Abstract
We describe here a GC/MS/MS method for the sensitive and concurrent determination of extracellular tryptophan and the kynurenine pathway metabolites kynurenine, 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN) in rat brain. This metabolic cascade is increasingly linked to the pathophysiology of several neurological and psychiatric diseases. Methodological refinements, including optimization of MS conditions and addition of deuterated standards, resulted in assay linearity to the low nanomolar range. Measured in samples obtained by striatal microdialysis in vivo, basal levels of tryptophan, kynurenine and QUIN were 415, 89 and 8 nM, respectively, but 3-HK levels were below the limit of detection (<2 nM). Systemic injection of kynurenine (100 mg/kg, i.p.) did not affect extracellular tryptophan but produced detectable levels of extracellular 3-HK (peak after 2-3 h: ~50 nM) and raised extracellular QUIN levels (peak after 2 h: ~105 nM). The effect of this treatment on QUIN, but not on 3-HK, was potentiated in the NMDA-lesioned striatum. Our results indicate that the novel methodology, which allowed the measurement of extracellular kynurenine and 3-HK in the brain in vivo, will facilitate studies of brain kynurenines and of the interplay between peripheral and central kynurenine pathway function under physiological and pathological conditions.
Keywords: 3-Hydroxykynurenine, Microdialysis, Quinolinic acid, Selective reaction monitoring, Tandem mass spectrometry, Tryptophan
INTRODUCTION
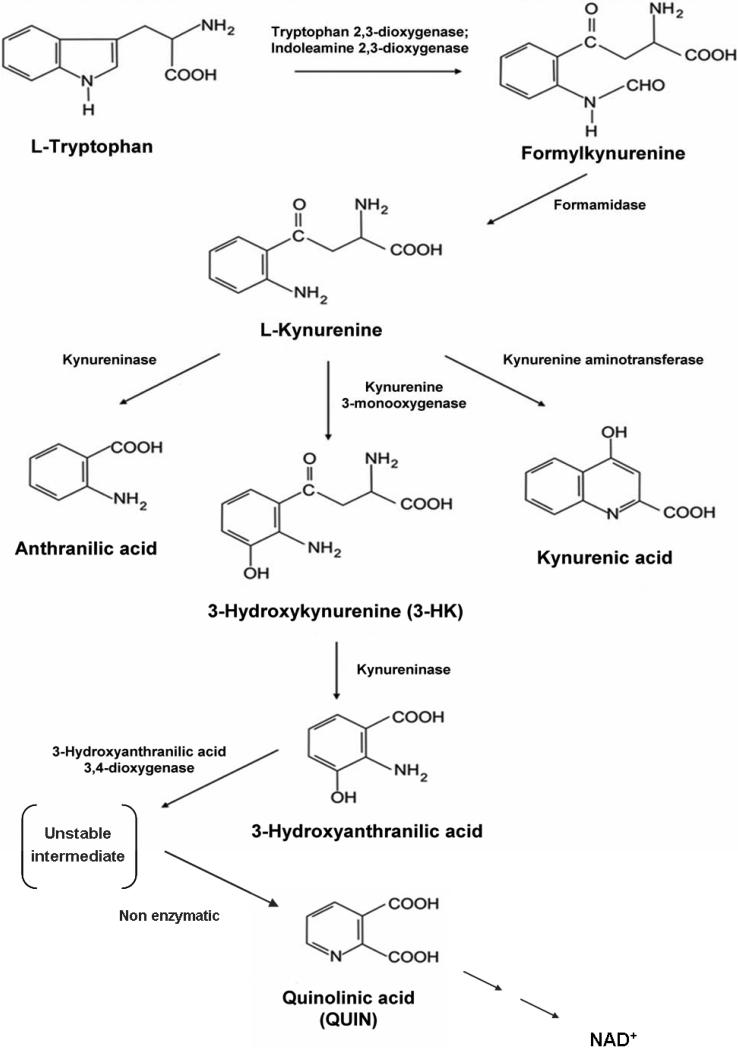
Mounting evidence indicates that metabolites of the kynurenine pathway (KP), the major catabolic route of dietary tryptophan in mammals (Fig. 1), play a significant role in biology. Although several of these metabolites (collectively termed kynurenines) have been credibly linked to peripheral immune function [1,2], age-associated endocrine disorders [3], regulation of vascular tone [4] and cancer [5], most major advances have come from studies of the central nervous system (CNS). Interest in the role of the KP in the CNS is mostly related to the neuroactive properties of kynurenic acid (a neuroprotective glutamate and α7 nicotinic receptor antagonist; [6,7,8]) and the excitotoxic NMDA receptor agonist quinolinic acid (QUIN; [9,10]). Also of interest to biologists is the ability of two other KP metabolites, 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid, to both generate and scavenge reactive free radicals [11,12]. Alone or jointly, these metabolites are involved in a number of important neurophysiological processes and participate causally in neurological and psychiatric diseases (see [13,14,15], for reviews). Pharmacological manipulation of the KP therefore offers novel, attractive approaches to influence brain physiology and pathology [16,17,18,19].
Figure 1.
The kynurenine pathway of tryptophan degradation.
The dynamics of the KP within the normal and the dysfunctional brain, and the effect of peripheral KP manipulations on the brain, have been explored in a series of studies, conducted mainly in rodents. Briefly, tryptophan, kynurenine and 3-HK readily enter the brain from the circulation, whereas the acidic metabolites kynurenic acid and QUIN appear to be largely precluded from crossing the blood-brain barrier [20,21]. In the brain, KP metabolism, initiated either by local synthesis or systemic influx of tryptophan, kynurenine or 3-HK, occurs mainly in glial cells (cf. [16]), though neurons have been implicated as well [22]. Interestingly, the two branches of the KP (Fig. 1) are segregated, with the formation of kynurenic acid occurring in astrocytes, and the competing cascade, leading to 3-HK and its downstream metabolites, taking place in microglial cells. However, the interplay between peripherally derived and locally produced kynurenines is complex, depending on the integrity of the brain tissue and, more generally, on the health status of the entire organism [23,24,25].
