Abstract
19F-modified bithiazole correctors and phenylglycine potentiators of the ΔF508-CFTR chloride channnels were synthesized and their function assayed in cells expressing human ΔF508-CFTR and a halide-sensitive fluorescent protein. Fluorine was incorporated into each scaffold using prosthetic groups for future biodistribution imaging studies using positron emission tomography (PET). The ΔF508-CFTR corrector and potentiator potencies of the fluorinated analogs were comparable to or better than those of the original compounds.
Keywords: Cystic fibrosis, PET, Corrector, Potentiator, ΔF508-CFTR
Cystic fibrosis (CF) is the most prevalent lethal hereditary disease among Caucasians, affecting approximately one in 2,500 individuals.1 The average life expectancy in CF is about 40 years. The principal cause of mortality in CF is deterioration of lung function caused by chronic lung infection.2,3 Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, the most common being a deletion of phenylalanine at position 508 (ΔF508).4,5 This mutation causes CFTR protein misfolding, resulting in retention and rapid degradation in the endoplasmic reticulum.6–9
Compounds have been synthesized that rescue defective ΔF508-CFTR protein processing (correctors) and defective chloride channel gating (potentiators).10–12 Bithiazole 1 (corr-4a) and indolylacetamidophenylacetamide 2 (PG-01) are the benchmark corrector and potentiator studied here, respectively (Figure 1). Structure-activity relationship studies based on corr-4a revealed that substitution of a 2-chloro-5-(N,N-dimethyl-amino)phenyl moiety for the 5-chloro-2-methoxyphenyl group improved water solubility (3; Figure 1)13 and that locking the bithiazole system into an s-cis conformation with a 7-membered ring (3; Figure 1) improved corrector efficacy.11
Figure 1.
ΔF508-CFTR correctors (1/3/4) and potentiator (2).
We recently reported the synthesis of a fluorescently-labeled bithiazole corrector 4 (Figure 1), which enabled the evaluation of its biodistribution in mice by fluorescence imaging.14 Though this approach produced information about the in vivo handling of a functional CF corrector, the inclusion of a fluorophore label resulted in poor compound metabolic stability, limiting the time over which the non-modified compounds could be studied. Also, the limited penetration of light into tissues precluded non-invasive whole-body imaging.
To address these issues, we report here a first step to apply positron emission tomography (PET) imaging to non-invasively visualize the biodistribution of a bithiazole corrector and a phenylglycine potentiator.15–17 Analogs suitable for PET imaging have the advantage of minimally modifying the original compound structure (replacing a H with an 18F),18 which increases the likelihood of maintaining activity while enabling in vivo monitoring of uptake and biodistribution. Indeed, imaging modalities can be relevant at all stages of CF drug development, with potential applications in animal models, tissue preparations, and human in vivo studies. Relevant imaging could enlighten at the basic mechanistic level leading to a better understanding CF pathophysiology, at the level of assessing the potential of new correctors or potentiators, at the effectiveness of treatment level, and/or at the level of measuring function to better drug delivery.19
PET produces images through the detection of positron emitting radioisotopes. The most commonly used radionucleotide is fluorine-18 due to its relatively long half-life (t1/2 = 110 min). Other elements, such as carbon (11C t1/2 = 20.4 min), nitrogen (13N t1/2 = 9.97 min), and oxygen (15O t1/2 = 2.04 min), have much shorter half-lives. To address the need for new disease-specific probes, prosthetic groups18 have been developed for the rapid incorporation of radioisotopes into the specific tracers. A variety of amine-reactive prosthetic groups have been developed, the most commonly being [18F]N-succinimidlyl-4-fluorobenzoate (5; Figure 2).20 Other 18F-acylating agents include the carboxylate reactive [18F]4-fluoroaniline prosthetic group (6; Figure 2).21 A strategy that has received considerable attention is the use of click chemistry to incorporate small molecular weight 18F-labeled alkynes such as [18F]5-fluoroalkyne (7; Figure 2) into azide substituted peptides.22 Copper(I)-catalyzed 1,2,3-triazole formation is fast, chemoselective, and performed under mild conditions.22
Figure 2.
