Abstract
As part of our program to develop new probes for the estrogen receptor binding domain, we prepared and evaluated a novel 17α-(rhenium tricarbonyl bipyridyl) vinyl estradiol complex. Preparation of the final compound was achieved using the Stille coupling between the preformed brominated rhenium tricarbonyl bipyridine complex and the tributylstannyl vinyl estradiol. Competitive receptor binding assays and stimulatory assays demonstrated that the final complex retained affinity and efficacy comparable to the corresponding pyridyl vinyl estadiol analog, but lower than that of the phenyl vinyl estradiol analog.
Keywords: synthesis, Stille coupling, bipyridyl rhenium complex, estradiol, estrogen receptor binding, molecular imaging
Detection of estrogen-responsive breast cancer using radiolabeled estrogens remains a major diagnostic goal of nuclear medicine in spite of over 30 years of research. Although initial efforts in the field focused on radio-iodinated derivatives of estradiol, which demonstrated considerable success at the pre-clinical stage,1–5 most of the later studies utilized [F-18]-estrogens, primarily because of the superior imaging and radiation dosimetry properties associated with the [F-18]-positron-emitting radionuclide.6–10 However, the need for a cyclotron within a reasonable distance to generate the radionuclide with a 110 min half-life limits the availability of such radiopharmaceuticals, thereby stimulating the search for alternatives. Technetium-99m is a readily available nuclide with highly desirable physical properties, i.e., a 6 h half life, 140 keV gamma ray emission energy and generator production, however, its chemical (metallic) properties limit its incorporation into many small bio-organic molecules. Initial efforts to prepare small Tc-chelating groups that did not significantly alter the physicochemical and biological properties of estrogenic ligands were unsuccessful.11–13 Smaller, more stable cyclopentadienyl Tc/Re tricarbonyl complexes retained many of the desired biological properties, however, the radiolabeled compounds were not accessible in appropriate time scales and overall radiochemical yields were low.14–22 Subsequent studies, primarily through use of its rhenium surrogate, indicated that Tc-99m tricarbonyl derivatives were readily prepared in an aqueous environment, could be coordinated to many small molecules and retain many of the desired biological properties.23–26 For example, studies by Arterburn, et al., using 17-α-substituted estradiol-pyridin-2-yl hydrazine conjugates suggested that such complexes may be designed in which the requisite ER-binding is not sacrificed.27–29 In this manuscript we report the preparation, via an innovative approach, of a rhenium tricarbonyl derivative of estradiol that retains significant affinity for the estrogen receptor-alpha subtype.
In developing the rhenium tricarbonyl derivative of estradiol we utilized our experience with both the synthesis of 17α-arylvinyl estradiols coupled with the chelating properties of 2,2'-bipyridines. Our ongoing research directed toward ER-ligands demonstrated that the ligand binding pocket (LBP) complementary to the 17α-position of estradiol can accommodate the substituted phenylvinyl groups with affinities comparable to the endogenous ligand, estradiol.30–32 Our synthetic approach for preparing these derivatives utilized Pd(0) coupling of the stannylvinyl estradiol with the requisite substituted aryl halide. Subsequent Stille coupling of tri-n-butylstannylvinyl estradiol with heteroaryl halides generated a series of derivatives that displayed an affinity for the estrogen receptor ligand binding domain (ERα-LBD), roughly comparable to the phenylvinyl derivative.33 In this study, we appreciated that the final complex would retain one labile coordination site which may compromise the ultimate utility of the agent, but the transition from a monopyridine to a bipyridine was a logical step which would provide both synthetic and biological information.