As several cell types are involved in cerebral KP metabolism, and as the blood-borne metabolites must enter the extracellular space before being accumulated by cells, the dynamics of neuroactive KP metabolites in the brain are best studied by in vivo microdialysis. This method has been successfully used to elaborate features of kynurenic acid and QUIN neurochemistry in experimental animals, providing valuable information on the role of these compounds in health and disease [26,27,28,29,30]. Other KP metabolites, on the other hand, have so far not been studied by microdialysis, and there is only sporadic mention of extracellular brain tryptophan in the literature [31,32].
Because the concentration of neuroactive kynurenines in the mammalian brain is in the nanomolar range, sensitive detection methods, e.g. fluorescence or gas chromatography/mass spectrometry (GC/MS), are required for analysis. Here, we used GC/MS/MS detection with negative chemical ionization (CI), rather than the traditional electron impact (EI) ionization or positive CI, focusing on the simultaneous measurement of tryptophan, kynurenine and the two microglial KP metabolites 3-HK and QUIN in brain microdialysate samples. This methodology, adapted from Eckstein et al. [33], provides improved selectivity and sensitivity compared to other ionization techniques [34] and was selected because our molecules of interest, after derivatization, can stabilize a negative charge and produce a stable negative ion. We now report the successful application of this methodology to a paradigmatic microdialysis study that was performed in the normal and excitotoxin-lesioned rat striatum. Our goal was to simultaneously identify tryptophan and kynurenines in the brain's extracellular compartment, to determine the effects of systemic kynurenine administration on these analytes, and to evaluate these features in a widely used animal model of the neurodegenerative disorder, Huntington's disease (HD).
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (200-250 g; Charles River Laboratories, Kingston, NY, USA) were used in all experiments. Animals were housed in a temperature-controlled, AAALAC-approved animal facility on a 12/12h-light/dark cycle with free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Chemicals and gases
L-Tryptophan, 3-hydroxy-DL-kynurenine (3-HK), [2H6]L-kynurenine, quinolinic acid (QUIN), N-methyl-D-aspartate (NMDA), ascorbic acid, ethyl acetate, as well as the derivatizing reagents pentafluoropropionic anhydride (PFAA) and 2,2,3,3,3-pentafluoro-1-propanol (PFP), were obtained from Sigma-Aldrich (St. Louis, MO, USA). L-Kynurenine sulfate (“kynurenine”; purity: 99.4%) was obtained from Sai Advantium (Hyderabad, India). [2H3]Quinolinic acid was purchased from Synfine Research (Richmond Hill, Ontario, Canada), and [2H5]L-tryptophan was obtained from CDN Isotopes (Pointe-Claire, Quebec, Canada). Formic acid was purchased from JT Baker (Phillipsburg, NJ, USA). Helium, nitrogen and methane (all 99.999% purity) were obtained from Airgas (Salem, NH, USA). The tuning standard perfluoro-5,8-dimethyl-3,6,9-trioxidodecane (PFDTD) was purchased from Agilent Technologies (Santa Clara, CA, USA).
Instrument conditions
Some selected reaction monitoring (SRM) transitions used in this study were initially described by Eckstein and colleagues [33], and instrument conditions were optimized as described in “Results”. All data were collected using an Agilent 7890A-7000B gas chromatograph (GC) tandem mass spectrometer (MS) equipped with a 7693A auto-sampler (Agilent Technologies), operated in electron capture negative ionization (ECNI) mode. The system was configured with a linear optical rail, and methane and nitrogen were used as the ECNI reagent and collision gas, respectively. To mitigate the chemical noise created by the meta-stable helium that can impede low level detection of the analytes of interest, atomic helium was introduced into the collision cell.
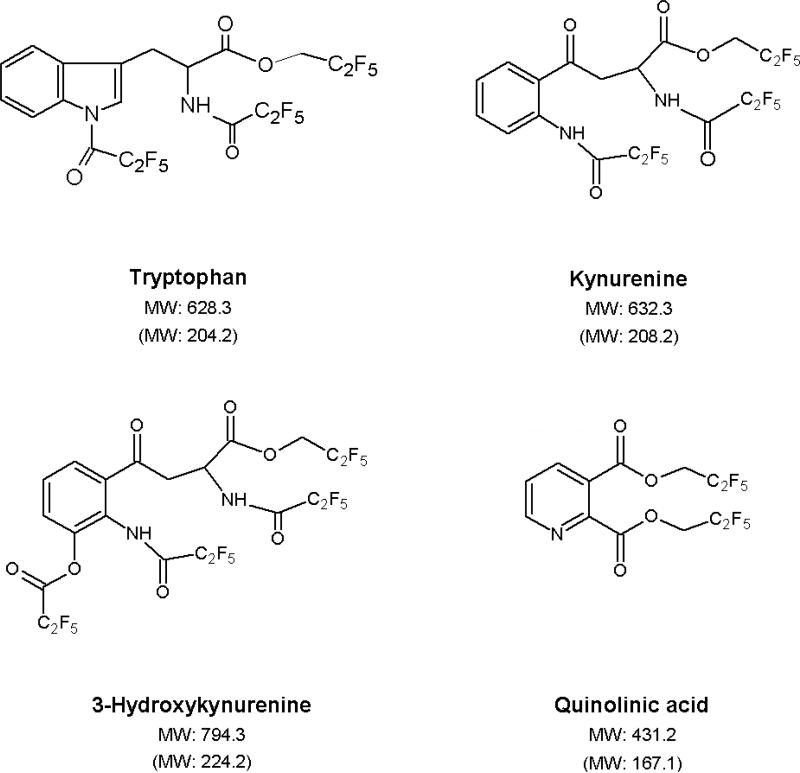
The GC was configured linearly with 3 column sections consisting of a 50 cm × 0.25 mm inner diameter (ID) HP-5MS film and two 15 m HP-5MS (0.25 mm ID × 0.25 μm film thickness) columns connected with an Ultimate Union and a Purged Ultimate Union, respectively (all obtained from Agilent Technologies). Helium was used as a carrier gas with flow rates of 1.1 mL/min and 1.2 mL/min for columns 1 and 2, respectively. Nitrogen flow in the collision cell was 1.5 mL/min, and the helium quench flow was 2.25 mL/min. One μL injection volumes were made into a 250°C split/splitless inlet in pulsed, splitless mode. The oven temperature program was as follows: 60°C for 1 min, 13°C/min to 230°C, for a total run time of 14.08 min. A post-run backflush program was configured with an oven temperature of 300°C, an auxiliary pressure of 35 psi and an inlet pressure of 1 psi for 4 min. The temperature of the CI source and of the quadrupoles (Q1and Q3) was 150°C. The ECNI reagent gas flow was 40% (2.0 mL/min), and the filament emission current was 50 μA. The mass spectrometer was tuned and calibrated using PFDTD on m/z 185, 351 and 449. SRM transitions for each analyte are listed in Table 1, and the predicted structures of the derivatized compounds are shown in Figure 2.
