Abstract
Background
Hepatitis C virus (HCV) has a lower prevalence in children and knowledge is limited regarding the natural outcome of HCV infection in children.
Aim
To study the risk factors of HCV acquisition and predictors of persistence in Egyptian children.
Methods
Children, one to 9 years of age, were evaluated for acquisition of HCV (anti-HCV positive regardless of viremia) and persistence of HCV (anti-HCV and HCV-RNA positive) at two pediatric hepatology clinics in Cairo at enrollment and at three-monthly intervals. Spontaneous clearance of HCV was defined as ≥2 positive anti-HCV antibody tests with negative HCV-RNA at least six months apart.
Results
Over a 33-month-period a total of 226 children <9 years of age were screened for HCV antibodies. Of those, 146 (65%) were anti-HCV positive of which 87 (60%) were HCV-RNA positive. HCV acquisition was more likely to occur in older children (p=0.003) with co-morbid conditions (p<0.01) compared to anti-HCV negative children. In a multivariate logistic regression analysis, the highest risk factors for HCV acquisition were surgical interventions [odds ratio (OR): 4.7] and blood transfusions (OR: 2.3). The highest risk factor for HCV persistence was dental treatment (OR: 16.9) and male gender (OR: 7.5). HCV persistence was also strongly associated with elevated baseline alanine aminotransaminase (ALT) levels (OR: 4.9) and fluctuating aspartate aminotransferase (AST) levels (OR: 8.1).
Conclusion
While surgical interventions and blood transfusion are significant risk factors for HCV acquisition in Egyptian children, dental treatment remains the highest risk factor for HCV chronic persistence in children.
Keywords: Children, Egypt, HCV, Persistent HCV infection, Spontaneous clearance of HCV
INTRODUCTION
Egypt has the highest worldwide prevalence of hepatitis C virus (HCV) infection (15%). [1–3] Prevalence rises steeply with age with anti-HCV antibodies detected in 2 to 7% in children under 10 years, about 10% in those 10–20 years of age, and in more than half the individuals between the age of 40 and 50 years in rural areas in the Nile Delta region.[4–7]
The progression of HCV infection to such serious outcomes as cirrhosis and hepatocellular cancer is well studied in adults [8], but little is known about the long-term outcome of infection in children. Early studies suggested that childhood infection is relatively benign, but the duration of follow-up in these studies was limited. [9–11] In Egypt few studies highlighted the epidemiological data and disease progression of HCV-infected children. In 1995, El-Nanawy and colleagues reported an HCV seroprevalence rate of 12%, 44%, and 29% in a random sample of normal children, thalassemic children, and in children with insulin dependent diabetes mellitus (IDDM) respectively. [12] More recently, a study reported that anti-HCV antibody was detected in 82% of children with haematological disorders (of which 49% were HCV-RNA positive) and 18% of children with malignancies (24% HCV-RNA positive). [13]
Another study retrospectively analyzing data from 105 anti-HCV-positive Egyptian children found that HCV RNA was detected in more than half of those tested. Sixty percent of the HCV RNA-positive children underwent a diagnostic liver biopsy that showed chronic hepatitis in 73%, cirrhosis in only one, while seven children (27%) had normal liver biopsies. Thus, suggesting that HCV infection is frequently not a benign disease during childhood. [14]
Iatrogenic infection remains the highest risk factor for HCV acquisition in Egypt, [15, 16] which has prompted multiple national programs to raise public awareness and establish infection control programs at healthcare facilities throughout Egypt.[17] However, most of these programs are aimed towards adults where the risk is perceived to be highest, and have mainly targeted larger healthcare facilities. Smaller healthcare units such as pediatric and school-based clinics remain largely unchanged and are a potential source of exposure to HCV and other bloodborne infections.[18]
In this study, we describe a unique pediatric population where infection was mostly acquired iatrogenically and many have co-morbid illnesses that could affect or exacerbate the liver injury associated with chronic HCV. The primary objective of this descriptive longitudinal study was to investigate risk factors for acquisition of HCV infection and to determine the predictors of HCV persistence in this pediatric population.
MATERIAL AND METHODS
Subjects
Children from both genders, one to 9 years of age, who were known to be positive for anti-HCV antibody, or who were at risk of exposure to HCV infection (defined as those living with HCV-infected family members, or who are exposed to blood-products, or had undergone surgical procedures) were referred to two specialty clinics for testing. These clinics were established to provide care for HCV-infected children, and are located at Cairo University Pediatric Hospital (CUPH) and at the National Hepatology and Tropical Medicine Research Institute (NHTMRI) affiliated to the Egyptian Ministry of Health. The children followed in this cohort were mostly referred from Cairo and Giza, and to a lesser degree from other Egyptian governorates. Although they were not selected by random sampling of the general population, they were quite likely a representative sample of HCV-infected children in Egypt, given the wide variety of referral sources.
