Abstract
Objective
To determine whether or not the N-terminal fragment of B-type natriuretic peptide (NTproBNP) was a biomarker of clinical, laboratory, and echocardiographic abnormalities in children with homozygous sickle cell disease (SCD).
Study design
A single-center retrospective study consisted of analysis of data from November, 2007 to December, 2010. We correlated serum NTproBNP with clinical and laboratory findings, echocardiographic data, and New York Heart Association (NYHA) functional class.
Results
NTproBNP levels from 42 children (median age 9 years, 52% female) had significant correlations with hemoglobin (r= −0.63, p<0.05), and echocardiographic measurements including tricuspid regurgitant velocity (r=0.46, p<0.05), lateral E’ (r=−0.52, p<0.05) and lateral E/E’ ratio (r=0.60, p<0.05) suggesting diastolic dysfunction. In addition, NTproBNP levels increased from NYHA functional class I to class III and had a significant linear correlation with the NYHA functional class (r=0.69, p <0.05).
Conclusions
NTproBNP correlated with low hemoglobin and tissue Doppler data as indicators of diastolic dysfunction. Elevated NTproBNP may be a prognostic biomarker for the presence of diastolic dysfunction related to anemia in children with SCD.
The N-terminal fragment of B-type natriuretic peptide (NTproBNP) is the inactive byproduct of the cleavage of pro-BNP, which was first identified in brain tissue. B-type natriuretic peptide is also produced in the atrium and right ventricle but is predominantly synthesized in the left ventricular (LV) myocardium. In a recent study of adults with sickle cell disease (SCD), elevated NTproBNP levels positively predicted the diagnosis of pulmonary hypertension and were independently associated with increased mortality.1–6 Previous reports indicated NTproBNP levels correlated with measures of LV diastolic function such as E/A ratio rather than systolic dysfunction in adult patients with SCD.4 LV diastolic dysfunction is common in the SCD population and diastolic LV filling abnormalities are also predictive of high rates of mortality in affected adults.7 LV diastolic dysfunction and pulmonary hypertension may develop independently in SCD patients and contribute to the disease-associated early mortality.7 The aim of our study was to determine whether or not NTproBNP levels could serve as a biomarker of clinical, laboratory, and echocardiographic abnormalities in children with SCD.
Methods
This single-center retrospective study consisted of data from November, 2007 to December, 2010. Data were analyzed from the medical records of children attending the Comprehensive Pediatric Sickle Cell Clinic at Colorado Children’s Hospital, Aurora, Colorado with the approval of the Colorado Multi-Institutional Review Board. NTproBNP levels were measured on an electrochemiluminescence immunoassay (Mayo medical laboratories; ProBNP II, Roche Diagnostics, Indianapolis, IN, USA). Children were routinely evaluated for the presence of pulmonary hypertension by two-dimensional Doppler echocardiography using the modified Bernoulli equation. As established by recent studies, an elevated tricuspid regurgitant (TR) jet velocity was defined as greater than 2.5 m/second in this population.8–10 The echocardiographic data included TR velocities, left and right ventricular diastolic dimension, mitral inflow E velocity, and LV fractional shortening by the modified Simpson’s method. Imaging of tissue Doppler was performed using spectral pulsed Doppler. In the apical 4-chamber view, pulsed Doppler sample volume was placed at the lateral mitral annulus. As previously reported, E’ (early diastolic myocardial relaxation) velocity and E/E′ ratio have been shown to be excellent echocardiographic predictors of diastolic dysfunction.11 We measured lateral E’ velocity and calculated lateral E/E’ ratio for evaluating LV diastolic dysfunction. For data analysis we included only echocardiographic data obtained within 30 days of the NTproBNP blood draw.
Statistical Analyses
All results are expressed as median and range or mean and standard deviation as specified. Spearman's non-parametric correlation test was used to determine possible correlations between NTproBNP level and clinical events, laboratory results, echocardiographic findings, and New York Heart Association (NYHA) functional class. NYHA functional class was evaluated in patients who were over 6 years-old. In addition, SCD patients were categorized into two groups by elevated (greater than or equal to 2.5 m/second) and normal TR velocity (less than 2.5 m/second). The Mann-Whitney U test, Student t-test, and Chi-square test were used to evaluate differences between the 2 groups defined by elevated and normal TR velocity. To compare mean values among the NYHA functional class I, class II, and class III, the differences were assessed by analysis of variance, with Bonferroni’s correction for multiple comparisons. The level of statistical significance was defined by a p value < 0.05. Analyses were conducted using Statmate III for Windows (Atoms Co., Tokyo, Japan).