Table 1.
Detection of tryptophan and its metabolites by GC/MS/MS. See text for experimental details.
| Metabolites | MW (amu) | Time segment | SRM transition Q1 → Q3 (m/z) / | Collision energy (V) | Rt (min) |
|---|---|---|---|---|---|
| Quinolinic acid | 431.2 | 1 | 431.0 → 298.0 | 10 | 8.8 |
| 3-Hydroxykynurenine | 794.3 | 2 | 630.0 → 219.0 | 10 | 12.3 |
| L-Kynurenine | 632.3 | 3 | 612.0 → 442.0 | 10 | 12.5 |
| L-Tryptophan | 628.3 | 3 | 608.2 → 351.0 | 20 | 12.5 |
| [2H3]Quinolinic acid | 434.2 | 1 | 434.0 → 301.0 | 10 | 8.8 |
| [2H6]L-Kynurenine | 638.3 | 3 | 618.0 → 447.0 | 10 | 12.5 |
| [2H5]L-Tryptophan | 633.3 | 3 | 613.2 → 351.0 | 20 | 12.5 |
MW: Molecular weight of analytes and deuterated standards after derivatization; Rt: Retention time; Q1, Q3: Quadrupoles. “Time segment” refers to the acquisition time periods depicted in Fig. 3.
Figure 2.
Predicted derivatized structures of the 4 analytes measured in this study. Molecular weights (MW) of the derivative and the parent compound (in parenthesis) are indicated.
Derivatization of tryptophan and kynurenines
The derivatization procedure described by Watson et al. [35] was adapted to determine tryptophan, kynurenine, 3-HK and QUIN. Standards were dissolved in 0.1 M NaOH containing 0.1% (w/v) ascorbic acid and diluted to the final concentration with 1% (v/v) aqueous formic acid. To determine the content of the analytes in brain microdialysate (see below), 50 μL of a solution containing internal standards (500 nM [2H5]L-tryptophan, 10 μM [2H6]L-kynurenine and 50 nM [2H3]quinolinic acid) in 1% aqueous formic acid were added to 50 μL of the biological sample. The mixture was dried down under vacuum in a glass tube (90 min; room temperature; SpeedVac, Thermo Scientific, Asheville, NC, USA). The samples were reconstituted using 120 μL of PFP and 130 μL of PFAA (75°C for 30 min), dried down again, and taken up in 50 μL of ethyl acetate. One μL was then injected into the GC.
In vivo microdialysis
Rats were anesthetized using chloral hydrate (360 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf, Tujunga, CA, USA). A guide cannula (0.65 mm o.d., SciPro, NY, USA) was positioned unilaterally or bilaterally above the striatum (AP: 1.0 anterior to bregma, L: ± 2.5 from the midline, V: 3.0 mm below the dura) and anchored to the skull with screws and dental cement. On the following day, a microdialysis probe (SciPro, membrane length: 2 mm) was inserted through the guide cannula, extending vertically 2 mm beyond the implanted guide. The probe was then perfused with Ringer solution (in mM: NaCl, 144; KCl, 4.8; MgSO4, 1.2; CaCl2, 1.7; pH 6.7) at a rate of 1 μL/min. Samples were collected hourly in Eppendorf tubes and placed on ice for the duration of the experiment.
Naïve rats were sampled for 5 consecutive hours. Microdialysates from animals that had received intrastriatal injections one week earlier (see below) were collected for 1-2 h to obtain baseline values. Animals then received an i.p. injection of kynurenine (100 mg/kg), and dialysis was continued for 7 h.
Samples were stored at -80°C until analysis. The concentrations of the analytes were not corrected for recovery from the microdialysis probe.
Striatal NMDA lesion
Rats were anesthetized with chloral hydrate and placed in a stereotaxic frame (see above). NMDA (8 μg/2 μL), dissolved in phosphate-buffered saline (PBS; pH 7.4), was then infused over 10 min into the striatum [36]. The injection coordinates were: AP: 1 mm anterior to bregma, L: ± 2.7 mm from the midline, V: 4.7 mm below the dura. The contralateral striatum received an identical injection of 2 μL PBS.
One week later, animals were implanted with bilateral guide cannulae. On the next day, microdialysis was performed simultaneously in the lesioned and contralateral striatum, as described above.
Following in vivo microdialysis, the animals were euthanized, and the success of the lesion was evaluated by determining striatal glutamate decarboxylase (GAD) activity [36].
Statistics
Results are expressed as the mean ± SEM. One-way or two-way ANOVA followed by Bonferroni's post-hoc test were used in microdialysis studies. Baseline metabolite values in lesioned and contralateral striata were compared using paired Student's t-test, and unpaired Student's t-test was applied to compare baseline metabolite levels in striata from naïve rats and lesioned striata. Differences in GAD activity were determined by paired Student's t-test. In all cases, a p value of <0.05 was considered significant.
RESULTS
Assay procedure
In addition to the SRM transitions previously used by Eckstein et al. [33], several modifications were introduced to improve assay quality. First, the optimal product ion for kynurenine was found to be 442.0 instead of 218.0, leading to a unique product ion for this analyte and thus optimizing the selectivity of the assay. Second, the transitions for all internal standards, which had not been used by Eckstein and his collaborators, were newly established (Table 1). Finally, we introduced hardware modifications such as the 3 column sections and column backflush to improve instrument performance and maintenance. As a result of the novel GC and MS instrumentation used, experimental conditions had to be optimized empirically, especially with respect to collision cell energies.