All children were screened for anti-HCV after obtaining a written informed consent from their parents. The relevant institutional Ethics Review Committees approved the study protocol.
All subjects were evaluated at baseline using a detailed standardized questionnaire describing demographic characteristics and potential risk factors. A detailed medical history that included past or present history of jaundice or hepatitis, and family history of HCV infection or other related liver diseases were recorded at baseline. All subjects underwent a complete physical examination. Laboratory investigations included a complete blood count, liver function tests including total and direct serum bilirubin, liver transaminases, total serum albumin, and prothrombin time and concentration. In addition, a detailed abdominal ultrasound examination using an FFsonic UF-4100 was performed at baseline. All anti-HCV positive children were retested for HCV-RNA.
Almost two-third of the anti-HCV positive subjects were followed for up to 36 months at three-month-monthly intervals. None had received treatment with antiviral drugs. Physical examination was performed at each visit along with laboratory evaluation for liver transaminases, synthetic and excretory liver functions. Complete blood count, HCV-RNA testing and an abdominal ultrasound were also performed annually. HCV acquisition was defined as the presence of anti-HCV antibodies regardless of viremia, while HCV persistence or chronic HCV infection, was defined as the presence of both anti-HCV antibodies and HCV-RNA viremia. HCV clearance was defined as ≥2 positive anti-HCV antibody tests but negative HCV-RNA on 2 consecutive visits at least 6 months apart. This is the same definition used by The European Paediatric Hepatitis C Virus Network in a recently published study. [19]
Serological testing
Serum samples were collected in plain vacutainer tubes (BD, USA,) and tested for HCV antibodies by AxSym® HCV version 3.0. This is a Microparticle Enzyme Immunoassay (MEIA) for the qualitative detection of anti-HCV in human serum or plasma.[20] MEIA is a variation of the enzyme immunoassay (EIA) technique. Viral markers for hepatitis B virus (HBV) were also tested. Hepatitis B surface antigen (HBsAg) and antibody to hepatitis B virus core antigen (anti-HBc) were tested by Axsym HBsAg version 2 and AxSym® CORE respectively. Both are Microparticle Enzyme Immunoassays for the qualitative detection of antibody in human serum or plasma.
Virological testing
Anti-HCV positive cases underwent HCV RNA detection by reverse transcriptase polymerase chain reaction (RT-PCR).[21]
Statistical methods
Risk factors, historical, clinical, and laboratory data were all analyzed in different groups using univariate and multivariate regression analysis where acquiring HCV infection was the dependent variable. Of particular interest was also comparing those children who were aviremic anti-HCV positive with those who had active HCV infection. Chi-square tests were performed for categorical data, while t-student test (or Mann Whitney U test when appropriate) was performed for continuous data.
RESULTS
Over a 33-month-period, a total of 226 subjects (59% males) between one and 9 years of age who were either known to be anti-HCV positive or who were at risk of acquiring the virus were enrolled in the study and tested for anti-HCV. Of those, 146/226 (65%) subjects were anti-HCV positive at the time of screening and were eligible for recruitment into the study. Demographic characteristics and laboratory indices in these children were compared with the anti-HCV negative subjects and are shown in Table 1. The anti-HCV positive children were significantly older (p=0.003) and were more likely to have co-morbid conditions (p<0.01) compared to the anti-HCV negative children. Not surprisingly ALT and AST levels were significantly higher in the anti-HCV positive group compared to the anti-HCV negative group (p<0.01), while other laboratory indices were comparable in both groups. Among the 146 anti-HCV positive subjects, serum ALT and AST levels were elevated in 59% (p<0.01) and 69% (p<0.008) of studied children respectively.
Table 1.