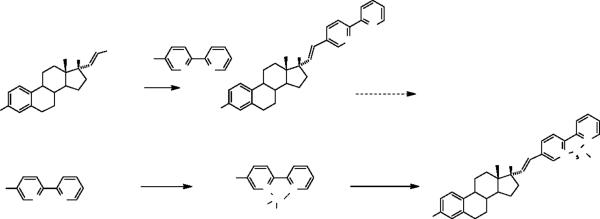
Bipyridines are a class of compounds capable of coordinating a wide variety of transition metals, including rhenium tricarbonyl species.34–37 Our strategy for demonstrating proof of principle therefore involved preparing the unsymmetrical 5-bromo-2,2'- bipyridine, and coupling it to the stannylvinyl estradiol via the Stille reaction. The resulting bipyridyl vinyl estradiol intermediate could ultimately be labeled with the corresponding rhenium tricarbonyl reagent (Scheme 1-Pathway A). Alternatively, the 5-bromo-2,2'-bipyridine could first undergo coordination with the rhenium tricarbonyl reagent followed by Stille coupling to give the final compound (Scheme 1-Pathway B). The first route would require that the bipyridine not undergo transchelation by the palladium catalyst, while the second would require the metallated bipyridine to be a successful coupling partner. There was no literature precedent for either pathway that proceeds through Stille coupling of a bromobipyridine derivative to a vinylstannane.
Scheme 1.
Routes to the Re(CO)3-bipyridyl-vinyl estradiol complexes.
The preparation of the stannylvinyl estradiol 1 proceeded via our established method30,32 while the unsymmetrical 5-bromo-2,2'-bipyridine 2 was generated by Stille coupling of 2, 5-dibromopyridine with 2-trimethylstannylpyridine.38,39 The rhenium derivative, 5-bromo-2, 2'-bipyridine)Re(CO)3Br 3, was obtained by reaction of 5-bromo-2, 2'-bipyridine 2 and [NEt4]2[Re(CO)3Br3] in methanol.40 Although synthesis of the model 3-pyridylvinyl estradiol 5 proceeded without difficulty,33 our efforts to couple the stannylvinyl estradiol with 5-bromo-2,2'-bipyidine to form the product proved to be unsuccessful, using a variety of Stille coupling procedures. Changes in catalyst [{(C6H5)3P}4Pd(0), Pd2dba3-(C6H5)3P, {(t-C4H9)3P}4Pd(0)], solvent (THF, 1,4-dioxane, toluene) and temperature (R.T., 60°C, reflux) did not yield detectable quantities of coupled product. The reasons behind the failure of the Stille coupling in the latter case are difficult to determine. Successful Stille coupling of bromobipyridines with arylstannanes and heteroarylstannanes in good yield have been reported.41 Coupling of Bu3Sn-functionalized Troger's base with 2-bromopyridine, however, required high temperatures (100°C) and proceeded in yields ranging from 0–64%, depending on the catalyst system used.42 Lower yields were ascribed to catalyst decomposition. We believe that the lower reactivity of vinylstannanes compared to arylstannanes and catalyst degradation may have contributed to the failure of the reaction between 5-bromo-2, 2'-bipyridine and the stannylvinyl estradiol.
Stille coupling of 5-bromo-2, 2'bipyridine)Re(CO)3Br 3 and the stannylvinyl estradiol 1 proved to be more successful, with the desired product 4 obtained in 30% isolated yield. The Re(CO)3 fragment in 5-bromo-2, 2'bipyridine)Re(CO)3Br acts as an electron withdrawing group when coordinated to the bromobipyridine ligand, promoting oxidative addition of the C-Br bond to Pd(0).43,44 Electron-withdrawing groups on aryl groups in Pd-aryl intermediates also promote reductive elimination of C-C bonds in the Stille reaction.45 Both of these effects may be contributing to the greater success of the cross-coupling reaction between 5-bromo-2, 2'bipyridine)Re(CO)3Br and stannylvinyl estradiol compared to the reaction of 5-bromo-2, 2'-bipyridine. The use of a polyfunctional and less reactive stannane (aryl > vinyl), as well as the limited solubility of the 5-bromo-2, 2'bipyridine)Re(CO)3Br in non-protic solvents that are compatible the Stille coupling, may also be contributing to lower yields for the reaction of 5-bromo-2, 2'bipyridine)Re(CO)3Br and stannyl vinyl estradiol than observed in Stille couplings of bromobipyridine with arylstannanes cited earlier.