Results
Forty two children with homozygous SCD (median age 9 years, 52% female) had NTproBNP measurements. Twenty nine children were born in Denver, thus the median time to live at moderate altitude was 8 years (2–19 years). Seventeen (40%) children were receiving hydroxyurea therapy and 6 (14%) patients were receiving chronic transfusions. No patients received bone marrow transplantation. Of 42 children, 27 (64%) had acute chest syndrome episodes and 33 children (79%) were hospitalized due to pain crisis or acute chest syndrome. Four (9%) children had previous ischemic stroke. Twenty four (57%) children had 39 echocardiogram examinations within 30 days of the NTproBNP evaluation. Because TR velocity could not be measured in 13 children (31%), we evaluated the correlation between NTproBNP and TR velocity in 20 samples. The median and range NTproBNP, hemoglobin, reticulocyte, lactate dehydrogenase, feriritin, and iron levels in all children were 92.9 pg/ml (8.5–839.7 pg/ml), 8.5 g/dl (4.8–12.7 g/dl), 13 % (1.2–33%), 558 U/l (230–1998 U/I), 240 ng/ml (27.1–6654 ng/ml), 89 µg/dl (26–236 µg/dl), respectively. Echocardiographic data included the median and range TR velocity (2.4 m/s, 1.6–3.3 m/s), right ventricular diastolic dimension (16.6 mm, 7.8–28.7 mm), LV diastolic dimension (44.1 mm, 29.4–59.6 mm), LV functional shortening (37%, 26–52%), LV inflow E velocity (1.1 m/s, 0.8–1.6 m/s), lateral E’ velocity (18 cm/s, 11–25 cm/s), and lateral E/E’ ratio 6.3 (3.6–13.5).
Correlation between laboratory, echocardiographic data and NTproBNP level
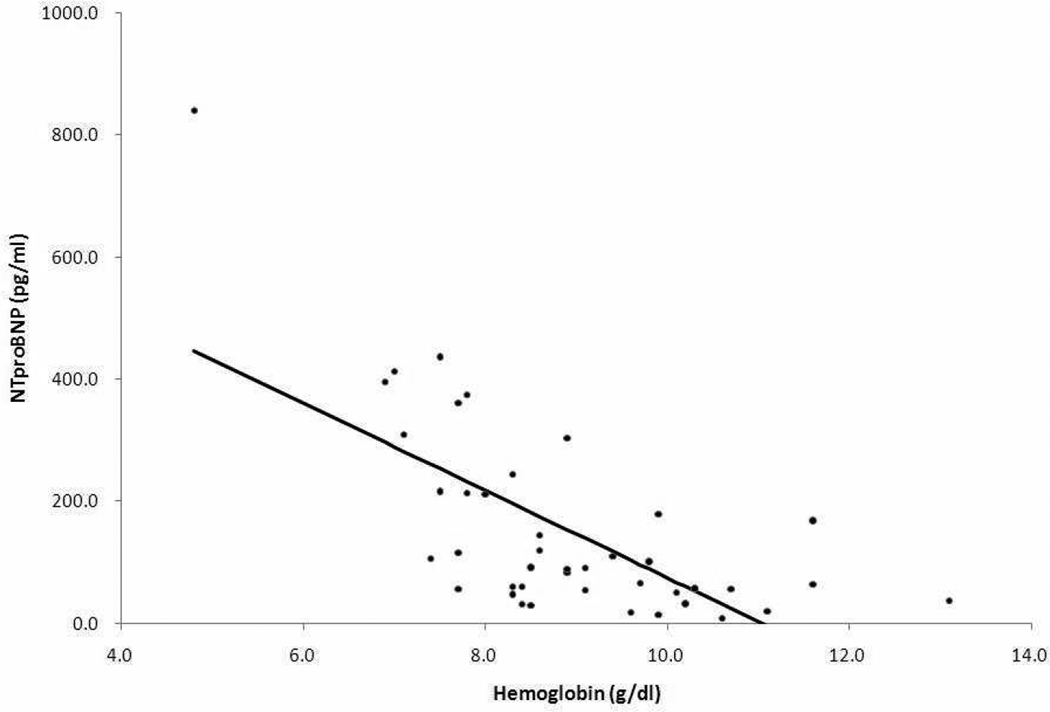