Demographic characteristics of children with or without anti-HCV antibodies at evaluation
| Anti-HCV negative (n=80) | Anti-HCV positive (n=146) | P-value | |
|---|---|---|---|
| Demographic features | |||
| Age in years (mean ±SD) | 5 ± 2 | 6 ± 2 | 0.003 |
| Gender: Male (n=134) | 49 (61) | 85 (58) | 0.89 |
| Female (n=92) | 31 (39) | 61 (41) | |
| Body mass index (mean ±SD) | 16±2 | 16± 2 | 0.74 |
| Residence : Urban (n=149) | 56 (74) | 93 (66) | 0.22 |
| Rural (n=69) | 20 (26) | 49 (35) | |
| History of HCV infection in family members | 39 (50.0%) | 39 (28.9%) | 0.002 |
| Comorbid conditions | 0 (0%) | 67 (45.9%) | <0.01 |
| Laboratory results | |||
| Elevated ALT levels | 9 (12) | 86 (59) | <0.01 |
| Elevated AST levels | 6 (24) | 100 (69) | <0.008 |
| ALT median (min-max) | 19 (12–276) | 51 (12–559) | <0.01 |
| AST median (min-max) | 31.5 (21–265) | 59.5 (22–617) | <0.01 |
| Total bilirubin median (min-max) | 0.5 (0.2–2) | 0.6 (0.1–32.5) | 0.31 |
| Direct bilirubin median (min-max) | 0.1 (0–0.6) | 0.1 (0–1.8) | 0.07 |
P values were calculated using chi-square tests for categorical data, and t-student test (or Mann Whitney U test when appropriate) for continuous data.
SD: standard deviation
Values are shown in number (%) if not mentioned otherwise
In a multivariate logistic regression analysis in which HCV acquisition is the dependent variable; surgical interventions seemed to be the most significant risk factor (odds ratio, OR:4.7, 95%CI: 1.6–14.1) followed by blood transfusion (OR: 2.3, 95%CI: 1.1–4.9) (Table 2). Few children underwent surgical procedures following motor vehicle accidents (e.g. splenectomy). Others had minor procedures (e,g. male circumcision, incisions, wound suturing, urinary catheterization, and peripheral venous catheterization).
Table 2.
Univariate and multivariate regression analysis of risk factors for acquisition of HCV infection in Egyptian children
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | p-value | CI | OR | p-value | CI | |
| Circumcision | 0.69 | 0.18 | 0.4–1.2 | |||
| Used syringes | 0.60 | 0.14 | 0.3–1.2 | |||
| Surgical Interventions | 6.42 | 0.01 | 2.9–14.3 | 4.7 | 0.006 | 1.5–14 |
| Peripheral venous catheter | 4.0 | 0.003 | 1.6–9.9 | 2.8 | 0.08 | 0.86–9.3 |
| Abscess evacuation | 7.2 | 0.06 | 0.9–56.3 | |||
| Urine catheter | 4.6 | 0.02 | 1.3–15.9 | |||
| Blood transfusion | 3.5 | 0.01 | 2–6.2 | 2.3 | 0.03 | 1.1–4.9 |
| Endoscopy | 2.4 | 0.13 | 0.8–7.4 | |||
| Urinary Schistosomiasis | 1.0 | 0.68 | 0.9–1.3 | |||
| Ear piercing | 0.96 | 0.31 | 0.9–1.0 | |||
| Cautery | 1.13 | 0.92 | 0.1–12.7 | |||
| Family member with HCV | 1.1 | 0.39 | 0.9–1.3 | |||
| Tattooing | 0.13 | 0.07 | 0.01–1.2 | 0.03 | 0.02 | 0.001–0.58 |
Comorbid conditions were present in 45.9% of anti-HCV-positive subjects and included chronic blood diseases such as thalassemia and hemophilia (59%), insulin-dependent diabetes mellitus (12%) and other comorbid conditions (29%). Children with co-morbid conditions had lower a body mass index (p= 0.02), and significantly higher incidence of abdominal pain, jaundice, pallor, hepatomegaly and splenomegaly (data not shown).
Of the 146 anti-HCV-positive subjects only 87 (60%) were HCV-RNA positive, and of those, 74% and 81% had high ALT and AST levels respectively, which was significantly higher than those who were anti-HCV positive but HCV-RNA negative (p<0.01). The risk ratio for elevated ALT levels was significantly higher among HCV-RNA positive children compared to HCV-RNA negative children [RR: 1.8; 95% CI: 1.3–2.6]. There were no significant differences among children in gender, age, body mass index (BMI), risk factors, clinical features or ultrasonographic findings compared to those who were HCV-RNA negative.
Of 146 anti-HCV positive children, 96 (66%) were followed for up to 36 months with a median follow up duration of 12 months (Min 6 months - Max 36 months). There was a median of 4 visits (range: 3–13), with approximately 3 months interval between visits. Among these 96 children, 62 (65%) were HCV-RNA positive and 34 (35%) were HCVRNA negative at baseline. Among the 62 HCV-RNA positive children 4 became HCVRNA negative during follow-up and 7 of 34 HCV-RNA negative children became positive during follow-up (Figure 1). Thus by the end of the follow-up period, a total of 31 of 96 anti-HCV positive subjects (32.3%) either remained or became HCV-RNA negative (spontaneous HCV clearance group) whereas 65 (67.7%) subjects had detectable HCV-RNA (persistent HCV infection group). A comparison of baseline characteristics between the patients who had spontaneous clearance and those with persistent HCV infection is presented in (Table 3).