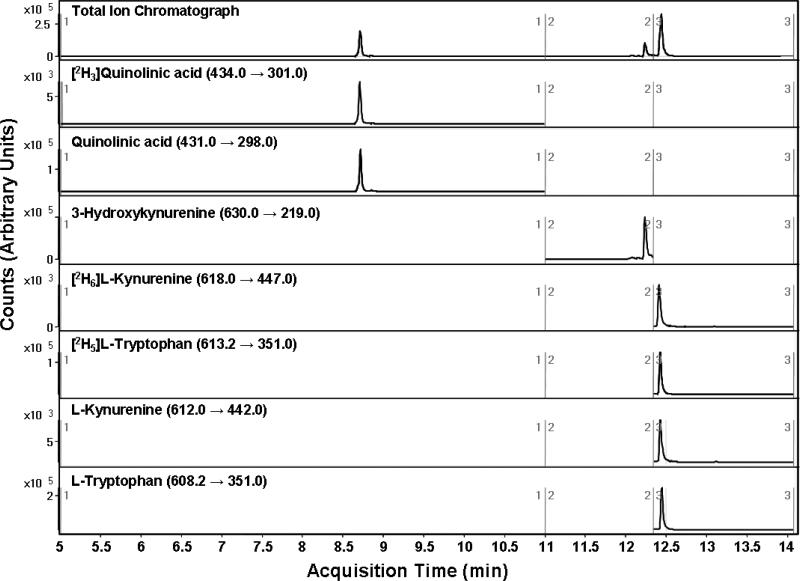
Three internal standards were added to all standard solutions and test samples before derivatization and sample preparation. Since their intensities co-varied, [2H6]L-kynurenine was used as the internal standard for both kynurenine and 3-HK. Standard curves were constructed by plotting the area ratio of each analyte relative to its deuterated internal standard versus concentration. Linearity of the response was determined for all compounds, and correlation coefficients (R2) were ≥0.995 over the concentration range of 10 to 1000 nM for tryptophan and kynurenine, and 1 to 1000 nM for 3-HK and QUIN. The percent relative standard deviation (%RSD, or coefficient of variation) was <2 for QUIN, kynurenine and tryptophan and <8 for 3-HK (8 replicates each). SRM chromatograms for standards are shown for each transition (Fig. 3).
Figure 3.
Representative GC/MS/MS chromatogram of analyte standards (1000 nM). “Counts” indicate the intensity of each trace. The top trace depicts the total ion chromatogram of the GC/MS run. Below are selected reaction monitoring (SRM) traces of individual standards and internal standards.
Simultaneous determination of extracellular tryptophan and kynurenines
Chromatography of samples derived from rat brain microdialysate resulted in well resolved symmetrical peaks that allowed the simultaneous determination of tryptophan, kynurenine, 3-HK and QUIN. However, we noted that the selectivity of the SRM transitions might have suffered from isobaric interference in the dialysate, causing slight inconsistencies when measuring the analytes in the biological samples. The routine use of internal standards was therefore required to optimize normalization of these effects and also increased accuracy and reproducibility of the assay.
We first determined the basal levels of tryptophan and its metabolites in striatal microdialysates from 5 naïve rats, measuring the content of the analytes in 5 consecutive hourly fractions. With the exception of 3-HK (levels were below the limit of detection, i.e. <2 nM), the compounds were readily detectable, measuring 415.0 nM (tryptophan), 88.9 nM (kynurenine) and 8.3 nM (QUIN) on average (Table 2).
Table 2.
Baseline concentrations of extracellular tryptophan, kynurenine, 3-HK and QUIN in the rat striatum.
| Metabolite (nM) | Naïve | lesioned (7 days) | Contralateral (7 days) |
|---|---|---|---|
| Tryptophan | 415.0 ± 28.4 | 621.6 ± 66.0 | 481.8 ± 100.0 |
| Kynurenine | 88.9 ± 3.0 | 113.4 ± 13.0 | 72.7 ± 14.2 |
| 3-HK | <2 | <2 | <2 |
| QUIN | 8.3 ± 0.5 | 11.4 ± 3.8 | 6.1 ± 1.0 |
Data from naïve animals (n = 5) are the average of 5 consecutive 1-h microdialysate fractions. The other samples were collected over 1-2 h from 6 animals that had received intrastriatal injections of NMDA (8 μg/2 μL; “lesioned”) or vehicle (PBS; “contralateral”) one week earlier. Data are the mean ± SEM. p>0.05, comparing lesioned striata to either contralateral striata (paired Student's t-test) or striata from naïve rats (unpaired Student's t-test).
Extracellular metabolite levels in the NMDA-lesioned striatum
Tryptophan, kynurenine and QUIN (but not 3-HK) were also readily measurable in microdialysates obtained from rats receiving intrastriatal injections of NMDA or PBS one week earlier. No significant differences in baseline levels were observed in NMDA-injected striata compared to either contralateral, PBS-injected striata or striata from naïve rats (p>0.05 each). In particular, baseline 3-HK levels remained <2 nM in all these animals (Table 2).
Compared to the contralateral, PBS-injected striatum, GAD activity in the NMDA-treated tissue was reduced by 40 ± 4 % (p<0.001), attesting to significant neuronal loss following the local injection of the excitotoxin.
Effects of systemic kynurenine administration
In animals receiving intrastriatal injections of NMDA or PBS one week earlier, systemic administration of kynurenine (100 mg/kg, i.p.) caused significant, transient changes in the extracellular levels of kynurenine and QUIN. Notably, we were also able to measure extracellular 3-HK in striatal microdialysates from these kynurenine-treated animals.