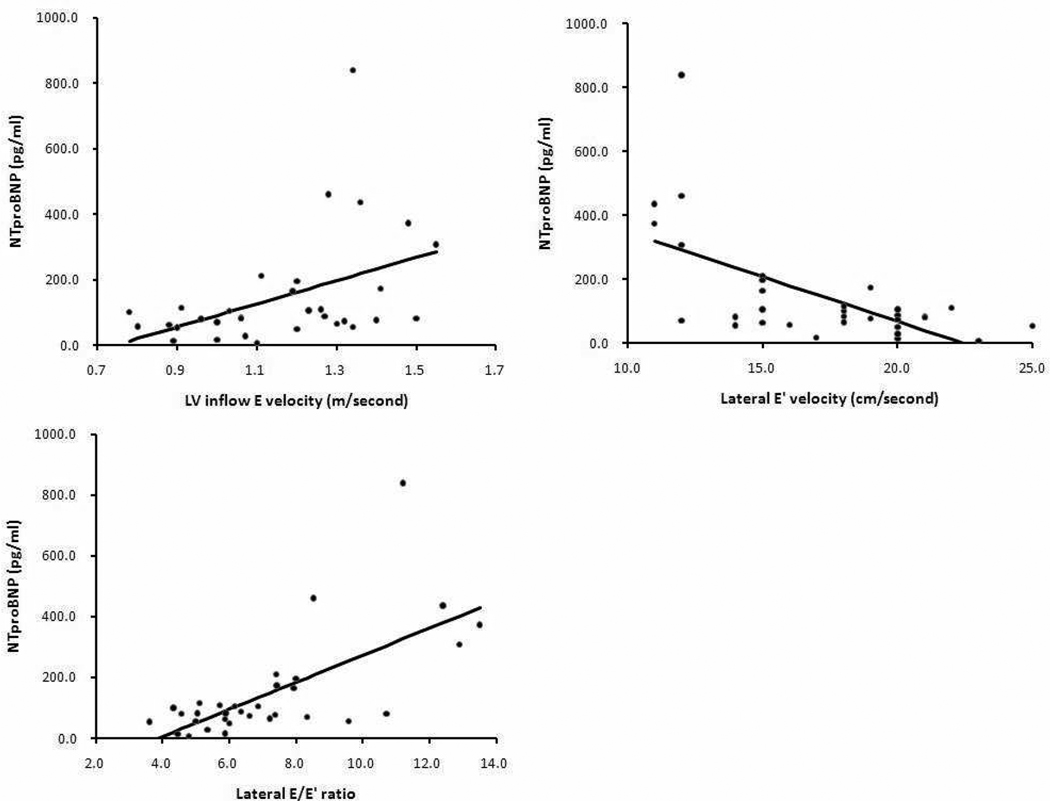
By Spearman rank correlation coefficients, the NTproBNP level had a significant negative correlation with lower hemoglobin (r=−0.63, p<0.05) (Figure 1). Even when excluding 6 patients who underwent chronic transfusion, we found a significant correlation between NTproBNP and hemoglobin (r=−0.60, p<0.05). There were no correlations between NTproBNP level and any other laboratory data including white blood cell, platelet, reticulocyte, lactate dehydrogenase, total bilirubin, direct bilirubin, ferritin, iron, aminotransferase, blood urea nitrogen, and creatinine. In addition, NTproBNP had no correlations with time to live at altitude, episodes of acute chest syndrome, and number of episodes of hospitalization due to pain crisis or acute chest syndrome. There were moderate, but significant correlations between NTproBNP level and LV inflow E velocity, lateral E’ velocity, and E/E’ ratio (r=0.49, p<0.05, r=−0.52, p<0.05, r=0.60, p<0.05, n=32, respectively) (Figure 2). Other echocardiographic variables (right ventricular diastolic dimension; r=0.12 p=0.45, LV diastolic dimension; r=−0.08, p=0.58, LV fractional shortening; r=0.02, p=0.92) did not correlate with NTproBNP. In addition, LV diastolic dimension did not correlate with hemoglobin (r=−0.03, p=0.84). Hemoglobin increased after hydroxyurea therapy in 17 children (mean +/− standard deviation; 8.0 +/− 0.8 versus 8.9 +/− 1.2 g/dl, p=0.02). Although 13 of 17 patients had acute chest syndrome before hydroxyurea treatment, only 1 patient had acute chest syndrome during follow-up (36+/−22 months).