Figure 1.
Study flow diagram: Flow chart of 96 anti-HCV positive children followed-up
Table 3.
Baseline characteristics of children with spontaneous HCV clearance and those with persistent HCV infection
| Characteristic (number [%] unless otherwise specified) | Total (n=96) | Spontaneous clearance group (n=31) | Persistent HCV infection group (n=65) | p-value | |
|---|---|---|---|---|---|
| Gender | Male | 53 (55.2) | 11 (35.5) | 42 (64.6) | 0.01 |
| Female | 43 (44.8) | 20 (64.5) | 23 (35.4) | ||
| Age | Mean ± SD | 5.9 ± 2.4 | 5.8 ± 2.6 | 5.9 ± 2.3 | 0.89 |
| Body mass index | Mean ± SD | 16.7 ± 2.7 | 16.5 ±2.7 | 16.7 ±2.7 | 0.74 |
| Residence | Rural | 32 (34.0) | 11 (35.5) | 21 (33.3) | 0.84 |
| Urban | 62 (66.0) | 20 (64.5) | 42 (66.7) | ||
| Abdominal pain | 46 (47.9) | 17 (54.8) | 29 (44.6) | 0.39 | |
| Abdominal distention | Mild | 35 (36.5) | 15 (48.4) | 20 (30.8) | 0.23 |
| Severe | 5 (5.2) | 1 (3.2) | 4 (6.2) | ||
| Jaundice | 26 (27.4) | 11 (35.5) | 15 (23.4) | 0.22 | |
| Elevated AST | 69 (71.9) | 17 (54.8) | 52 (80.0) | 0.01 | |
| Elevated ALT | 58 (61.1) | 13 (41.9) | 45 (70.3) | 0.008 | |
| Serum albumin | Mean ± SD | 3.6 ± 0.6 | 3.5 ± 0.8 | 3.8 ± 0.5 | 0.029 |
| Total bilirubin | Median (Min-Max) | 0.60 (0.1–9.3) | 0.60 (0.2–7) | 0.60 (0.1–9.3) | 0.61 |
| Direct bilirubin | Median (Min-Max) | 0.10 (0–5.7) | 0.10 (0–4.4) | 0.10 (0–5.7) | 0.42 |
| AST (ULN 40) | Median (Min-Max) | 59.0 (22–402) | 43.0 (22–357) | 66.0 (24–402) | 0.057 |
| ALT (ULN 40) | Median (Min-Max) | 51.0 (12–559) | 23.0 (12–281) | 57.0 (12–559) | 0.005 |
| Prothrombin time | Mean ± SD | 13.5 ± 1.5 | 13.9 ± 2.2 | 13.4± 1.0 | 0.66 |
| White cells count | Mean ± SD | 11.2 ± 10.6 | 10.3 ± 8.8 | 11.7 ± 11.4 | 0.83 |
| Hemoglobin | Mean ±SD | 10.7 ± 1.9 | 10.4 ± 1.5 | 10.8 ± 2.1 | 0.26 |
| Liver in midclavicular line (cm) by ultrasound | Median (Min-Max) | 0 (0–8) | 1 (0–8) | 0 (0–7) | 0.26 |
| Liver echo-pattern by ultrasound | Normal | 71 (76.3) | 21(72.4) | 50 (78.1) | 0.19 |
| Bright | 4 (4.3) | 0 (0) | 4 (6.3) | ||
| Coarse | 18 (19.4) | 8 (27.6) | 10 (15.6) | ||
| Spleen by ultrasound | Normal | 68 (73.1) | 21 (72.4) | 47 (73.4) | 0.97 |
| Enlarged | 18 (19.4) | 6 (20.7) | 12 (18.8) | ||
| Absent (removed) | 7 (7.5) | 2 (6.9) | 5 (7.8) |
Male gender, elevated baseline ALT and AST levels were significantly associated with persistent HCV infection in the studied children (p<0.05). Serum albumin level showed a significant decrease (though within normal level) in the group that spontaneously cleared the virus.