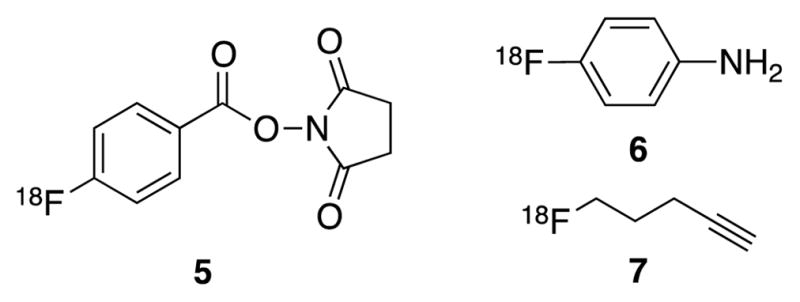
Potential 18F-prosthetics.
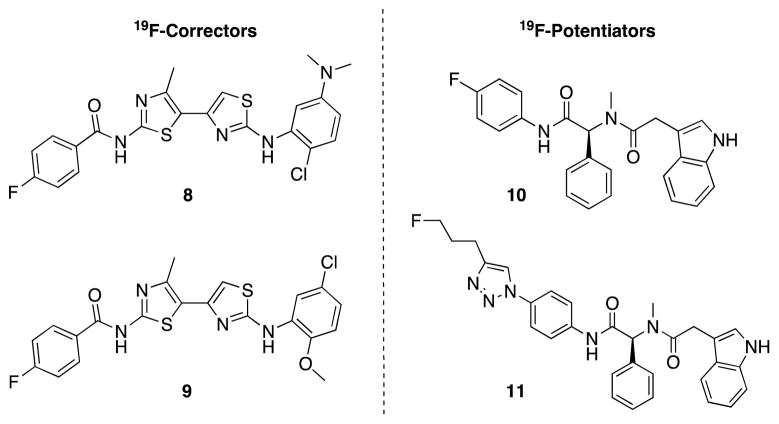
As a first step in enabling CF-relevant PET studies, we report here the design, synthesis, and ΔF508-CFTR activity of two 19F-labeled correctors (8 and 9; Figure 3) and two 19F-labeled potentiators (10 and 11; Figure 3). Since 18F has a 110 min half-life, late-stage introduction of the fluorinated moiety is essential and, consequently, targets 8–11 were designed with the aforementioned 18F-prosthetic groups in mind.
Figure 3.
Fluorine-containing corrector and potentiator analogs.
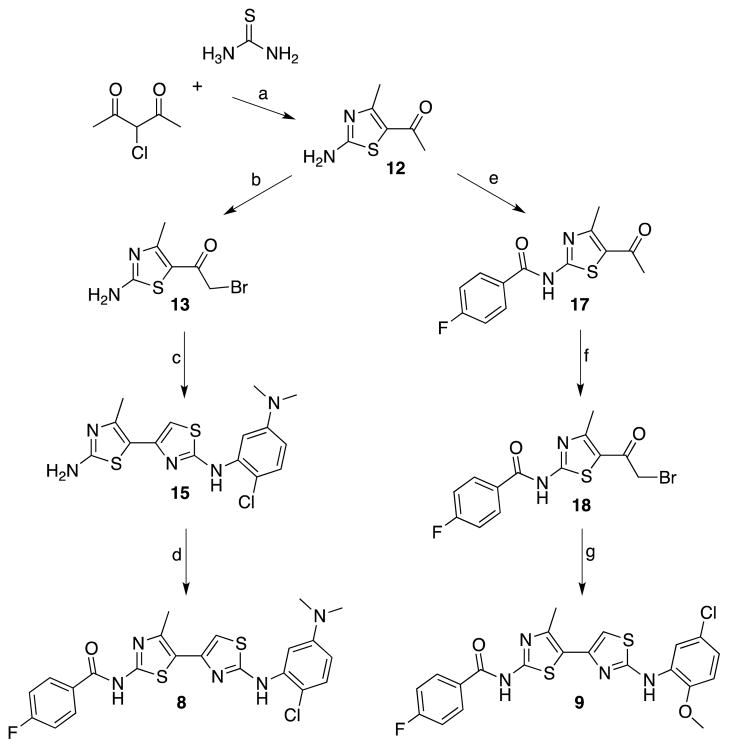
Targeting fluorobenzoyl analogs 19F-8 and 19F-9 (Scheme 1), the first synthetic step was the condensation of 3-chloropentane-2,4-dione with thiourea in refluxing ethanol over 12 h to afford aminothiazole 12 in 95% yield.11 The acetyl group of 12 was subsequently α-brominated with bromine in acetic acid to produce 13 in 86% yield. Aminothiazole building block 15 was obtained in 79% yield by the condensation of 13 with 1-(2-chloro-5-dimethylaminophenyl)thiourea (14) in refluxing ethanol. The final step in the synthesis of 19F-bithiazole 8 proved to be more challenging than expected as numerous attempts to couple it with fluorobenzoic acid by amide-coupling condensation with HATU, EDC, or CDI proved unsuccessful. Eventually, bithiazole 15 was acylated with 4-fluorobenzoyl chloride (prepared by treatment of 4-fluorobenzoic acid with oxalyl chloride) in the presence of triethylamine at room temperature (10 min) to afford 19F-8.
