, but not of sodium ions22. Ruggieri and coworkers4 showed a difference in permeability to 99mTc-diethlyenetriaminepentaacetic acid that was significant at p=0.07, but whether this represents a type II error due to the sample size of 10 patients and 9 controls is unclear. Ericksson showed a four-fold increase in permeability over controls following bladder distention of patients, and for the first time unambiguously demonstrating a difference in permeability7. The most unambiguous demonstration was a small study from Buffington3 showing altered kinetics of excretion of fluorescein by normals and IC patients due to recycling of fluorescein due to absorption of the excreted fluorescein back into the bladder.
Few therapies are effective in treating IC, but among the most effective has been intravesical administration of glycosaminoglycans (GAGs)18, 20 to replace the GAG layer that is missing on patient bladders. Animal studies using the acute acid-damaged mouse and rat bladder as a model for a leaky bladder showed that chondroitin sulfate bound specifically to areas lacking apical (“umbrella”) cells and restored the bladder impermeability to the K+ mimetic 86Rb+ to control levels11. However, restoration of the barrier function using exogenous GAG has not been shown to have a physiologic effect that suggests efficacy of “GAG replenishment therapy.”
In this paper we showed that in the acute acid-damaged bladder that treatment with chondroitin sulfate sharply inhibited the recruitment of neutrophils and mast cells to the suburothelial space, Chondroitin sulfate was used rather than pentosan polysulfate, heparin or the non-sulfated hyaluronan because chondroitin sulfate has negligible effect on the clotting system1 as well as minimal activity as an effector of signaling systems, as is the case with heparin14 or hyaluronan27. The effect on restoring barrier function will therefore more likely represent the physical action of restoring the barrier function. We conclude that not only does treatment of a “leaky” urothelium with intravesical chondroitin sulfate restore the impermeability to ions, this results in a depression of the acute inflammatory response that would normally occur.
Materials and Methods
Rat model for testing restoration of the permeability barrier by chondroitin sulfate
The animals were treated using the model previously described11 in an IACUC-approved protocol. Briefly, Sprague-Dawley rats (300 g) with a cannula surgically implanted in the dome of the bladder (Charles River Laboratories, Cambridge, MA) were anesthetized and treated by injecting 400 μL of 10 mM HCl through the cannula into the bladder. The negative control received 400 μL of PBS instead of the HCl solution. The solutions remained in the bladders for 10 min. The acid was then aspirated, the bladders were washed once with 0.15 M sodium bicarbonate to neutralize any remaining acid and then washed with PBS. On the following day, animals in the ChS group were treated with 400 μL of 20 mg/mL chondroitin sulfate in 0.154 M NaCl provided as a sterile solution for intravesical use (trade name Uracyst from Stellar Pharmaceuticals, London, Ontario). The negative control and the positive control animals both received 400 μL of PBS alone. The solutions were allowed to remain in the bladder for 20 minutes, at which time the solutions were removed. Two and four days following administration of chondroitin sulfate, two rats each from the positive and negative controls and chondroitin sulfate-treated groups were euthanized. The bladders were partially inflated with PBS containing 1% formalin and then were soaked in the 1% formalin solution overnight prior to mounting in paraffin and sectioning as 5 micron sections. Several sections at different levels in the bladder were cut from each bladder.
Immunohistochemical staining
Sections were selected at random from different levels of the bladder and immunohistochemical (IHC) staining was performed as previously described5. Sections were de-waxed with a graded xylene and ethanol series and re-hydrated with a graded ethanol water series. Anti-CD45RC antibody, anti-Myeloperoxidase antibody, and Toluidine Blue staining were used to detect lymphocytes, neutrophils, and mast cells, respectively. IHC labeling was performed with two primary antibodies, anti-CD45RC (AbD Serotec, MCA53Ga, mouse monoclonal, no retrieval 1:50), and anti-Myeloperoxidase (Dako, A0398, rabbit polyclonal, no retrieval, 1:600). Two secondary antibodies were used, pre-diluted anti-rabbit (Zymed Histostain-Plus Kit) and goat anti-mouse (Calbiochem, 401216, 1:100). Toluidine Blue (Polysciences Inc., #1234, 1g/100ml 70% ethanol stock) was performed by incubating rehydrated sections for 2 minutes with a working solution of stock Toluidine Blue diluted 1:10 with 1 g/ml aqueous NaCl) followed by 3 rinses with deionized water and coverslipping.