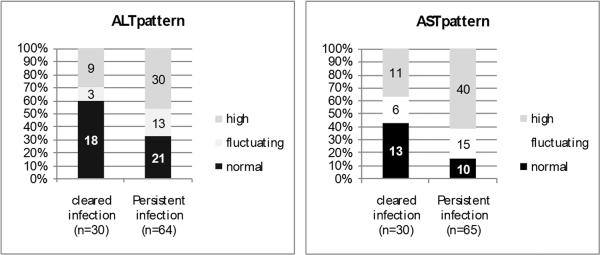
During the follow-up period, ALT and AST values varied widely among children in both groups, but were significantly higher and fluctuating in patients with persistent HCV infection compared to children who spontaneously cleared the virus (p=0.04 and 0.01 respectively) (Figure 2). There were no significant differences between groups in prothrombin time, serum albumin, total bilirubin and abdominal ultrasound findings.
Figure 2.
Pattern of transaminases in the studied groups. P value 0.04 and 0.01 for ALT and AST patterns (respectively) in the 2 studied groups.
All variables achieving significance at the level of p<0.25 on univariate analysis were identified and entered into a stepwise multivariate logistic regression analysis; where the dependent variable was persistent HCV infection. Dental treatment (OR:16.9, p=0.01), the fluctuating pattern of AST levels in follow up period (OR:8.1, p=0.024), male gender (OR:7.5, p=0.002), and high baseline ALT (OR:4.9, p=0.027) were significantly associated with persistence of HCV infection in the studied patients compared to anti-HCV positive/RNA-negative children.
DISCUSSION
HCV infection occurs less frequently in children than in adult patients, and the natural history, prognosis and clinical significance of HCV infection are poorly defined in childhood.[22, 23] In contrast, HCV infection in adults presents a high degree of chronicity, with up to 50% of all HCV-infected adults developing progressive liver disease [8]
In this study, using multivariate logistic regression analysis in which acquiring HCV infection is the dependent variable; surgical interventions and blood transfusions were almost five times and twice as common to have occurred among anti-HCV positive children, respectively. This is also true in other developing countries like Pakistan where blood transfusion, non-sterile surgical and dental procedures are the main risk factors for HCV acquisition [24, 25]. In the stepwise multivariate logistic regression analysis, we found that dental treatment was 16.9 times more likely to have occurred among children with chronic persistent HCV infection compared to anti-HCV negative children. This strong association with dental treatment persisted even after adjusting for all other variables including socioeconomic status, age, gender, and residence. However, although the route of infection is biologically plausible, it can still be a proxy for another unknown confounder (such as a common risk factor) shared by those who attended the dental clinic.
HCV infection remains a major public health challenge in Egypt prompting major policy changes and awareness campaigns in the last decade.[17] While infection control programs have been implemented across the country, they have not been applied consistently with the greatest improvements noted in the private sector, army and university hospitals and the larger Ministry of Health facilities where greater resources have been allocated. Meanwhile, rural healthcare units, urban pediatric government clinics and public school-based clinics remain underfunded and have been much slower to change.[26] Given that most major surgical interventions are done at the better-supplied hospitals and healthcare facilities, HCV exposures in our cohort due to surgical interventions were less likely to occur and when they did, the viral exposure was lower, resulting in a greater likelihood of spontaneous clearance. This may explain how surgical interventions were the highest risk for HCV acquisition in our study, but not for HCV persistence, where dental procedures were the highest.
Interestingly, in Egypt, most pediatric dental care is provided by outpatient facilities in rural, urban and school healthcare units that have been traditionally underfunded, and infection control practices have been lagging. While the Egyptian Medical Syndicate has published detailed guidelines for cleaning, disinfection and sterilization of dental equipment, they are difficult and expensive to implement.[27] Most dental tool kits are expensive and many clinics possess only one or two reusable kits. Furthermore, the required equipment such as autoclaves are not always available at these clinics, and many rural sites have intermittent electricity that is needed to power these units even if they were available. Given that an average clinic has to serve several dozen children within a few hours, there is no time or manpower to wash, scrub, disinfect and autoclave these kits before being used in the next child. While HCV virus on a dry surface can become nonviable with a few hours,[25] the short interval between patients rarely permits time for the virus to dry, and increases the risk of infecting the next child. Furthermore, given the lack of sufficient dental care clinics, most children are taken by their parents only for substantial interventions such as cavities and tooth extraction, which carry a very high risk of parenteral exposure. These interventions are also more likely to expose the child to a high viral dose and thereby result in a chronic persistent infection as we noted in our study, and has been shown in other studies [14, 25–28]
Risk factors identified in this unique pediatric population are similar to previously published studies by Saleh et al [16], El-Raziky et al [29] and Shebl et al [30] describing the risk factors of HCV infection in Egyptian children. The fact that these studies also noted such an association further strengthens our findings specially that our pediatric population is unique and not necessarily similar to children studied in other studies.