Figure 1.
Negative correlation between NTproBNP and hemoglobin was observed in 42 samples by Spearman rank correlation.
NTproBNP; N-terminal fragment of B-type natriuretic peptide
Figure 2.
Significant correlations between NTproBNP level and echocardiographic measurements of LV diastolic dysfunction including LV inflow E velocity, lateral E’ velocity, and lateral E/E’ ratio were observed in 32 samples by Spearman rank correlation.
LV; left ventricular, E’; early diastolic myocardial relaxation velocity
TR velocity and NTproBNP level
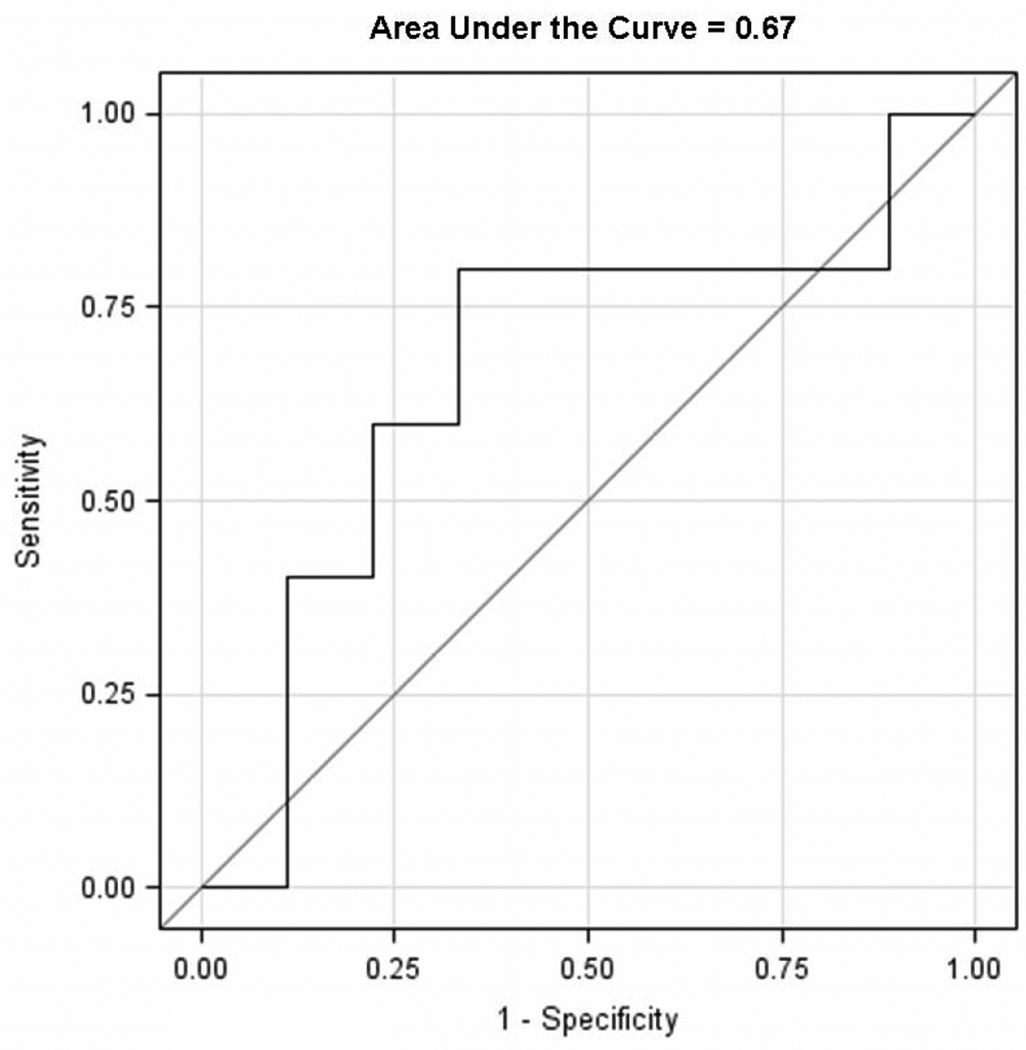
Ten children had elevated pulmonary artery systolic pressures as evidenced by a TR velocity of greater than or equal to 2.5 m/second. One-third of these children (3) had TR velocity ≥ 3.0 m/second. The Table shows the clinical variables in children with and without elevation of TR velocity. In the elevated TR velocity group, right ventricular diastolic dimension, the number of hospitalizations due to crisis, and time at altitude were significantly higher than those in < 2.5 m/second TR group (p<0.05). In contrast, 2 children who had stroke episodes were in the < 2.5 m/second TR group. NTproBNP level had a significant correlation with TR velocity (r=0.46, p<0.05, n=20) when evaluated as a continuous variable. However, in children with elevation of TR velocity ≥ 2.5 m/second, the NTproBNP levels tended to be higher than those without elevation of TR velocity, but this was not statistically significant (median and range; 105.5 (28–839.7) pg/ml versus 81.9 (18.1–373.7) pg/ml, p=0.44). NTproBNP was positively associated with having a TR velocity ≥ 2.5 m/second with an area under the Receiver Operating Characteristic curve = 0.67 (Figure 3; available at www.jpeds.com). NTproBNP had a higher predictive ability compared with hemoglobin, which was negatively associated with having a TR velocity ≥ 2.5 m/second, Receiver Operating Characteristic curve = 0.56.