Slides were examined by microscopy, and stained cells located in the lamina propria were scored as positive. To avoid bias, the circumference of the entire bladder of each section was analyzed. Two rats were analyzed for each condition. Individual cells of interest were counted without knowledge of how the animals had been treated. Data were analyzed by one-way ANOVA to identify whether statistically significant differences existed in the mast cell and neutrophil datasets, and Mann-Whitney analysis was performed to test for statistical significances of differences between individual pairs at day 2 and day 4 using a one-tailed analysis because our hypothesis is that the PBS treatment will always produce higher counts than the chondroitin sulfate treatment.
RESULTS
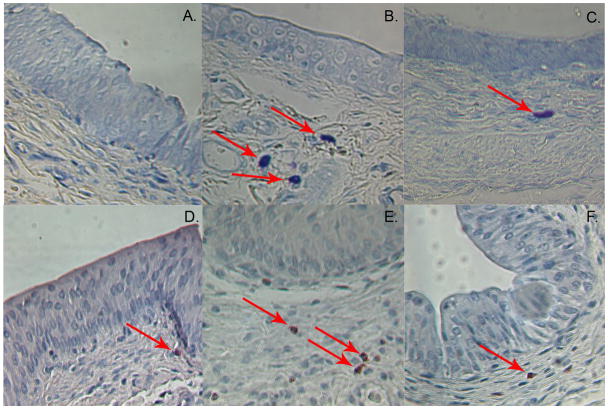
Fig. 1 illustrates representative examples of sections from bladders that were damaged with acid and then treated with chondroitin sulfate or PBS the following day and harvested either 2 or 4 days after treatment with chondroitin sulfate (3 and 5 days post damage). As has been shown previously, the acid-damage model removed most of the “umbrella cell” layer, exposing the intermediate cell layer. This produced leakiness, edema, and inflammation in the area underlying the urothelium. Mast cells (Fig. 1A) and neutrophils (Fig. 1D) were present at low levels in the undamaged control bladder. After treatment of the bladder with HCl, the numbers of mast cells (Fig. 1B) and neutrophils (Fig. 1E) were dramatically increased. The amount of edema and inflammation appeared less pronounced in the chondroitin sulfate-treated bladders, and the numbers of mast cells (Fig. 1C) and neutrophils (Fig. 1F) were significantly reduced by treatment with chondroitin sulfate to restore barrier function.
Figure 1.
Photographs illustrating examples of positive cells and their distribution as a function of treatment. (A) Control slide showing rare mast cells in untreated bladder. (B) Increased number of mast cells 4 days after HCl treatment of bladder to induce leakiness. (C) The number of mast cells is decreased 4 days following intravesical chondroitin sulfate treatment (D) Control slide showing relatively rare neutrophils in untreated bladder. (E) Increased number of neutrophils 4 days after HCl treatment of bladder to induce leakiness. (F) The number of neutrophils is decreased 4 days following intravesical chondroitin sulfate treatment. Magnification is 400X.