Elevated transaminases were present in three quarters of the actively HCV infected children (anti-HCV-positive, HCV-RNA positive), and the risk ratio for elevated ALT levels was significantly higher among HCV-RNA positive children compared to HCVRNA negative children [RR: 1.8; 95% CI: 1.3–2.6]. This clearly reflects hepatic injury and the high risk of continuous parenchymal hepatic damage in this unique population where half the patients have co-morbid illnesses that could affect or exacerbate the liver injury associated with chronic HCV infection.
Earlier reports suggested that children infected with HCV are more likely than infected adults to spontaneously clear the virus and are more likely to have normal ALT levels [31]. Our data demonstrated that elevated baseline ALT, and fluctuating AST throughout the follow up period, were observed significantly more frequent among patients with persistent HCV infection. This is consistent with studies that have shown that ALT normalization is more common among those with self-limited infection [32, 33].
In a recent report of 157 children with HCV infection, 28% cleared infection after 10 years of follow-up, while among neonatal cases 25% had spontaneous clearance by 7.3 years, concluding that younger age at follow up and normal ALT values both favored spontaneous clearance [34]. In our series, 32.3% of our patients cleared the virus and remained persistently aviremic throughout the follow up period. Of note, 21% of the children who were originally HCV-RNA negative were later found to be HCV RNA positive by the end of the follow up period. This intermittently positive test results has been reported by other investigators [35] and could be either secondary to the fluctuating nature of viremia in HCV-infected individuals or due to reinfection given that the majority of those children had chronic blood diseases and were subject to repeated exposure.
The incidence of spontaneous viral clearance in our series was similar to that reported by Yeung [34], Posthouwer [36], and Vogt [37]; 28%, 35% and 45% respectively but was much higher than that previously reported by Jara [38], and Bortolotti [39]. This difference in the rate of spontaneous viral clearance may be due to different routes of HCV acquisition, and/or other host- and virus-related factors. While genotype 4 is the commonest HCV genotype in Egypt, causing >90% of infections, it is unlikely to be different from studies of other genotypes given the similarities in infection, clearance and persistence between genotypes.[40–42]
In our study, females cleared the virus spontaneously more frequently, similar to what has been observed in other studies.[43–46]
Hepatomegaly and/or splenomegaly were not prominent findings in our studied population, indicating that the absence of hepato/spelnomegaly in a child with risk factors of acquisition of HCV infection does not obviate screening for HCV.
Our study had many strengths, including the large number of HCV-infected children, and the number of children who spontaneously cleared their infection. Also, our study participants had several causes of infection and were referred from many sites from Cairo, Giza and several other governorates. Some limitations should be noted including the shorter duration of follow-up, the fact that this was not a randomly selected sample of children from the population, and hence are more representative of the tertiary care center clinics to which the children were referred. Another limitation is our use of qualitative rather than quantitative PCR testing which could have allowed us to comment on the level of viremia, particularly among those who cleared compared to those who developed persistent infection. Finally, the absence of genetic testing on these children was not done and we could not comment on the effect of specific polymorphisms such as that of the Il-28B gene on spontaneous clearance. [47]
In conclusion
Among Egyptian children, parenteral acquisition of HCV infection remains a major route for acquiring the virus and a predictor of the outcome of the infection, with dental treatment being the predictor of utmost significance for HCV persistence. Additional factors include male gender, elevated baseline ALT and fluctuating AST levels in the current studied cohort of Egyptian children. Given that spontaneous HCV clearance is favored by female gender and a normal baseline ALT level hence, these children should be given the opportunity to clear their infection while male children with elevated ALT levels deserve consideration for earlier treatment. Furthermore, infection control practices should aggressively target dental facilities providing care for children given that preventing transmission at these facilities would not only reduce initial HCV acquisition, but also chronic persistent infections that are more likely to progress to chronic liver disease.
ACKNOWLEDGMENT
Financial support for this project was provided by the National Institute of Allergy and Infectious Diseases (NIAID), NIH (Grant number 5R03AI058971-03). We thank Dr. Nabiel Mikhail and Dr. Maged el Setouhy for their tremendous help with the data management and with the statistical analysis of the results of this study. We are immensely appreciative for Dr. Amr Abouzied, and Dr. G. Thomas Strickland for their pivotal role in writing the protocol, for their advice and tremendous support throughout the study.