Table 1.
Characteristics of children with and without elevated TR velocity
| TR velocity >2.5 m/s (N=10) |
TR velocity <2.5 m/s (N=19) |
P value | |
|---|---|---|---|
| NTproBNP (pg/ml); median (range) | 105.5 (28–839.7) | 81.9 (18.1–373.7) | 0.44§ |
| Age (years); median (range) | 12 (4–19) | 8 (3–19) | 0.20* |
| Sex (male : female) | 4:6 | 8:11 | 0.58¶ |
| Time to live at altitude; median (range) | 11 (2–19) | 7.5 (3–19) | <0.05§ |
| Acute chest syndrome; case (%) | 7 (70) | 14(73) | |
| (median times of episode) | 1 (0–3) | 1 (0–5) | 0.85§ |
| Admission for crisis; case (%) | 9 (90) | 17 (89) | |
| (median times of hospitalization for crisis) | 7 (0–30) | 3 (0–10) | <0.05 § |
| Stroke; case (%) | 0 (0) | 2 (11) | 0.38¶ |
| Hydroxyurea; case (%) | 5 (50) | 7 (37) | 0.42¶ |
| Chronic blood transfusion; case (%) | 2 (20) | 3 (16) | 0.79¶ |
| Laboratory data | Median (range) | ||
| Hemoglobin (g/dl) | 8.9 (4.8–13.1) | 8.5 (7.4–11.6) | 0.96* |
| White blood cell (×103/µl) | 12.4 (5.4–351) | 12.6 (4.3–18.9) | 0.64§ |
| Platelet (×103/µl) | 369.5 (194–559) | 393 (303–522) | 0.93* |
| Reticulocyte (%) | 11.8 (1.2–20.3) | 12.5 (4.6–20.1) | 0.59* |
| Lactate dehydrogenase (U/l) | 532 (267–1406) | 500 (351–1998) | 0.82* |
| Total bilirubin (mg/dl) | 2.2 (0.7–3.2) | 3.9 (1.1–7) | 0.08* |
| Direct bilirubin (mg/dl) | 0.2 (0–0.4) | 0.3 (0–0.6) | 0.22* |
| Ferritin (ng/ml) | 204.7 (40.2–1477) | 264.4 (27.1–797.4) | 0.93§ |
| Iron (µg/dl) | 86.5 (77–132) | 95.5 (49–123) | 0.46§ |
| Alanine aminotransferase (U/l) | 57.5 (21–108) | 44.5 (28–77) | 0.33* |
| Aspartate aminotransferase (U/l) | 27 (12–52) | 22 (9–39) | 0.46* |
| Blood urea nitrogen (mg/dl) | 8.2 (5–9.9) | 7.5 (4.3–11) | 0.73* |
| Creatinine (mg/dl) | 0.4 (0.2–0.5) | 0.3 (0.2–0.6) | 0.73§ |
| Echocardiographic data | Median (range) | ||
| Right ventricular diastolic dimension (mm) | 18.3 (9.8–24.9) | 13.4 (7.8–23.5) | <0.05* |
| Left ventricular diastolic dimension (mm) | 43.7 (32.7–54.7) | 41.7 (30.8–49.8) | 0.52* |
| Left ventricular fractional shortening (%) | 42 (33–49) | 38 (26–45) | 0.10* |
| Left ventricular inflow E velocity (m/s) | 1.3 (0.8–1.6) | 1.0 (0.7–1.5) | 0.09§ |
| Lateral E’ velocity (cm/s) | 19 (12–22) | 20 (11–25) | 0.55* |
| Lateral E/E’ ratio | 6.7 (4.3–12.9) | 5.9 (3.6–13.5) | 0.34§ |
NTproBNP; N-terminal fragment brain natriuretic peptide, TR; Tricuspid regurgitation velocity
Two-sample t-test for normal distribution data for normally distributed data
Mann-Whitney U test for non-normally distributed data
Chi-square test
Figure 3.