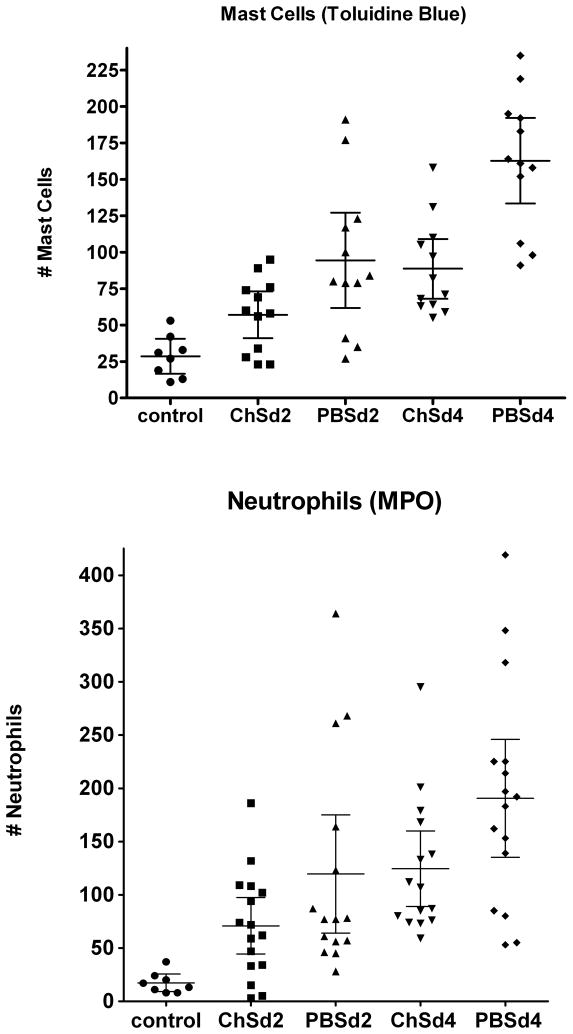
The counts of mast cells and neutrophils are shown in Figure 2. At two and at four days post administration of chondroitin sulfate (three and five days post damage with HCl), the number of mast cells per section increased four- and six-fold respectively in the absence of chondroitin sulfate. The increase in neutrophils was actually larger (7-fold at day 3 and 11-fold at day 5), but was more variable per section. The differences within the data sets were highly significant (p<0.0001) by ANOVA. All of the cell counts for treated animals were significantly different from the controls (p at least <0.05). Treatment with chondroitin sulfate immediately inhibited the recruitment of mast cells, and the difference was significant even 2 days following treatment with chondroitin sulfate (p-values are listed in the figure caption). Four days after treatment with chondroitin sulfate, the mast cell counts were reduced by 43%. The effect of chondroitin sulfate on recruitment of neutrophils was also significant, showing a trend at day 2 and statistical significance at day 4; neutrophils were reduced by 35% four days after treatment. There were no differences in the numbers of lymphocytes (CD 45+)(data not shown).
Figure 2.
Comparison of the effect of intravesical chondroitin sulfate on recruitment of mast cells (top) and neutrophils (bottom) two and four days following treatment with acid to increase bladder permeability. Each symbol represents the number of cells seen in the lamina propria of a single, entire cross section. A total of two rats was sampled for each condition. Shown are the means and the 95% confidence intervals. Testing for statistical significance of differences showed the decrease in neutrophils produced by chondroitin sulfate was not significant (p = 0.13) at two days but was significant (p = 0.024) at four days. For mast cells, the difference was significant (p = 0.015) at two days and highly significant at four days (p = 0.0004).
DISCUSSION
Although interstitial cystitis does not generally manifest with a robust immune cell response, evidence of inflammation is still present in the bladder as compared to healthy individuals12, 15. The morphologic features actually suggest physical loss of the umbrella cell layer and variable loss of intermediate cells as well up to and including complete denudation of the urothelium12, 15, which are features partially duplicated by the acute acid damage model used here or the protamine sulfate model used by other investigators25. The presence of urine is necessary for eliciting an immune response from these physical damage models25, indicating that loss of impermeability is a key factor in producing an inflammatory response. Because interstitial cystitis also involves loss of impermeability, it is likely that the immune effector cell response observed in interstitial cystitis also has its origins in penetration of the bladder by urinary solutes. Since similar responses are seen in these models and human interstitial cystitis, it appears that these physical damage models can serve as a useful model, at least for the acute response to loss of impermeability. However, the model is not intended to duplicate IC itself, and only provides a means to assess the effects of correcting bladder permeability in an acute model.