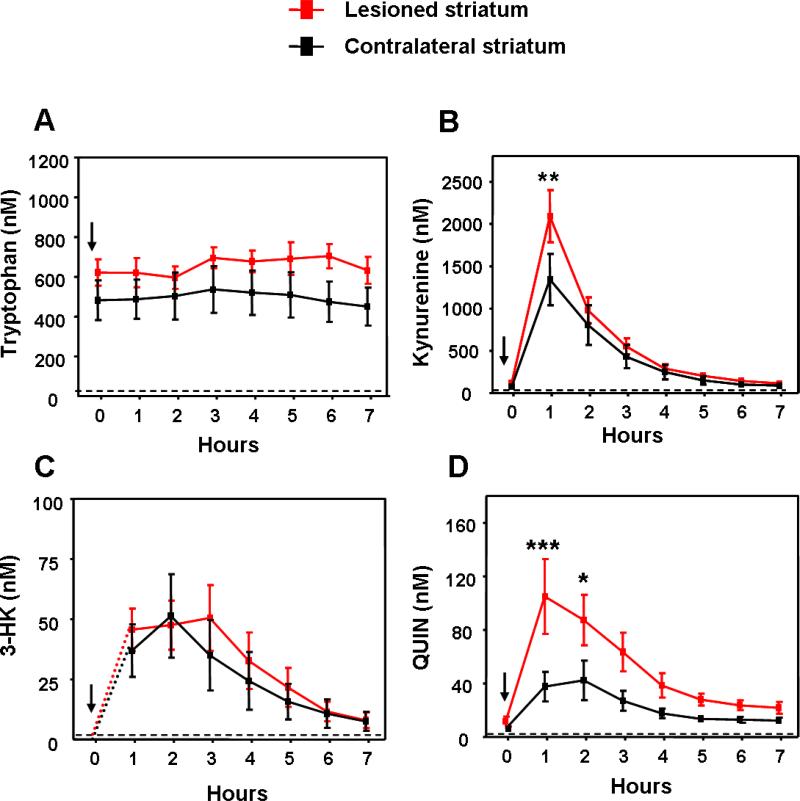
As expected, tryptophan levels remained unaffected by the systemic administration of kynurenine (p>0.05; Fig. 4A). In contrast, reflecting prompt brain influx of peripherally applied kynurenine [37], extracellular kynurenine levels in both the PBS-treated (control) and the NMDA-lesioned striatum rose quickly and dramatically, peaking in the first microdialysis fraction. This rise in kynurenine levels was more pronounced in the NMDA-lesioned tissue (p<0.01), reaching a zenith of 2090 ± 308 nM after 1 h (Fig. 4B).
Figure 4.
Effects of systemically administered kynurenine (100 mg/kg, i.p.; arrows) on the extracellular concentration of tryptophan (A) kynurenine (B) 3-HK (C) and QUIN (D) in the striatum of rats that had received intrastriatal injections of NMDA (“lesioned striatum”) or PBS (“contralateral striatum”) one week earlier (n = 6). Concentrations were not corrected for recovery from the microdialysis probe. With the exception of 3-HK (levels <2 nM, i.e. below the detection limit), baseline values for these animals are reported in Table 2. All metabolites were determined in the same samples. Data are the mean ± SEM. *p<0.05, ** p<0.01, ***p<0.001 vs. the contralateral striatum (two-way ANOVA followed by Bonferroni's post-hoc test).
As shown in Fig. 4C, 3-HK concentrations reached a peak of ~50 nM 2 h following the kynurenine injection, yet no differences were observed between the lesioned and the control striatum. In both tissue, the levels of extracellular 3-HK remained relatively stable until 3 h after i.p. kynurenine administration and then declined slowly during the next 4 h. Notably, extracellular 3-HK levels in both tissues were still measurable after 7 h, i.e. at the end of our collection period.
Extracellular QUIN levels also increased following the systemic administration of kynurenine (Fig. 4D). Compared to baseline values, QUIN levels were elevated 6- and 10-fold during the first hour in PBS- and NMDA-lesioned striata (p<0.05 and 0.001, respectively). The difference in these increases between the two tissues was highly significant (p<0.001). In both cases, QUIN levels gradually returned to baseline between 2 h and 7 h after the kynurenine injection.
DISCUSSION
The present paper describes a sensitive method that can be used for the simultaneous determination and quantification of tryptophan, kynurenine, QUIN, and, conditionally, 3-HK in microdialysate samples from rat brain. To meet the analytical challenge, we used GC/MS/MS in ECNI mode, an established technique for measuring amino acids and their acidic metabolites concurrently and with high sensitivity [38,39], and refined the method of Eckstein et al. [33] by adding internal standards for each analyte and optimizing MS conditions. Compared to Eckstein et al., we replaced methyl t-butyl ether with ethyl acetate, which enhanced the stability of the derivatized analytes due to its higher boiling point. The use of ethyl acetate also allowed us to start the GC run at 60°C, i.e. 10°C below the boiling point of the solvent, creating a tight band of analytes at the column head and resulting in superior peak shapes. Moreover, we replaced argon with the equally effective, but more readily available and less costly, nitrogen as the collision gas. Finally, a new product ion for kynurenine (442 instead of 218) was determined empirically to provide a more selective ion transition for this analyte, boding well for future applications in tissue and other biological matrices. These modifications produced significant improvements in selectivity and reproducibility, resulting in linear responses for each analyte down to the low nanomolar range, as required for the present study.
Further advances were made by introducing a pre-column connected from the inlet to the first column by a zero-dead volume stainless steel union. This configuration provided two significant benefits compared to previous assays: a) quick and ventless replacement of the first column yielded highly reproducible retention times; and b) column backflush further reinforced retention time precision, mitigated matrix build-up on the columns over subsequent injections, improved spectral (SRM) quality and greatly reduced the need for source maintenance. Using this configuration, the chromatographic run time was 14 min, permitting three runs per hour, including the 4 min post-run backflush. Taken together, these methodological improvements resulted in a sensitive, selective and accurate method for the simultaneous measurement of tryptophan and its catabolic products in a biological matrix. It should be added that, in principle, analysis can also be performed on a 30 m column connecting the inlet to the mass spectrometer. However, under these conditions, we encountered retention time instability, increased need for column maintenance (i.e. “clipping” the column head) and reduction in overall method robustness. The use of intra-column backflush addressed these issues through efficient removal of heavy matrix build-up on the analytical column, thus avoiding rapidly decreasing column performance. Additionally, the use of a coated pre-column eliminated the need for column clipping, as it is possible to quickly install a new 50 cm section of column without venting the system.