The synthesis of the vinyl-bipyridine estradiol-Re(CO)3 complex adds to the limited examples of Stille coupling between transition-metal coordinated arenes or aromatic heterocyclic ligands. Stille coupling of (η6-chlorobenzene)Cr(CO)3 and (η6-p-chloroanisole)Cr(CO)3 with 2-tributylstannyl-thiophene yielded the cross-coupled products in 55 and 40%, respectively.46 Cationic (η6-chloroarene)Mn(CO)3+ complexes readily reacted with Pd(PPh3)4 to form a stable intermediate that was inert toward further reaction, an observation attributed to the strong electron withdrawing effect of the Mn(CO)3+ group.47,48 The Cr(CO)3 also acts as an electron withdrawing group when coordinated to arene ligands. The synthesis of the vinyl-bipyridine estradiol-Re(CO)3 complex is unique in using a vinylstannane rather than an arylstannane.
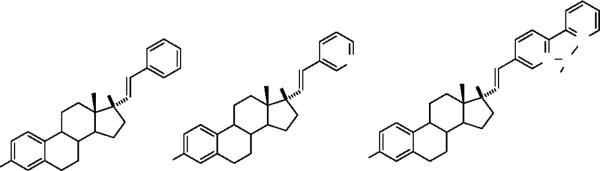
The receptor binding affinity of the Re(CO)3-bipyridyl-vinyl estradiol complex 5 for the ER -LBD was determined by radiometric assays with [H-3] estradiol, expressed as relative binding affinity (RBA) compared to estradiol (100%).49,50 [Figure 1] The nature of the aryl group had only a modest effect on the binding affinity of the compound for the ER -LBD. As previous studies have shown, introduction of the terminal phenyl ring reduced receptor binding compared to estradiol,30 however, replacement of the phenyl ring by the isosteric pyridyl group did not dramatically reduce the RBA value, 4.0% versus 10.3%.33 As the binding results indicate, further modification by appending the second pyridine ring para to the first and introducing the metal carbonyl moiety had no additional effects on the RBA value. This observation is similar to that reported by Arterburn, et al.,27–29 with their complexes and suggests that the ER -LBD can accommodate significant structural diversity, including heterocyclic and metallated groups at the 17 -position.51
Figure 1.
Relative Binding Affinity (RBA) and Stimulatory Activity (RSA) values for the phenylvinyl, pyridylvinyl and Re(CO)3-bipyridylvinyl estradiols. RBA = 100 × [E]/[C] where [E] is the concentration of unlabeled estradiol necessary to reduce the specific binding of tritiated estradiol to the ER -HBD by 50% and [C] is the concentration of the competitive ligand necessary to reduce specific binding by 50%. The RBA of estradiol is 100% at 25 °C. Curves for ligand and estradiol had correlation coefficients >95%. RSA = Relative Stimulatory activity compared to E2 = 100%, in stimulation of alkaline phosphatase (AlkP) in the Ishikawa cell line. EC50 for E2 = 0.9 ± 0.2 nM.
To determine the functional response of this complex we used the ligand-induced alkaline phosphatase activity in endometrial adenocarcinoma (Ishikawa) cells, expressed as relative stimulatory activity (RSA) compared to estradiol (100%).52 The parent compound, 17α-phenylvinyl estradiol, demonstrated an RSA value that was comparable to its observed RBA values (10.3 vs 9.5%). The 3-pyridyl analog 5 and the Re(CO)3Br complex 4, on the other hand, had a significantly reduced efficacy (RSA) compared to parent phenyl vinyl estradiol and compared to their observed RBA values (approximately 0.25–0.4% vs. 4%). The difference between the ability to bind to the isolated receptor (RBA) and ability to stimulate it in cells (RSA) may be due to a variety of factors. Steric factors should not be significant in modulating binding at the ERα-LBD, and therefore the reduced efficacy may represent an influence on the ability to recruit the co-regulatory factors. Alternatively, the ligands 4 and 5 may be less able to cross the cellular membrane compared to the phenyl analog or may be unstable toward cellular metabolism. In this example, extension of the pyridyl moiety to the rhenium tricarbonyl coordinated bipyridyl analog produced no further alteration in function, suggesting a similar mode of binding and response modulation for the two derivatives.