The inflammatory response is complex and is modulated by several sets of effector cells. Neutrophils are associated with tissue injury as well as pathogen invasion and have been identified in elevated numbers in the urine of IC patients6. Mast cells are generally associated with an allergic-type response and also have been strongly implicated in IC28. However, the mast cells in this study are not degranulated, as shown by their metachromatic reaction of the Toluidine Blue with intracellular heparin, indicating that histamine and other vasoactive amines are intact within the granules. At two and at four days post treatment, the number of mast cells per section was increased four- and six-fold respectively in the absence of GAG replenishment therapy. The increase in neutrophils was actually larger, but was more variable per section.
Although administration of chondroitin sulfate intravesically restored the impermeability of the bladder surface to control levels11, the effector cell response was muted but not abolished completely. One reason for this is that the chondroitin sulfate was not administered until 24 hours later because cells killed by the acid treatment slough from the urothelium during the first 24 hours, thereby exposing the urothelium to urinary solutes. If chondroitin sulfate is administered within the first 24 hours it will be lost along with these sloughed cells. Alternately, the effector cell response could still have been triggered by the tissue damage through other mechanisms. Nonetheless, “GAG replenishment therapy” with chondroitin sulfate clearly diminished the inflammatory response due to loss of impermeability and presumably would with other GAGs as well. Interestingly, the response rate for GAG replenishment therapy is only about 60%17, whereas 91% of patients had a positive potassium sensitivity test in one study with intravesical chondroitin sulfate18 The optimal dosing interval is not known, and once weekly may be insufficient in some patients to maintain a functional GAG layer. Also, the prevalence of “leakiness” is unknown and is inferred from potassium sensitivity test data. Clearly the role of loss of permeability in the bladder and how it might relate to comorbidities (e.g. bowel symptoms) is unknown and in need of further study.
In summary, these results demonstrate a clear muting of the inflammatory response induced by loss of the permeability barrier of the urinary bladder and support the use of intravesical chondroitin sulfate or other GAG for treatment of bladder disorders that involve the loss of the full impermeability barrier of the bladder. In addition to interstitial cystitis, other disorders such as radiation cystitis and overactive bladder could involve this etiologic factor because the potassium sensitivity test is positive as well in over 60% of patients5, 22.
Acknowledgments
The authors thank Jean Coffman for her excellent technical assistance.
This work was supported in part by R01 DK069808 (REH) and by a grant from Stellar Pharmaceuticals, Inc.
REH has no financial interest other than as a recipient of the research grant from Stellar. No other authors have any financial interest. The chondroitin sulfate used for these studies was donated by Stellar Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Brecher AS, Adamu MT. Coagulation protein function: enhancement of the anticoagulant effect of acetaldehyde by sulfated glycosaminoglycans. Dig Dis Sci. 2001;46 (9):2033–2042. doi: 10.1023/a:1010668005729. [DOI] [PubMed] [Google Scholar]
- 2.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172(4 Pt 1):1242–1248. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- 3.Buffington CA, Woodworth BE. Excretion of fluorescein in the urine of women with interstitial cystitis. J Urol. 1997;158(3 Pt 1):786–789. doi: 10.1097/00005392-199709000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Chelsky MJ, Rosen SI, Knight LC, et al. Bladder permeability in interstitial cystitis is similar to that of normal volunteers: direct measurement by transvesical absorption of 99mtechnetium-diethylenetriaminepentaacetic acid [see comments] J Urol. 1994;151(2):346–349. doi: 10.1016/s0022-5347(17)34945-5. [DOI] [PubMed] [Google Scholar]