This work was conducted with financial support provided by NIH 5R03 A1058971-03. Part of the information contained in this article has been presented at; “15th International Symposium on Hepatitis C Virus and Related Viruses” held in San Antonio, Texas from 5–9 October, 2008 and at; “28th Annual Meeting of the European Society for Paediatric Infectious Diseases which took place in Nice, France, May 4–8, 2010”.
Footnotes
Potential conflicts of interest
G.E.: No conflict
M.H.: No conflict
M. E-R.: No conflict
W.E-A.: No conflict
S.E-N.: No conflict
N.E-K.: No conflict
R.E-S.: No conflict
R.A.: No conflict
M.A.A. No conflict
M.A-H.: No conflict
S. S. E-K.: No conflict
H.E-K.: No conflict
REFERENCES
- 1.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–91. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 2.Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43:915–22. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- 3.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Ministry of Health (El-Zanaty and Associates and Macro International, Cairo); Egyptian: 2009. p. 431. [Google Scholar]
- 4.Aziz F, Habib M, Mohamed MK, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology. 2000;32:111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 5.Habib M, Mohamed MK, Abdel-Aziz F, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–53. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 6.Medhat A, Shehata M, Magder LS, et al. Hepatitis C in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hygiene. 2002;66:633–8. doi: 10.4269/ajtmh.2002.66.633. [DOI] [PubMed] [Google Scholar]
- 7.Nafeh MA, Medhat A, Shehata M, et al. Hepatitis C in a community in Upper Egypt: I. Cross-sectional survey: Am J Trop Med Hygiene. 2000;63:236–41. [PubMed] [Google Scholar]
- 8.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 10.Rodger AJ, Roberts S, Lanigan A, et al. Assessment of long-term outcomes of community-acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology. 2000;32:582–587. doi: 10.1053/jhep.2000.9714. [DOI] [PubMed] [Google Scholar]
- 11.Villano SA, Vlahov D, Nelson KE, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 12.El-Nanawy AA, el Azzouni OF, Soliman AT, et al. Prevalence of hepatitis-C antibody seropositivity in healthy Egyptian children and four high risk groups. J Trop Pediatr. 1995;41:341–3. doi: 10.1093/tropej/41.6.341. [DOI] [PubMed] [Google Scholar]
- 13.Said ZN, El-Sayed MH, El-Bishbishi IA, et al. High prevalence of occult hepatitis B in hepatitis C-infected Egyptian children with haematological disorders and malignancies. Liver Int. 2009;29(4):518–24. doi: 10.1111/j.1478-3231.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- 14.El-Raziky MS, El-Hawary M, El-Koofy N, et al. Hepatitis C virus infection in Egyptian. J Viral Hepat. 2004;11:471–6. doi: 10.1111/j.1365-2893.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 15.Paez Jimenez A, Sharaf Eldin N, Rimlinger F, et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59(11):1554–60. doi: 10.1136/gut.2009.194266. [DOI] [PubMed] [Google Scholar]
- 16.Saleh DA, Shebl FM, El-Kamary SS, et al. Incidence and risk factors for community-acquired hepatitis C infection from birth to 5 years of age in rural Egyptian children. Trans R Soc Trop Med Hyg. 2010;104(5):357–363. doi: 10.1016/j.trstmh.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talaat M, Kandeel A, Rasslan O, et al. Evolution of infection control in Egypt: achievements and challenges Evolution of infection control in Egypt: achievements and challenges. Am J Infect Control. 2006 May;34(4):193–200. doi: 10.1016/j.ajic.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Talaat M, Afifi S, Dueger E, El-Ashry N, et al. Effects of Hand hygiene campaigns on incidence of laboratory-confirmed Influenza and absenteeism in schoolchildren, Cairo, Egypt. EID. 2011;17(4):619–25. doi: 10.3201/eid1704.101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The European Paediatric Hepatitis C Network Three Broad Modalities in the Natural History of Vertically Aquired Hepatitis C Infection. CID. 2005;41(1):45–51. doi: 10.1086/430601. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Hamid M, El-Daly M, El-Kafrawy S, et al. Comparison of second- and third-generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002;40:1656–1659. doi: 10.1128/JCM.40.5.1656-1659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Hamid M, Edelman DC, Highsmith WE, et al. Optimization, assessment, and proposed use of a direct nested reverse transcription-polymerase chain reaction protocol for the detection of hepatitis C virus. J Hum Virol. 1997;1:58–65. [PubMed] [Google Scholar]