In summary, we have demonstrated the preparation of a new class of rhenium tricarbonyl coordinated ligands for the estrogen receptor through the novel Stille coupling of the 5-bromo-2,2'-bipyridine- Re(CO)3 complex and the vinylstannane. Although incorporation of the metal ion into the final product requires two steps, the process would still be compatible with the 6h half-life of the clinically relevant technetium-99m radionuclide. Initial in vitro evaluation indicated that although the observed affinity for the initial examples is lower compared to estradiol, it has been demonstrated that in vitro binding and in vivo activity for this type of 17α-substituted estradiol derivatives can be significantly enhanced by appropriate 11β-substituents, such as methoxy, ethyl or vinyl.53,54 Incorporation of an additional coordination site to replace the bromide may also enhance stability of the complex and reduce susceptibility toward metabolism. Replacement of the rhenium by technetium-99m, the gamma-emitting radionuclide, should then provide a radiopharmaceutical with potential for in vivo imaging ER-containing tissues, such as hormone responsive breast cancer.
Supplementary Material
Acknowledgments
Funding Sources Support of this research was provided by grants from the National Institutes of Health [PHS 1R01 CA81049 (R.N.H.), PHS 1R01 CA 37799 and R21 MH082252 (R.B.H.)], the U.S. Army Breast Cancer Research Program [DAMD 17-00-1-00384 and W81HW0410544(R.N.H.)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hochberg RB. Science. 1979;205:1138. doi: 10.1126/science.472733. [DOI] [PubMed] [Google Scholar]
- 2.Hanson RN, Franke LA. J. Nucl Med. 1984;25:998. [PubMed] [Google Scholar]
- 3.Symes EK, Coulson WF, Das R, Scurr JH. J. Steroid Biochem. 1990;35:641. doi: 10.1016/0022-4731(90)90303-a. [DOI] [PubMed] [Google Scholar]
- 4.Bennink RJ, Rijks LJ, van Tienhoven G, Noorduyn AL, Janssen AG, Sloof GW. Radiology. 2001;220:774. doi: 10.1148/radiol.2203001639. [DOI] [PubMed] [Google Scholar]
- 5.Bennink RJ, van Tienhoven G, Rijks LJ, Noorduyn AL, Janssen AG, Sloof GW. J. Nucl. Med. 2004;45:1. [PubMed] [Google Scholar]
- 6.VanBrocklin HF, Carlson KE, Katzenellenbogen JA, Welch MJ. J. Med. Chem. 1993;36:1619. doi: 10.1021/jm00063a012. [DOI] [PubMed] [Google Scholar]
- 7.Mankoff DA, Peterson LM, Tewson TJ, Link JM, Gralow JR, Graham MM, Krohn KA. J. Nucl. Med. 2001;42:679. [PubMed] [Google Scholar]
- 8.Seimbille Y, Ali H, van Lier JE. J. Chem. Soc. Perkin Trans. I. 2002:657. [Google Scholar]
- 9.Vijaykumar D, Al-Qahtani MH, Welch MJ, Katzenellenbogen JA. Nucl. Med. Biol. 2003;30:397. doi: 10.1016/s0969-8051(02)00446-8. [DOI] [PubMed] [Google Scholar]
- 10.Seo JW, Comninos JS, Chi DY, Kim DW, Carlson KE, Katzenellenbogen JA. J. Med. Chem. 2006;49:2496. doi: 10.1021/jm0512037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuest F, Carlson KE, Katzenellenbogen JA, Spies H, Johannsen B. Steroids. 1998;63:665. doi: 10.1016/s0039-128x(98)00079-8. [DOI] [PubMed] [Google Scholar]