The most recent set of matched NTproBNP and TR velocities (n=14) were used to generate the Receiver Operating Characteristic curve analysis. A logistic regression was used to test the association between TR velocity ≥ or < 2.5 m/second with NTproBNP and hemoglobin.
TR; tricuspid regurgitant
NYHA functional class and NTproBNP level
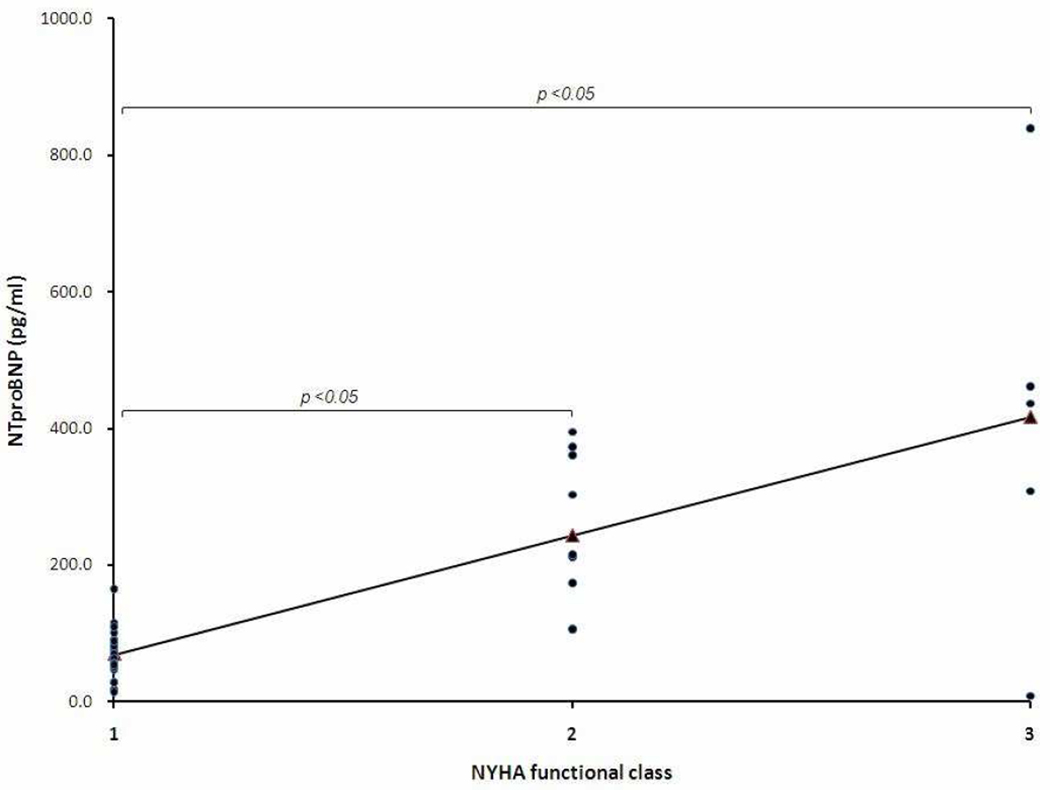
As shown in Figure 4, serum NTproBNP levels increased from NYHA functional class I (mean +/− standard deviation; 67.7+/−33.0 pg/ml, n=28), class II (249.6+/−112.5 pg/ml, n=9), to class III (410.9+/−299.8 pg/ml n=5). NTproBNP in NYHA class II and III were significantly higher than in class I (p <0.05), but not each other. Moreover, NTproBNP had a significantly positive correlation with NYHA functional class (r=0.69, p <0.05). In contrast, there was no significant correlation between TR velocity and functional class (r=0.36, p =0.15)
Figure 4.
NTproBNP had a significant correlation with NYHA functional class in children who are greater than 6 years-old. NTproBNP in NYHA class II (n=9) and III (n=5) were significantly higher compared with in class I (n=28) by Student t-test.