- 22.Kelly D, Skidmore S. Hepatitis C-Z: recent advances. Arch Dis Child. 2002;86:339–343. doi: 10.1136/adc.86.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zein NN. Hepatitis C in children: recent advances. Curr Opin Pediatr. 2007;19:570–4. doi: 10.1097/MOP.0b013e3282f04ea8. [DOI] [PubMed] [Google Scholar]
- 24.Jafri W, Jafri N, Yakoob J, et al. Hepatitis B and C: prevalence and risk factors associated with seropositivity among children in Karachi, Pakistan. BMC Infect Dis. 2006;23(6):101. doi: 10.1186/1471-2334-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waheed Y, Shafi T, Safi SZ, et al. Hepatitis C virus in Pakistan: a systematic review of prevalence, genotypes and risk factors World. J Gastroenterol. 2009;15:5647–53. doi: 10.3748/wjg.15.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamili S, Krawczynski K, McCaustland K, et al. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28(5):519–24. doi: 10.1086/513727. [DOI] [PubMed] [Google Scholar]
- 27.Egyptian Medical Syndicate (Cleaning, Disinfection, and Sterilization of Medical Equipment) Cairo: 2008. pp. 137–167. http://www.ems.org.eg/esic_home/Guideline_part1.htm. [Google Scholar]
- 28.Madwar MA, El-Gindy I, Fahmy HM, et al. Hepatitis C virus transmission in family members of Egyptian patients with HCV related chronic liver disease. J Egypt Public Health Assoc. 1999;74(3–4):313–32. [PubMed] [Google Scholar]
- 29.El-Raziky MS, El-Hawary M, Esmat G, et al. Prevalence and risk factors of asymptomatic hepatitis C virus infection in Egyptian children. World J Gastroenterol. 2007;13(12):1828–32. doi: 10.3748/wjg.v13.i12.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shebl FM, El-Kamary SS, Saleh DA, et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J. Med. Virol. 2009;81:1024–1031. doi: 10.1002/jmv.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin: Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 32.Hoofnagle JH. Therapy for acute hepatitis C. NEJM. 2001;345:1495–1497. doi: 10.1056/NEJM200111153452013. [DOI] [PubMed] [Google Scholar]
- 33.Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol. 2004;19:1357–1362. doi: 10.1111/j.1440-1746.2004.03463.x. [DOI] [PubMed] [Google Scholar]
- 34.Yeung LT, To T, King SM, et al. Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat. 2007;14:797–805. doi: 10.1111/j.1365-2893.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 35.Pirisi M, Toniutto P, Fabris C, et al. Factors associated with serum HCV RNA positivity in anti-HCV antibody positive intravenous drug users. J Clin Epidemiol. 1998;51(5):423–7. doi: 10.1016/s0895-4356(97)00305-3. [DOI] [PubMed] [Google Scholar]
- 36.Posthouwer D, Fischer K, van Erpecum KJ, et al. The natural history of childhood-acquired hepatitis C infection in patients with inherited bleeding disorders. Transfusion. 2006;46:1360–6. doi: 10.1111/j.1537-2995.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 37.Vogt M, Lang T, Frösner G, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–70. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 38.Jara P, Resti M, Hierro L, et al. Chronic hepatitis C virus infection in childhood: Clinical patterns and evolution in 224 white children. Clin Infect Dis. 2003;36:275–80. doi: 10.1086/345908. [DOI] [PubMed] [Google Scholar]
- 39.Bortolotti F, Verucchi G, Cammà C, et al. Italian Observatory for HCV Infection and Hepatitis C in Children. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. 2008;134:1900–7. doi: 10.1053/j.gastro.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 40.Ray SC, Arthur RR, Carella A, et al. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 2000;182:698–707. doi: 10.1086/315786. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Agha S, Saudy N, et al. Genetic epidemiology of hepatitis C virus throughout Egypt. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol. 2004;58(2):191–5. doi: 10.1007/s00239-003-2541-3. [DOI] [PubMed] [Google Scholar]
- 42.Simmonds P, McOmish F, Yap PL, et al. Sequence variability in the 5' non-coding region of hepatitis C virus: identification of a new virus type and restrictions on sequence diversity. J Gen Virol. 1993;74(Pt 4):661–8. doi: 10.1099/0022-1317-74-4-661. [DOI] [PubMed] [Google Scholar]
- 43.Gerlach JT, Diepolder HM, Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4 (+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 44.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 45.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 46.Jauncey M. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–4. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- 47.Thomas DL, Thio CL, Martin ML, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009 Oct 8;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]