Scheme 1.
Synthesis of fluorinated corrector analogs.a
aReagents: (a) EtOH, reflux; (b) Br2, AcOH; (c) 1-(2-chloro-5-dimethylaminophenyl)thiourea (14), EtOH, reflux; (d) i. 4-fluorobenzoic acid, CH2Cl2, oxalyl chloride, ii. 4-fluorobenzoyl chloride (16), CH2Cl2, TEA; (e) 4-fluorobenzoic acid, CDI, DMF, 100 °C; (f) PyrH+Br3−, 33% HBr in AcOH; (g) 1-(5-chloro-2-methoxyphenyl)thiourea (19), EtOH, reflux.
Given these difficulties, a new strategy was developed for the synthesis of fluorinated bithiazole 9. Work began here with the CDI-mediated coupling of aminothiazole 12 with 4-fluorobenzoic acid. Thiazole 17 was markedly easier to purify than 8. Bromination of 17 proved significantly more difficult than 12, requiring the use of a stronger acidic medium (HBr vs. AcOH) with pyridinium tribromide to afford 18 (91% yield). The final reaction involved condensation of 18 with 1-(5-chloro-2-methoxyphenyl)thiourea (19), delivering 19F-9 in 76% yield.
Yield and purification difficulties were due to the poor solubility of aminobithiazole intermediate 15 as well as the corresponding fluorinated bithazoles 8 and 9. Because [18F]N-succinimidlyl-4-fluorobenzoate (18F-SFB) would be used in picomolar quantities for the final coupling to aminobithiazole intermediates, the reaction solution would be quite dilute and solubility is not expected to be a significant issue.
The ΔF508-CFTR corrector activity of 19F-8 and 19F-9 was assayed using a cell-based fluorescence assay in Fischer rat thyroid (FRT) cells co-expressing human ΔF508-CFTR and the halide-sensitive fluorescent protein YFP-H148Q/I152L.11 The concentration-dependence data show slightly greater potency of 19F-8 and 19F-9, compared to benchmark corrector corr-4a, as shown in Figure 4. EC50 values (in μM) were: 8 = 1.4; 9 = 1.4; corr-4a = 2.3].
Figure 4.

Concentration-dependence of ΔF508-CFTR corrector activity of 19F-8 and 19F-9 compared to corr-4a.
Synthesis of fluoroaniline potentiator 19F-10 (Scheme 2) began with an EDC-mediated coupling of 4-fluoroaniline to Boc-N-methyl-L-phenylglycine (Scheme 2).10,23 Boc-protected 21, obtained in 61% yield, was then deprotected by treatment with TFA and subsequent EDC-activated coupling with 3-indole acetic acid and DMAP gave 19F-10 in 72% yield over two steps.
Scheme 2.
Synthesis of fluorinated potentiator 19F-10 and azido intermediate 23.a
aReagents: (a) CuI, NaN3, L-proline, NaOH, DMSO, 60 °C; (b) EDC, HOBt, DMF, DCM, 0 °C to rt; (c) i. TFA, DCM, ii. EDC, DMAP, DCM, DMF, 0 °C to rt.
Synthesis of 4-(3-fluoropropyl)-1H-1,2,3-triazole analog 11 began with the synthesis of 4-azidoaniline via a CuI-catalyzed, proline-promoted coupling reaction24 followed by an EDC-mediated condensation of 20 (Scheme 2) with Boc-N-methyl-L-phenylglycine to afford 22 (20, 74%; and 22, 97%, respectively). Subsequent Boc removal (TFA) followed by treatment with EDC-activated 3-indole acetic acid and DMAP afforded azido phenylglycine intermediate 23 (90% yield).