NYHA; New York Heart Association
Discussion
We found that NTproBNP levels had a significant inverse correlation with hemoglobin levels in children with SCD. This has been reported in adults with SCD.4 The inverse relationship is not well understood, although it is known that chronic anemia leads to activation of the renin–angiotensin–aldosterone system and hyperactivity of the sympathetic nervous system. In response, cardiac output, LV filling pressure, and LV end diastolic volume may be increased.12,13 In addition, myocardial ischemia associated with tissue hypoxia due to anemia may cause vasodilation and low systemic vascular resistance mediated by endothelium derived nitric oxide.14–17 Importantly, we also found that NTproBNP levels correlated with TR velocity, left ventricular inflow E velocity, mitral annulus E’ velocity, and E/E’ ratio suggesting LV diastolic dysfunction. Danzmann et al reported that left ventricular inflow E velocity is highly sensitive to preload and left atrial pressure can change dramatically as diastolic dysfunction progresses.18 In addition, E’ velocity reflects the velocity of early myocardial relaxation during early rapid LV filling. Decreased E’ velocity is an early marker for detecting diastolic dysfunction.11,19,20 LV filling pressures are correlated with the ratio of the LV inflow E wave to lateral E’ wave (E/E’). Elevation of the ratio due to reduced E’ and increased mitral E suggests LV diastolic dysfunction. Therefore, E’ velocity and E/E′ ratio have been shown to be excellent echocardiographic predictors of diastolic dysfunction.11,19–22 In adult patients with chronic anemia secondary to beta thalassemia major, elevated E/E′ ratios significantly correlated with elevated NTproBNP levels and diastolic dysfunction.23,24 Our study extends these findings to pediatric patients with SCD and suggests that elevated NTproBNP levels are a biomarker for LV diastolic dysfunction. The findings suggest the elevation of NTproBNP may be in response to LV overload and diastolic dysfunction due to chronic anemia. Although B-type natriuretic peptide has also been investigated, NTproBNP rather than B-type natriuretic peptide may be a more accurate biomarker because of its stability and longer half-life.25,26
Several SCD studies have suggested that NTproBNP levels predict the development of elevated TR velocity and perhaps pulmonary hypertension in adult patients with SCD.1,3,4 Elevated NTproBNP levels are associated with exercise intolerance (defined by the 6 minute walk test) as well as an independent risk factor for early mortality in adults with SCD.4,27,28 Similarly, we found that high NTproBNP levels significantly correlated with TR velocity in children with SCD. Previous reports suggested elevated NTproBNP levels may be secondary to hemolysis-related secondary pulmonary hypertension, but we did not find a significant difference in NTproBNP level between with and without elevation of TR velocity groups. Our results suggest the elevated NTproBNP is mainly due to LV diastolic dysfunction with chronic anemia as the NTproBNP level was not different between patients with or without an elevated TR velocity.
Previous studies have reported that serum NTproBNP concentration was increased in patients with advanced heart failure and was closely related to disease progression.29,30 Similarly, we found that NTproBNP may provide prognostic information for functional deterioration with LV diastolic dysfunction. In contrast, the TR velocity was not associated with functional class. Our findings suggest that screening for cardiac dysfunction with NTproBNP may be useful to identify children who should have a cardiac evaluation, thereby potentially improving the management of children with SCD by preventing functional status deterioration. Although cardiac management should not be based solely on the basis of NTproBNP levels, future research might find that NTproBNP can help guide evaluation for cardiac dysfunction in children with SCD.
Our study was limited by small numbers and was an observational cohort study from a single center. Therefore, a larger study involving pediatric patients with SCD is needed to determine whether the results we observed can be generalized to the larger pediatric sickle cell population. Due to the limited number of patients with available information on TR velocity in our study, further study should be performed to clarify whether NTproBNP level can predict TR velocity. In addition, hydoxyurea and transfusions therapies might potentially influence the natural history. However, despite these limitations, we suggest that NTproBNP measurements are easily obtained and may serve as a useful prognostic marker for the presence of diastolic dysfunction related chronic anemia in children with SCD.
Acknowledgment
We would like to thank Brandie D Wagner PhD, Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado Denver, for her advice in performing the statistical analysis in this manuscript.
Supported by the Jayden DeLuca Foundation, The Leah Bult Foundation, M01-RR00069 General Clinical Research Center, National Center for Research Resources, and National Institutes of Health.