- 12.Storr T, Thompson KH, Orvig C. Chem. Soc. Rev. 2006;35:534. doi: 10.1039/b514859f. [DOI] [PubMed] [Google Scholar]
- 13.Vessieres A, Top S, Beck W, Hillard E, Jaouen G. Dalton Trans. 2006:529. doi: 10.1039/b509984f. [DOI] [PubMed] [Google Scholar]
- 14.Wuest FR. Current Topics in Steroid Research. 2004;4:197. [Google Scholar]
- 15.Skaddan MB, Wust FR, Jonson S, Syhre R, Welch MJ, Spies H, Katzenellenbogen JA. Nucl. Med. Biol. 2000;27:269. doi: 10.1016/s0969-8051(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 16.Reisgys M, Wust FR, Alberto R, Schibli R, Schubiger PA, Pietzsch H-J, Spies H, Johannsen B. Bioorg. Med. Chem. Lett. 1997;7:2243. [Google Scholar]
- 17.Jackson A, Davis J, Pither RJ, Rodger A, Hannon MJ. Inorg. Chem. 2001;40:3964. doi: 10.1021/ic010152a. [DOI] [PubMed] [Google Scholar]
- 18.Jaouen G, Top S, Vessieres A, Alberto R. J. Organomet. Chem. 2000;600:23. [Google Scholar]
- 19.Bigott HM, Parent E, Luyt LG, Katzenellenbogen JA, Welch MJ. Bioconj Chem. 2005;16:255. doi: 10.1021/bc049770g. [DOI] [PubMed] [Google Scholar]
- 20.Mull ES, Sattigeri VJ, Rodriguez AL, Katzenellenbogen JA. Bioorg. Med. Chem. 2002;10:1381. doi: 10.1016/s0968-0896(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 21.Masi S, Top S, Boubekeur L, Jaouen G, Mundwiler S, Spingler B, Alberto R. Eur. J. Inorg. Chem. 2004:2013. [Google Scholar]
- 22.Luyt LG, Bigott HM, Welch MJ, Katzenellenbogen JA. Bioorg.Med. Chem. 2003;11:4977. doi: 10.1016/j.bmc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Schibli R, Schwarzbach R, Alberto R, Ortner K, Schmalle H, Dumas C, Egli A, Schubiger PA. Bioconj. Chem. 2002;13:750. doi: 10.1021/bc015568r. [DOI] [PubMed] [Google Scholar]
- 24.Schibli R, Schubiger PA. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1529. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 25.Garcia R, Paulo A, Domingos A, Santos I, Ortner K, Alberto R. J. Am. Chem. Soc. 2000;122:11240. [Google Scholar]
- 26.Stichelberger A, Waibel R, Dumas C, Schubiger PA, Schibli R. Nucl. Med. Biol. 2003;30:465. doi: 10.1016/s0969-8051(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 27.Arterburn JB, Rao KV, Perry MC. Angew. Chemie, Intern. Ed. 2000;39:771. [PubMed] [Google Scholar]
- 28.Arterburn JB, Corona C, Rao KV, Carlson KE, Katzenellenbogen JA. J. Org. Chem. 2003;68:7063. doi: 10.1021/jo034780g. [DOI] [PubMed] [Google Scholar]
- 29.Ramesh C, Bryant BJ, Nayak T, Revankar CM, Anderson T, Carlson KE, Katzenellenbogen JA, Sklar LA, Norenberg JP, Prossnitz ER, Arterburn JB. J Am. Chem. Soc. 2006;128:14476. doi: 10.1021/ja066360p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson RN, Lee CY, Friel CJ, Dilis R, Hughes A, DeSombre ER. Journal of Medicinal Chemistry. 2003;46:2865. doi: 10.1021/jm0205806. [DOI] [PubMed] [Google Scholar]
- 31.Hanson RN, Lee CY, Friel C, Hughes A, DeSombre ER. Steroids. 2003;68:143. doi: 10.1016/s0039-128x(02)00165-4. [DOI] [PubMed] [Google Scholar]