The assay procedure described here permitted the concurrent determination of basal levels of tryptophan, kynurenine and QUIN levels in 60-min microdialysis fractions from the rat striatum. The concentrations of extracellular tryptophan, kynurenine and QUIN in naïve rats were in line with studies using various detection methods, including ECNI [31,32,40,41]. Interestingly, ambient kynurenine levels in brain microdialysates from normal rats (~500 nM when corrected for the ~20% recovery from the dialysis probe) were only slightly lower than kynurenine concentrations in rat brain tissue and serum, respectively [42,43]. This suggests that the kynurenine concentration in the brain's extracellular compartment is homeostatically controlled by blood-borne kynurenine [20] and, possibly, efflux from brain cells [44].
In contrast to kynurenine, we were unable to measure basal extracellular 3-HK with our methodology (detection limit: ~2 nM). In light of the fact that 3-HK levels in serum are approximately 30 times lower than those of kynurenine [45], this was not entirely unexpected, assuming that brain 3-HK, if it is present extracellularly at all, is exclusively blood-derived. These data support the hypothesis that 3-HK enters the brain from the circulation using the same large neutral amino acid transporter as kynurenine and tryptophan, albeit with a somewhat lower efficiency [20,21]. In the brain, 3-HK is avidly accumulated [46] for further sequestration en route to QUIN. Our inability to detect the metabolite in brain dialysate therefore also indicates that no, or only very little, 3-HK is normally released into the extracellular compartment after being accumulated from the circulation or synthesized locally in microglia [47,48,49].
After establishing baseline values of extracellular tryptophan, kynurenine and QUIN, we used our methodology to examine the acute effects of a systemic administration of kynurenine, which has long been known to increase the levels of neuroactive KP metabolites in the brain [50]. As anticipated, this treatment caused a prompt surge in extracellular kynurenine in the brain, with levels reaching 1.2 μM within one hour (Fig. 4B). In the same samples, no changes were seen in the concentration of extracellular tryptophan, which remained constant throughout the entire 7-h collection period. Notably, however, systemic kynurenine application allowed us to detect 3-HK in the extracellular fluid. Levels were already measurable after one hour, peaked during the second hour and then decreased gradually, but were still quantifiable after 7 h. Although definitive differentiation between blood-borne and locally produced 3-HK must await further experimentation, preliminary studies indicated that striatal inhibition of 3-HK neosynthesis [51] failed to prevent the rise of extracellular 3-HK and QUIN in the brain after systemic kynurenine application (data not shown). This favors a scenario in which extracellular 3-HK in the brain derives from kynurenine in the periphery, and then crosses the blood-brain barrier through the common large neutral amino acid transporter [21].
Kynurenine-induced increases in extracellular QUIN levels followed a similar time course as 3-HK. Since QUIN crosses the blood-brain barrier poorly [21], and since intracerebral application of even millimolar kynurenine fails to augment extracellular QUIN locally [52], the surge was very likely triggered by local QUIN synthesis from 3-HK or 3-hydroxyanthranilic acid, secondary to the brain uptake of the blood-borne precursors (see above and [25]). In support of this interpretation, extracellular QUIN levels in the brain can be readily increased by reverse dialysis of 10 μM 3-hydroxyanthranilic acid, the immediate product of 3-HK degradation and bioprecursor of QUIN (Fig. 1; [52]). Notably, increased levels of extracellular 3-hydroxyanthranilic acid are also seen after systemic kynurenine administration [53].
Excitotoxic striatal lesions provide a heuristically useful model of HD, a disorder associated with increased metabolism along the QUIN branch of the KP (see [54], for review). It therefore seemed sensible to compare the dynamics of our 4 analytes in the lesioned striatum and the contralateral, vehicle-treated striatum using the newly developed methodology. Somewhat surprisingly, in view of the substantial increase in extracellular kynurenic acid and the pronounced activation of several KP enzymes in the lesioned striatum [55,56], extracellular tryptophan, kynurenine and QUIN showed only a modest, non-significant trend towards elevated baseline levels, and extracellular 3-HK remained unmeasurable (Table 1). With the exception of a relatively minor increase in extracellular kynurenine in the lesioned striatum in the first 60-min fraction, extracellular tryptophan, kynurenine and 3-HK levels in the lesioned tissue also did not differ from those measured in the contralateral, PBS-treated striatum after a systemic kynurenine injection (Fig. 4). This indicates that the large neutral amino acid transporter is not substantively impaired when blood-brain barrier permeability is increased due to excitotoxic tissue damage [57]. Moreover, our results suggest that extracellular 3-HK in the brain emanates mostly from 3-HK in the blood. Thus, in spite of the fact that the biosynthetic enzyme of 3-HK, kynurenine 3-monooxygenase, is activated after a lesion and causes a significant increase in 3-HK neosynthesis locally [54], extracellular 3-HK was not elevated in lesioned tissue after systemic kynurenine administration (Fig. 4C). It follows that 3-HK formed within the brain does not appear to enter the extracellular compartment, though it is readily degraded to QUIN intracellularly. In contrast, systemic kynurenine administration resulted in significantly higher extracellular QUIN levels in the NMDA-lesioned compared to the contralateral control striatum. This potentiation is likely related to the pronounced microglial activation and/or macrophage infiltration at the lesion site. These cells harbor the two enzymes that are sequentially responsible for the conversion of 3-HK to QUIN (kynureninase and 3-hydroxyanthranilic acid 3,4-dioxygenase; [58]), and both these enzyme activities, like kynurenine 3-monooxygenase (see above), are dramatically increased in response to an excitotoxic insult [56]. Notably, increased formation and release of QUIN has been shown in the excitotoxically lesioned rat striatum after local application of the immediate bioprecursor 3-hydroxyanthranilic acid, using both microscopic and biochemical outcome measures [52,59,60].
