Abstract
A three-stage context amplification model was tested with a sample of 345 African American parent-child dyads. The model combined the conceptual structure of stress generation with recent findings regarding genetic susceptibility. Because the 7R+ allele of the dopamine transporter (DRD4) has the potential to enhance contextual priming and arousal, this allele was examined as a potential moderator of each stage of the amplification process. Particular attention was given to the hypothesized influence of parental negative arousal on valence of parent-child interactions. The literature on genetic susceptibility led to the hypothesis that DRD4 would moderate each stage of the model in a “for better or for worse” manner. The model was partially supported. DRD4 moderated effects at all three stages of the model and, as hypothesized, DRD4 moderated contextual effects on negative arousal in a “for better or for worse” manner. Effects on parent-child interaction, however, were moderated in a “for worse” manner only. These results indicate that parenting interactions may amplify the effects of positive and negative contexts in a stress-generating manner, and that a susceptibility framework captures the way in which DRD4 moderates the impact of context on negative arousal.
Keywords: DRD4, stress generation, parenting, arousal, susceptibility
Parent-child relationships are a source of concern for many parents, in part because of the expectation that difficulties in parent-child interactions may confer risk for future problems among youth (Goodman, 2007). Compounding this concern, contextual factors may set the stage for parenting difficulties, and several chronic contextual stressors that forecast parenting problems occur at a higher rate for African American than for European American families. These stressors include instability and dissatisfaction in the primary coparenting relationship, community disorder/ crime, and financial stressors/economic pressure. Specifically, African American spouses report lower marital satisfaction than do European American spouses (Roebuck, & Brown, 2007), and marital difficulties have been found to be associated with heightened rates of anxiety (Overbeek, Vollebergh, de Graaf, Scholte, de Kemp, & Engels, 2006) and parenting problems (Jouriles, Pfifner, and O’Leary, 1988). Likewise, neighborhood crime and personal victimization are associated with chronic elevation of perceived threat, which in turn is associated with negative arousal (Cutrona, Russell, Hessling, Brown, & Murry, 2000), a risk factor for negative parenting interactions. Finally, financial stress and economic pressure increase negative arousal (Cutrona et al., 2003) and negatively influence parenting (e.g. Conger & Elder, 1994). Likewise, positive contextual factors may counteract negative influences. In particular, positive life events, supportive social networks, and positive work experiences may counteract the impact of negative contextual stressors on negative arousal, supporting positive parenting and mitigating the impact of negative context on arousal and parent-child interactions.
As the stress generation framework illustrates (Hammen, 2006), the impact of acute and chronic stressors on physical health, mental health, or adjustment outcomes can be extended or compounded if an individual’s reactions to stress create additional stressors. Interpersonal stressors are particularly important in the stress generation process because they have the potential to produce greater subjective distress than do noninterpersonal stressors (Frans, Rimmo, Aberg, & Fredrikson, 2005), amplifying initial level of distress. It is equally plausible, although less examined, to hypothesize that the impact of benign environments may be extended or amplified by positive, engaged interpersonal processes, compounding the impact of positive contextual factors as well.
Susceptibility to environmental influences may be influenced by genetic predispositions (see Belsky & Pluess, 2009; Ellis, Boyce, Belsky, Bakermans-Kranenburg & van IJzendoorn, 2011). This possibility has led proponents of the susceptibility perspective to propose that those persons most vulnerable to adverse social environments may also reap the greatest benefit from more benign environments. If so, such individuals would experience greater overall sensitivity to environmental contexts, for better or for worse (e.g., Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007). Differential susceptibility is evident when the slopes for a gene by environment (G×E) interaction show a “crossover effect.” This effect occurs when a susceptible group shows worse outcomes than a comparison group in the context of environmental risk or stress but shows better outcomes than the comparison group in low-risk or supportive environments (Belsky et al., 2007; Belsky & Pluess, 2009). As Ellis et al. (2011) noted, substantial evidence demonstrates susceptibility effects for several genes, including DRD4 (e.g. Bakermans-Kranenburg & van IJzendoorn, 2006; Seeger, Schloss, Schmidt, Ruter-Jungfleisch, & Henn, 2004). For example, low maternal sensitivity displayed to young children with the 7-repeat DRD4 allele predicted greater externalizing problems 2 years later whereas high maternal sensitivity predicted fewer externalizing problems among children with the 7-repeat allele (Bakermans-Kranenburg & van IJzendoorn, 2006). Belsky and Pluess (2009) review this and a number of other G×E studies identifying crossover patterns indicative of susceptibility effects that, in most cases, the studies’ authors did not recognize or discuss. Accordingly, it appears important to implement the susceptibility framework in analyzing G×E research, requiring attention to hypothesized G×E interaction effects in the absence of genetic main effects (Belsky & Pluess, 2009).
Combining the conceptual structure of stress generation with recent developments in the understanding of genetic susceptibility suggests the novel hypothesis that contextual effects may be amplified due to their influence on interpersonal processes, and that genetic factors may influence the magnitude of such effects. The dopamine D4 receptor (DRD4) is a candidate gene of particular interest in this regard because of its effects on attention and negative arousal. DRD4 contains a 48bp variable nucleotide repeat (VNTR) polymorphism in exon III of chromosome 11 that is highly polymorphic, with 2 to 11 repeat units. Individuals with at least one DRD4 allele containing 7 or more repeats (7R+) show reduced gene expression (Schoots & Van Tol, 2003). This genotype sets the stage for enhanced attention to the environment (Wang, Zhong & Yan, 2002), emotional and behavioral reactivity to environmental deprivation, and heightened anxiety in response to unconditioned stimuli (Falzone, Gelman, Young, Grandy, Low & Rubinstein, 2002).