Abbreviations
- LV
left ventricular
- NYHA
New York Heart Association
- NTproBNP
N-terminal fragment of B-type natriuretic peptide
- SCD
sickle cell disease
- TR
tricuspid regurgitant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 2.Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 4.Voskaridou E, Tsetsos G, Tsoutsias A, Spyropoulou E, Christoulas D, Terpos E. Pulmonary hypertension in patients with sickle cell/beta thalassemia: incidence and correlation with serum N-terminal pro-brain natriuretic peptide concentrations. Haematologica. 2007;92:738–743. doi: 10.3324/haematol.11136. [DOI] [PubMed] [Google Scholar]
- 5.Souza R, Jardim C, Julio Cesar Fernandes C, Silveira Lapa M, Rabelo R, Humbert M. NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med. 2007;101:69–75. doi: 10.1016/j.rmed.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Aliyu ZY, Suleiman A, Attah E, Mamman AI, Babadoko A, Nouraie M, et al. NT-proBNP as a marker of cardiopulmonary status in sickle cell anaemia in Africa. Br J Haematol. 2010;150:102–107. doi: 10.1111/j.1365-2141.2010.08195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 9.Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, et al. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177:646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 10.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–782. doi: 10.1542/peds.2007-0730. [DOI] [PubMed] [Google Scholar]
- 11.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 13.Okonko DO, Anker SD. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. 2004;10:S5–S9. doi: 10.1016/j.cardfail.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. 1972;83:415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 15.Anand IS, Chandrashekhar Y, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70:357–362. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand IS, Chandrashekhar Y, Wander GS, Chawla LS. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol. 1995;25:1402–1407. doi: 10.1016/0735-1097(95)00007-Q. [DOI] [PubMed] [Google Scholar]
- 17.Desai AS, Bibbins-Domingo K, Shlipak MG, Wu AH, Ali S, Whooley MA. Association between anaemia and N-terminal pro-B-type natriuretic peptide (NT-proBNP): findings from the Heart and Soul Study. Eur J Heart Fail. 2007;9:886–891. doi: 10.1016/j.ejheart.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Danzmann LC, Bodanese LC, Kohler I, Torres MR. Left atrioventricular remodeling in the assessment of the left ventricle diastolic function in patients with heart failure: a review of the currently studied echocardiographic variables. Cardiovasc Ultrasound. 2008;6:56. doi: 10.1186/1476-7120-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 20.Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396–e398. doi: 10.1161/CIRCULATIONAHA.105.579268. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 23.Hamdy AM. Use of strain and tissue velocity imaging for early detection of regional myocardial dysfunction in patients with beta thalassemia. Eur J Echocardiogr. 2007;8:102–109. doi: 10.1016/j.euje.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Kremastinos DT, Tsiapras DP, Kostopoulou AG, Hamodraka ES, Chaidaroglou AS, Kapsali ED. NT-proBNP levels and diastolic dysfunction in beta-thalassaemia major patients. Eur J Heart Fail. 2007;9:531–536. doi: 10.1016/j.ejheart.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Pemberton CJ, Johnson ML, Yandle TG, Espiner EA. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension. 2000;36:355–359. doi: 10.1161/01.hyp.36.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Alibay Y, Beauchet A, El Mahmoud R, Brun-Ney D, Alexandre JA, Benoit MO, et al. Analytical correlation between plasma N-terminal pro-brain natriuretic peptide and brain natriuretic peptide in patients presenting with dyspnea. Clin Biochem. 2004;37:933–936. doi: 10.1016/j.clinbiochem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 28.Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Richards AM, Doughty R, Nicholls MG, MacMahon S, Sharpe N, Murphy J, Espiner EA, Frampton C, Yandle TG. Plasma n-terminal pro-brain natriuretic peptide and adrenomedullin: Prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Australia-new zealand heart failure group. J Am Coll Cardiol. 2001;37:1781–1787. doi: 10.1016/s0735-1097(01)01269-4. [DOI] [PubMed] [Google Scholar]
- 30.Kirk V, Bay M, Parner J, Krogsgaard K, Herzog TM, Boesgaard S, Hassager C, Nielsen OW, Aldershvile J, Nielsen H. N-terminal probnp and mortality in hospitalised patients with heart failure and preserved vs. Reduced systolic function: Data from the prospective copenhagen hospital heart failure study (chhf) Eur J Heart Fail. 2004;6:335–341. doi: 10.1016/j.ejheart.2004.01.002. [DOI] [PubMed] [Google Scholar]