The synthesis of 5-fluoroalkyne for the triazole-forming cycloaddition with azido-phenylglycine intermediate 23 proved to be unsuccessful. Initially, we focused on utilizing diethylaminosulfur trifluoride (DAST) for the conversion of the alcohol moiety of 4-pentyne-1-ol into an alkyl fluoride.25 Unfortunately, no fluorinated alkyne was isolated. Das et al. reported that ionic liquids can be used in an improved purification procedure of the dehydroxy-fluorination reaction with DAST; again, no desired product was isolated.26 Likewise, a modified procedure employing tosylate 24 (synthesized by the treatment of 4-pentyn-1ol with p-toluenesulfonyl chloride and triethylamine27) and nucleophilic fluoride [KF plus 18-Crown-6 or Bu4N+F−(tBuOH)428] was unsuccessful.
With this as a backdrop, tosylate 24 was clicked to azido intermediate 23 in a Huisgen copper-catalyzed 1,3-dipolar cycloaddition to afford 25 in 85% yield (Scheme 3). Finally, potentiator analog 19F-11 was obtained by treatment of 25 with Bu4N+F−(tBuOH)4 (26) in 31% yield.
Scheme 3.
Synthesis of fluorinated potentiator 19F-11.
aReagents: (a) TsCl, TEA, DCM, 0 °C to rt; (b) Na ascorbate, CuSO4, 23, DCM, tBuOH, H2O; (c) i. Bu4N+F− H2O, tBuOH, hexane, 90 °C, 30 min forms Bu4N+F−(tBuOH)4 (26), ii. 25, 26, ACN, 70 °C, 1 h.
The ΔF508-CFTR potentiator activity of 19F-10 and 19F-11 was assayed in FRT cells co-expressing ΔF508-CFTR and YFP-H148Q/I152L after rescue of ΔF508-CFTR by 24 h culture at 27 °C.11,12 Fluorinated phenylglycine analogs had EC50 of 0.09 μM (19F-10) and 1.1 μM (19F-11), comparable to that of 0.3 μM reported previously for PG-01,10 and much better than that of 7.0 μM for benchmark potentiator genistein (Figure 5).
Figure 5.
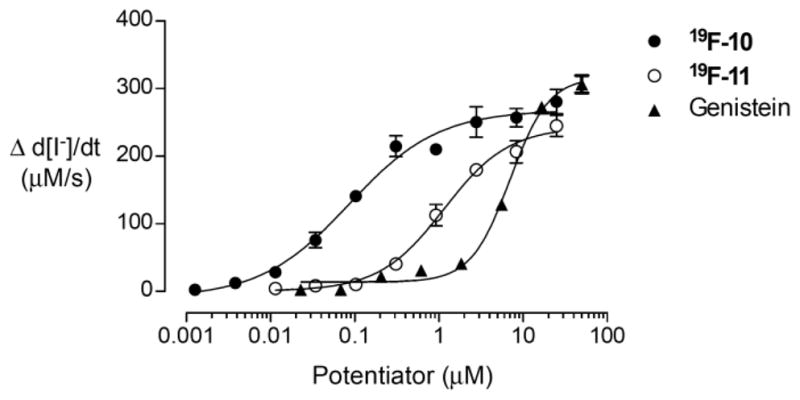
Concentration-dependence of ΔF508-CFTR potentiator activity of 19F-10 and 19F-11 compared to genistein.
The retained corrector activity of 19F-8 and 19F-9 and potentiator activity of 19F-10 is likely the consequence of the enhanced binding interactions of fluorine.29 Indeed, fluorine is found in approximately 5–15% of drugs that have come to market over the past 50 years.29 Fluorine has also been shown to improve metabolic stability and generally enhance physicochemical properties,29 features which would be useful in future in vivo studies.
In conclusion, two active fluorinated corrector analogs and two active fluorinated potentiator analogs were synthesized and the introduction of a fluorine atom was found to improved potency. Both fluorinated correctors (19F-8 and 19F-9) and a fluorinated potentiator (19F-10) showed improved or comparable activity to the original compounds. These active, fluorinated analogs are potentially useful for non-invasive PET imaging of in vivo compound uptake and biodistribution. Given the short half-life of 19F (t1/2 = 110 min), the next step en route to PET studies with these compounds will be to develop synthetic routes to 19F-8, 19F-9, and 19F-10 where the 19F moiety is introduced in the last synthetic step. Current efforts are focused on developing viable and high-yielding routes to 19F-8 from N2-(2-chloro-5-(dimethylamino)phenyl)-4′-methyl-[4,5′-bithiazole]-2,2′-diamine (15) + 7 and 19F-10 from (S)-2-(2-(1H-indol-3-yl)-N-methylacet-amido)-2-phenylacetic acid + 6.