- 5.Chung MK, Butrick CW, Chung CW. The overlap of interstitial cystitis/painful bladder syndrome and overactive bladder. JSLS. 2010;14(1):83–90. doi: 10.4293/108680810X12674612014743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd LG, Tello J. Cytologic examination of urine from patients with interstitial cystitis. Acta Cytol. 1998;42(4):923–927. doi: 10.1159/000331969. [DOI] [PubMed] [Google Scholar]
- 7.Erickson DR, Herb N, Ordille S, et al. A new direct test of bladder permeability. J Urol. 2000;164(2):419–422. [PubMed] [Google Scholar]
- 8.Evans RJ, Sant GR. Current diagnosis of interstitial cystitis: an evolving paradigm. Urology. 2007;69(4 Suppl):64–72. doi: 10.1016/j.urology.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep. 2006;7(6):440–446. doi: 10.1007/s11934-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 10.Hanno P, Nordling J, van OA. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 2008;18(4):353–358. doi: 10.1097/MOU.0b013e3282fcea88. [DOI] [PubMed] [Google Scholar]
- 11.Hauser PJ, Buethe DA, Califano J, et al. Restoration of the Barrier Function to Acid-Damaged Bladder by Intravesical Chondroitin Sulfate. J Urol. 2009;182(5):2477–2482. doi: 10.1016/j.juro.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser PJ, Dozmorov MG, Bane BL, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008;179(2):764–769. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks RM, Ketterer B, Warren RC. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Phil Trans R Soc Lond B Biol Sci. 1974;268:23–38. doi: 10.1098/rstb.1974.0013. [DOI] [PubMed] [Google Scholar]
- 14.Kuschert GS, Coulin F, Power CA, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38 (39):12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 15.Leiby BE, Landis JR, Propert KJ, et al. Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol. 2007;177(1):142–148. doi: 10.1016/j.juro.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 16.Malykhina AP, Qin C, Greenwood-Van MB, et al. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18(10):936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 17.Nickel JC. Interstitial cystitis: a chronic pelvic pain syndrome. Med Clin North Am. 2004;88(2):467–481. doi: 10.1016/S0025-7125(03)00151-2. [DOI] [PubMed] [Google Scholar]
- 18.Nickel JC, Ergerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103(1):56–60. doi: 10.1111/j.1464-410X.2008.08028.x. [DOI] [PubMed] [Google Scholar]
- 19.Noronha R, Akbarali H, Malykhina A, et al. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293(5):F1461–F1467. doi: 10.1152/ajprenal.00311.2007. [DOI] [PubMed] [Google Scholar]
- 20.Parsons CL. Current strategies for managing interstitial cystitis. Expert Opin Pharmacother. 2004;5(2):287–293. doi: 10.1517/14656566.5.2.287. [DOI] [PubMed] [Google Scholar]
- 21.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69(4 Suppl):9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 22.Parsons CL, Stein PC, Bidair M, et al. Abnormal sensitivity to intravesical potassium in interstitial cystitis and radiation cystitis. Neurourol Urodyn. 1994;13(5):515–520. doi: 10.1002/nau.1930130503. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran M, Stein P, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol. 2006;13(4):409–414. doi: 10.1111/j.1442-2042.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 24.Slobodov G, Feloney M, Gran C, et al. Abnormal Expression of Molecular Markers for Bladder Impermeability and Differentiation in Urothelium of Interstitial Cystitis Patients. J Urol. 2004;171(4):1554–1558. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 25.Soler R, Bruschini H, Freire MP, et al. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J Urol. 2008;180(4):1527–1531. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Stanford EJ, Dell JR, Parsons CL. The emerging presence of interstitial cystitis in gynecologic patients with chronic pelvic pain. Urology. 2007;69(4 Suppl):53–59. doi: 10.1016/j.urology.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 27.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83(7):317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- 28.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57(6 Suppl 1):47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- 29.Veranic P, Jezernik K. Succession of events in desquamation of superficial urothelial cells as a response to stress induced by prolonged constant illumination. Tissue Cell. 2001;33(3):280–285. doi: 10.1054/tice.2001.0175. [DOI] [PubMed] [Google Scholar]