The methodological advance described here also has significant pathophysiological ramifications. Thus, impaired KP metabolism does not only appear to play a role in the etiology of HD and other neurodegenerative diseases, but is increasingly understood to participate actively in major psychiatric diseases including depressive disorders and schizophrenia ([61,62]; cf. Introduction). Because of the well-established connection between the KP and the immune system, hypotheses linking KP dysfunction to brain pathology frequently focus on neuro-immune interactions as core pathogenic mechanisms. Exciting new discoveries, for example the demonstration that kynurenine directly affects endothelial cells and thus influences vascular relaxation and blood pressure [4], further emphasize the need to study the dynamics of the entire KP, and especially the interplay between peripheral and central KP function, in greater detail. Our improved GC/MS/MS method should facilitate these studies and should also prove to be of value for the development and assessment of therapeutic interventions that are based on the manipulation of KP metabolism within or outside the brain.
ACKNOWLEDGEMENTS
This study was supported in part by NIH grant MH085554. We thank Mr. Kyle Horning for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez A, Varo N, Alegre E, Diaz A, Melero I. Immunosuppression routed via the kynurenine pathway: a biochemical and pathophysiologic approach. Adv. Clin. Chem. 2008;45:155–197. doi: 10.1016/s0065-2423(07)00007-8. [DOI] [PubMed] [Google Scholar]
- 3.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann. N. Y. Acad. Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Jr., Hunt NH, Stocker R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costantino G. New promises for manipulation of kynurenine pathway in cancer and neurological diseases. Expert. Opin. Ther. Targets. 2009;13:247–258. doi: 10.1517/14728220802665734. [DOI] [PubMed] [Google Scholar]
- 6.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984;48:273–278. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 7.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J. Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 8.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 9.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981;72:411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 10.Schwarcz R, Whetsell WO, Jr., Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- 11.Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem. Biophys. Res. Commun. 2003;300:719–724. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 12.Leipnitz G, Schumacher C, Dalcin KB, Scussiato K, Solano A, Funchal C, Dutra-Filho CS, Wyse AT, Wannmacher CM, Latini A, Wajner M. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem. Int. 2007;50:83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth H, Toldi J, Vecsei L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005;2:249–260. doi: 10.2174/1567202054368326. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Meininger V, Guillemin GJ. Recent advances in the treatment of amyotrophic lateral sclerosis. Emphasis on kynurenine pathway inhibitors. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:32–39. doi: 10.2174/187152409787601941. [DOI] [PubMed] [Google Scholar]
- 16.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 17.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 18.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vamos E, Pardutz A, Klivenyi P, Toldi J, Vecsei L. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J. Neurol. Sci. 2009;283:21–27. doi: 10.1016/j.jns.2009.02.326. [DOI] [PubMed] [Google Scholar]
- 20.Gal EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem. Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 21.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 22.Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyes MP, Morrison PF. Quantification of local de novo synthesis versus blood contributions to quinolinic acid concentrations in brain and systemic tissues. J. Neurochem. 1997;68:280–288. doi: 10.1046/j.1471-4159.1997.68010280.x. [DOI] [PubMed] [Google Scholar]
- 24.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J. Neurochem. 2002;82:258–268. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard JF, Jr., Erickson JB, Flanagan EM. Quinolinic acid in neurological disease: opportunities for novel drug discovery. Adv. Pharmacol. 1994;30:85–127. doi: 10.1016/s1054-3589(08)60173-8. [DOI] [PubMed] [Google Scholar]
- 26.Bergqvist PB, Heyes MP, Apelqvist G, Butterworth RF, Bengtsson F. Brain extracellular quinolinic acid in chronic experimental hepatic encephalopathy as assessed by in vivo microdialysis: acute effects of L-tryptophan. Neuropsychopharmacology. 1996;15:382–389. doi: 10.1016/0893-133X(95)00256-D. [DOI] [PubMed] [Google Scholar]
- 27.Beagles KE, Morrison PF, Heyes MP. Quinolinic acid in vivo synthesis rates, extracellular concentrations, and intercompartmental distributions in normal and immune-activated brain as determined by multiple-isotope microdialysis. J. Neurochem. 1998;70:281–291. doi: 10.1046/j.1471-4159.1998.70010281.x. [DOI] [PubMed] [Google Scholar]
- 28.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HQ, Baran H, Ungerstedt U, Schwarcz R. Kynurenic Acid in the Quinolinate-lesioned Rat Hippocampus: Studies In Vitro and In Vivo. Eur. J. Neurosci. 1992;4:1264–1270. doi: 10.1111/j.1460-9568.1992.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 30.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutson PH, Sarna GS, Kantamaneni BD, Curzon G. Monitoring the effect of a tryptophan load on brain indole metabolism in freely moving rats by simultaneous cerebrospinal fluid sampling and brain dialysis. J. Neurochem. 1985;44:1266–1273. doi: 10.1111/j.1471-4159.1985.tb08753.x. [DOI] [PubMed] [Google Scholar]
- 32.Landolt H, Lutz TW, Langemann H, Stauble D, Mendelowitsch A, Gratzl O, Honegger CG. Extracellular antioxidants and amino acids in the cortex of the rat: monitoring by microdialysis of early ischemic changes. J. Cereb. Blood Flow Metab. 1992;12:96–102. doi: 10.1038/jcbfm.1992.12. [DOI] [PubMed] [Google Scholar]