Supplementary Material
Acknowledgments
The authors thank Prof. Julie Sutcliffe and Ms. Robin Cumming (UC Davis, Department of Biomedical Engineering) for helpful suggestions in selecting prosthetic groups and, for financial support, the Tara K. Telford Fund for Cystic Fibrosis Research at the University of California/Davis, the National Institutes of Health (Grants DK072517 and GM076151), and the National Science Foundation [Grants CHE-0910870, CHE-0443516, CHE-0449845, and CHE-9808183 (NMR spectrometers)].
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:#####.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Bobadilla J, Macek M, Fine JP, Farrell PM. Hum Mutat. 2002;19:575. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Dankert-Roelse JE, te Meerman GJ. Thorax. 1995;50:712. doi: 10.1136/thx.50.7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJV, Verkman AS. J Clin Invest. 2005;115:2564. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilewski JM, Frizzell RA. Physiol Rev. 1999;79:S215. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard DN, Welsh MJ. Physiol Rev. 1999;79:S23. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 6.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Nature. 1992;358:761. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 7.Lukacs GL, Mohamed A, Kartner N, Chang AB, Riordan JR, Grinstein S. EMBO J. 1994;13:6076. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopito RR. Physiol Rev. 1999;79:S167. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 9.Du K, Sharma M, Lukacs GL. Nat Struct Mol Biol. 2005;12:17. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 10.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, Kurth MJ, Galietta LJV, Verkman AS. Mol Pharmacol. 2005;67:1797. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 11.(a) Yoo CL, Yu GJ, Yang B, Robins LI, Verkman AS, Kurth MJ. Bioorg Med Chem Lett. 2008;18:2610. doi: 10.1016/j.bmcl.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu GJ, Yoo CL, Yang B, Lodewyk MW, Meng L, El-Idreesy TT, Fettinger JC, Tantillo DJ, Verkman AS, Kurth MJ. J Med Chem. 2008;51:6044. doi: 10.1021/jm800533c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, Galietta LJ, Verkman AS. J Biol Chem. 2003;278:35079. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 13.Yu GJ. Golden Bridge to Cystic Fibrosis: Heterocycles. University of California; Davis: 2009. [Google Scholar]
- 14.Davison HR, Taylor S, Drake C, Phuan P-W, Derichs N, Yao C, Jones EF, Sutcliffe J, Verkman AS, Kurth MJ. Bioconjugate Chem. 2011 doi: 10.1021/bc2004457. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambhir S. Nat Rev Cancer. 2002;2:683. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 16.Judweid ME, Cheson BD. N Engl J Med. 2006;354:496. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 17.Kubota K. Ann Nuc Med. 2001;15:471. doi: 10.1007/BF02988499. [DOI] [PubMed] [Google Scholar]
- 18.Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem Int Ed. 2008;47:8998. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 19.King GG. Pulm Pharmacol Ther. 2011;24:497. doi: 10.1016/j.pupt.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Wester HJ, Schottelius M. PET Chemistry. 2007;64:79. doi: 10.1007/978-3-540-49527-7_4. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Gifford A, Liu Q, Thotapally R, Ding YS, Makriyannis A, Gatley SJ. Nucl Med Biol. 2005;32:361. doi: 10.1016/j.nucmedbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Marik J, Sutcliffe JL. Tetrahedron Lett. 2006;47:6681. [Google Scholar]
- 23.Mills AD, Yoo CY, Butler JD, Yang B, Verkman AS, Kurth MJ. Bioorg Med Chem Lett. 2010;20:87. doi: 10.1016/j.bmcl.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, Ma D. Chem Commun. 2004:888. doi: 10.1039/b400878b. [DOI] [PubMed] [Google Scholar]
- 25.Hausner SH, Marik J, Gagnon KJ, Sutcliffe JL. J Med Chem. 2008;51:5901. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Chandrasekhar S, Yadav JS, Gree R. Tetrahedron Lett. 2007;48:5305. [Google Scholar]
- 27.Aucagne V, Berna J, Crowley JD, Goldup SM, Hanni KD, Leigh DA, Lusby PJ, Ronaldson VE, Slawin AMZ, Viterisi AL, Walker DB. J Am Chem Soc. 2007;129:11950. doi: 10.1021/ja073513f. [DOI] [PubMed] [Google Scholar]
- 28.Kim DW, Jeong H, Lim ST, Sohn M. Angew Chem Int Ed. 2008;47:8404. doi: 10.1002/anie.200803150. [DOI] [PubMed] [Google Scholar]
- 29.Hagmann WK. J Med Chem. 2008;51:4359. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.