- 32.Hanson RN, Tongcharoensirikul P, Dilis R, Hughes A, DeSombre ER. J. Med. Chem. 2007;50:472. doi: 10.1021/jm060940f. [DOI] [PubMed] [Google Scholar]
- 33.Hanson RN, Olmsted SL, Tongcharoensirikul P, McCaskill E, Gandiaga K, Labaree D. 2012. In Press. [DOI] [PMC free article] [PubMed]
- 34.Bossert J, Daniel C. Chem.--A Eur. Jour. 2006;12:4835. doi: 10.1002/chem.200501082. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher NC, Brown RT, Doherty AP. Inorg. Chem. 2006;45:6132. doi: 10.1021/ic060966x. [DOI] [PubMed] [Google Scholar]
- 36.Gibson DH, Mashuta MS, Yin X. Acta Cryst. Sect. E. 2003;E59:m911. [Google Scholar]
- 37.Gelling A, Orrell KG, Osborne AG, Sik V. J. Chem. Soc., Dalton Trans: Inorg. Chem. 1994:3545. [Google Scholar]
- 38.Schubert US, Eschbaumer C, Heller M. Org. Lett. 2000;2:3373. doi: 10.1021/ol006473s. [DOI] [PubMed] [Google Scholar]
- 39.Schwab PFH, Fleischer F, Michl J. J. Org. Chem. 2002;67:443. doi: 10.1021/jo010707j. [DOI] [PubMed] [Google Scholar]
- 40.Cesati RR, Tamagnan G, Baldwin RM, Zoghbi SS, Innis RB, Kula NS, Baldessarini RJ, Katznellenbogen JA. Bioconj. Chem. 2002;13:29. doi: 10.1021/bc010011x. [DOI] [PubMed] [Google Scholar]
- 41.Champouret YDM, Chaggar RK, Dadhiwala I, Fawcett J, Solan GA. Tetrahedron. 2006;62:79–89. Gavina, P.; Tatay, S. Tetr. Lett. 2006, 47: 3471. Inorg. Chem 2001, 40: 630. [Google Scholar]
- 42.Solano C, Svensson D, Olomi Z, Jensen J, Wendt OF, Warnmark K. Eur. J. Org. Chem. 2006:3510. [Google Scholar]
- 43.Fitton P, Rick EA. J. Organometal. Chem. 1971;28:287. [Google Scholar]
- 44.Jutand A, Msleh A. Organometallics. 2004;23:1810. [Google Scholar]
- 45.Culkin DA, Hartwig JF. Organometallics. 2004;23:3398. [Google Scholar]
- 46.Prim D, Giner Planas J, Auffrant A, Rose-Munch F, Rose E, Vaissermann J. J. Organomet. Chem. 2003;688:73. [Google Scholar]
- 47.Trouillet L, De Nicola A, Guillerez S. Chem. Mat. 2000;12:1611. [Google Scholar]
- 48.Dunne SJ, Constable EC. Inorg. Chem. Commun. 1998;1:167. [Google Scholar]
- 49.Labaree DC, Shang J, Harris HA, O'Connor C, Reynolds TY, Hochberg RB. J. Med. Chem. 2003;46:1886. doi: 10.1021/jm0204340. [DOI] [PubMed] [Google Scholar]
- 50.Green S, Walter P, Kumar V, Krust A, Bonert JM, Argos P, Chambon P. Nature. 1986;320:134. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 51.Nettles KW, Bruning JB, Gil G, O'Neill EE, Nowak J, Hughes A, Kim Y, DeSombre ER, Dilis R, Hanson RN. EMBO Reports. 2007;8:563. doi: 10.1038/sj.embor.7400963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. Endocrinology. 1990;127:2757. doi: 10.1210/endo-127-6-2757. [DOI] [PubMed] [Google Scholar]
- 53.Hanson RN, Napolitano E, Fiaschi R. J. Med. Chem. 1998;41:4686. doi: 10.1021/jm9801051. [DOI] [PubMed] [Google Scholar]
- 54.Hanson RN, Napolitano E, Fiaschi R. Steroids. 1998;63:479. doi: 10.1016/s0039-128x(98)00052-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.