- 33.Eckstein JA, Ammerman GM, Reveles JM, Ackermann BL. Simultaneous profiling of multiple neurochemical pathways from a single cerebrospinal fluid sample using GC/MS/MS with electron capture detection. J. Mass. Spectrom. 2008;43:782–790. doi: 10.1002/jms.1376. [DOI] [PubMed] [Google Scholar]
- 34.Risby TH. Use of negative chemical ionization mass spectrometry for the trace analysis of metals. Environ. Health Perspect. 1980;36:39–46. doi: 10.1289/ehp.803639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson E, Wilk S. Derivatization and gas chromatographic determination of some biologically important acids in cerebrospinal fluid. Anal. Biochem. 1974;59:441–451. doi: 10.1016/0003-2697(74)90297-8. [DOI] [PubMed] [Google Scholar]
- 36.Schwarcz R, Scholz D, Coyle JT. Structure-activity relations for the neurotoxicity of kainic acid derivatives and glutamate analogues. Neuropharmacology. 1978;17:145–151. doi: 10.1016/0028-3908(78)90127-2. [DOI] [PubMed] [Google Scholar]
- 37.Gal EM, Sherman AD. Synthesis and metabolism of L-kynurenine in rat brain. J. Neurochem. 1978;30:607–613. doi: 10.1111/j.1471-4159.1978.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 38.Naritsin DB, Boni RL, Markey SP. Pentafluorobenzylation method for quantification of acidic tryptophan metabolites using electron capture negative ion mass spectrometry. Anal. Chem. 1995;67:863–870. doi: 10.1021/ac00101a012. [DOI] [PubMed] [Google Scholar]
- 39.Smythe GA, Braga O, Brew BJ, Grant RS, Guillemin GJ, Kerr SJ, Walker DW. Concurrent quantification of quinolinic, picolinic, and nicotinic acids using electron-capture negative-ion gas chromatography-mass spectrometry. Anal. Biochem. 2002;301:21–26. doi: 10.1006/abio.2001.5490. [DOI] [PubMed] [Google Scholar]
- 40.During MJ, Freese A, Heyes MP, Swartz KJ, Markey SP, Roth RH, Martin JB. Neuroactive metabolites of L-tryptophan, serotonin and quinolinic acid, in striatal extracellular fluid. Effect of tryptophan loading. FEBS Lett. 1989;247:438–444. doi: 10.1016/0014-5793(89)81387-0. [DOI] [PubMed] [Google Scholar]
- 41.Malone MA, Zuo H, Lunte SM, Smyth MR. Determination of tryptophan and kynurenine in brain microdialysis samples by capillary electrophoresis with electrochemical detection. J. Chromatogr. A. 1995:73–80. [Google Scholar]
- 42.Saito K, Fujigaki S, Heyes MP, Shibata K, Takemura M, Fujii H, Wada H, Noma A, Seishima M. Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am. J. Physiol. Renal Physiol. 2000;279:F565–572. doi: 10.1152/ajprenal.2000.279.3.F565. [DOI] [PubMed] [Google Scholar]
- 43.Okuno A, Fukuwatari T, Shibata K. High tryptophan diet reduces extracellular dopamine release via kynurenic acid production in rat striatum. J. Neurochem. 2011;118:796–805. doi: 10.1111/j.1471-4159.2011.07369.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 2007;5:e257. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawlak D, Tankiewicz A, Buczko W. Kynurenine and its metabolites in the rat with experimental renal insufficiency. J. Physiol. Pharmacol. 2001;52:755–766. [PubMed] [Google Scholar]
- 46.Eastman CL, Guilarte TR, Lever JR. Uptake of 3-hydroxykynurenine measured in rat brain slices and in a neuronal cell line. Brain Res. 1992;584:110–116. doi: 10.1016/0006-8993(92)90883-b. [DOI] [PubMed] [Google Scholar]
- 47.Heyes MP, Saito K, Markey SP. Human macrophages convert L-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1992;283(Pt 3):633–635. doi: 10.1042/bj2830633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 49.Guillemin GJ, Kerr SJ, Pemberton LA, Smith DG, Smythe GA, Armati PJ, Brew BJ. IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J. Interferon. Cytokine Res. 2001;21:1097–1101. doi: 10.1089/107999001317205231. [DOI] [PubMed] [Google Scholar]
- 50.Chiarugi A, Carpenedo R, Moroni F. Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and of kynureninase. J. Neurochem. 1996;67:692–698. doi: 10.1046/j.1471-4159.1996.67020692.x. [DOI] [PubMed] [Google Scholar]
- 51.Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009;109:316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speciale C, Ungerstedt U, Schwarcz R. Production of extracellular quinolinic acid in the striatum studied by microdialysis in unanesthetized rats. Neurosci. Lett. 1989;104:345–350. doi: 10.1016/0304-3940(89)90601-0. [DOI] [PubMed] [Google Scholar]
- 53.Cannazza G, Baraldi M, Braghiroli D, Tait A, Parenti C. High-performance liquid chromatographic method for the quantification of anthranilic and 3-hydroxyanthranilic acid in rat brain dialysate. J. Pharm. Biomed. Anal. 2003;32:287–293. doi: 10.1016/s0731-7085(03)00091-8. [DOI] [PubMed] [Google Scholar]
- 54.Schwarcz R, Guidetti P, Sathyasaikumar KV, Muchowski PJ. Of mice, rats and men: Revisiting the quinolinic acid hypothesis of Huntington's disease. Prog. Neurobiol. 2010;90:230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu HQ, Fuxe K, Schwarcz R. Neuronal A1 receptors mediate increase in extracellular kynurenic acid after local intrastriatal adenosine infusion. J. Neurochem. 2004;90:621–628. doi: 10.1111/j.1471-4159.2004.02531.x. [DOI] [PubMed] [Google Scholar]
- 56.Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J. Neurochem. 1995;65:2621–2632. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds DS, Morton AJ. Changes in blood-brain barrier permeability following neurotoxic lesions of rat brain can be visualised with trypan blue. J. Neurosci. Methods. 1998;79:115–121. doi: 10.1016/s0165-0270(97)00168-4. [DOI] [PubMed] [Google Scholar]
- 58.Alberati-Giani D, Ricciardi-Castagnoli P, Kohler C, Cesura AM. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J. Neurochem. 1996;66:996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- 59.Speciale C, Schwarcz R. On the production and disposition of quinolinic acid in rat brain and liver slices. J. Neurochem. 1993;60:212–218. doi: 10.1111/j.1471-4159.1993.tb05840.x. [DOI] [PubMed] [Google Scholar]
- 60.Lehrmann E, Molinari A, Speciale C, Schwarcz R. Immunohistochemical visualization of newly formed quinolinate in the normal and excitotoxically lesioned rat striatum. Exp. Brain Res. 2001;141:389–397. doi: 10.1007/s002210100887. [DOI] [PubMed] [Google Scholar]
- 61.Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry. 2011;68:665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]