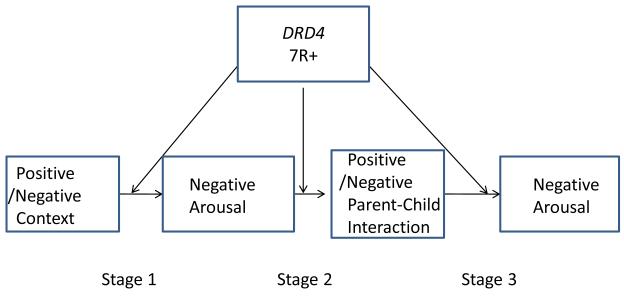
Figure 1 illustrates our expectation that genetic variation at DRD4 will moderate each of three key stages of contextual amplification. As a direct consequence of enhanced attention to the immediate environment, we hypothesize genetic moderation of chronic positive and negative contextual effects on negative arousal, presented in stage 1 of Figure 1. In addition, the DRD4 7R+ allele causes inhibitory neurons that use the associated receptor to require more dopamine to function normally (Swanson et al., 2000). This requirement can contribute to a heightened sense of urgency to engage in direct approach behavior (Cyders & Smith, 2008), a focus on immediate reinforcement, and susceptibility to the priming effects of negative arousal on behavioral choices. This expectation is presented in stage 2 of Figure 1, in which DRD4 is hypothesized to moderate the impact of negative arousal on observed parent-child interaction. Finally, consistent with heightened attention to the environment, we also expected individuals with the DRD4 7R+ allele to show a heightened impact of parenting on their own subsequent negative arousal levels, as presented in stage 3 of Figure 1. Another reason for examining DRD4 as a factor in both susceptibility and stress generation is that genetic variation in DRD4 has been found to influence responses to parenting interventions (Beach, Brody, Lei & Philibert, 2010), with DRD4 7R+ carriers showing less change in intervention targeted parenting in response to intervention. In sum, the model guiding the current examination of contextual amplification combines the stress-generation framework (e.g., Hammen, 2006) with a genetic susceptibility perspective (Belsky & Pluess, 2009). It further suggests that positive and negative contexts will influence negative arousal, which in turn will influence parent-child interactions, which will influence subsequent negative arousal. Each stage of this process is hypothesized to be amplified by the presence of the DRD4 7R+ allele.
Figure 1.
Theoretical model linking compound chronic stressors/supports to negative arousal and parent - child interaction. The model indicates that DRD4 is associated with greater stress generation at all three stages of the context amplification process.
In the current study, we adopt a single ethnic group strategy. We do so because comparative studies often pathologize members of racial minority groups (Bryant, Wickrama, Bolland, Bryant, Cutrona & Stanik, 2010), a danger that is particularly pertinent in studies that include genetic variables. In addition, studies that are designed to identify genetic effects among multiple ethnic groups can identify spurious effects arising from background variations in gene frequencies across groups (see the caution from Hamer & Sirota, 2000, about finding the gene that predicts the use of chopsticks). Known differences in allele frequencies at DRD4 across various racial groups makes spurious findings a particular concern. Accordingly, a single ethnic group model provides a better initial approach for construction of complex G×E models that may identify useful points of intervention for African American parents.
Method
Participants
The current investigation utilizes data from four waves (1, 2, 4, and 5) of the Family and Community Health Study (FACHS), a multisite (Georgia and Iowa) investigation of neighborhood and family processes that contribute to African American children’s development (see Gibbons, Gerrard, Cleveland, Wills & Brody, 2004). Wave 3 was not included because it was not needed to test long-term outcome of parent-child interactions on later negative arousal1. The first wave of the FACHS data was collected in 1997-1998 from 889 African American children in the fifth grade (411 boys and 478 girls; 467 from Iowa and 422 from Georgia), their primary caregivers (PC), and secondary caregivers in homes in which they were present. Households were randomly selected from the sampling frame using rosters of fifth grade students in the public school system and rosters provided by community liaisons compiled from private schools, clubs, and word of mouth. Candidates who declined were removed from the rosters, and other households were randomly selected until the required number of households had been recruited. The second, third, fourth, and fifth waves of data were collected in 1999-2000, 2001-2002, 2004-2005, and 2007-2008 when target youths were ages 12-13, 14-15, 17-18, and 20-21, respectively. Within the sample, PCs self-identified as single parents in 54.9% of cases. Of the 889 PCs interviewed at Wave 1, 693 were interviewed again at Wave 5 (77.26% of the original sample), and 508 had data at all waves used in the current investigation. As part of Wave 5 data collection, PCs provided DNA using a blood or saliva sample. Of the PCs eligible for the current investigation, 367 (72.24%) agreed to provide a sample for genetic analysis; 345 individuals were successfully genotyped.
Comparison of those individuals excluded from the current analyses due to missing data with those retained did not identify any significant differences with regard to PCs’ age, sex, education, single-parent status, household income, or negative arousal at any wave; stressful/positive environment at Wave 1; or parent-child observed interactions at Wave 1 or Wave 2. The resulting sample had a mean age of 37.52 years, SD = 8.34, and an average family per capita income of $6624.79/year. Of the families, 39% lived below the poverty line.
Materials and Procedures
As described elsewhere, to enhance rapport and cultural understanding, African American university students and community members served as field researchers to collect data from the families in their homes. The instruments were presented on laptop computers, allowing participants to enter anonymous responses.
Self-report measures
To assess compound chronic stressors/supports (Wave 1), we examined three negative contexts of particular importance in forecasting parenting problems and three positive, supportive contexts. Four items assessed relationship instability (Booth, Johnson & Edward, 1983); e.g. “Have you thought your relationship might be in trouble?” Cronbach’s alpha was .89. Consistent with prior research, those not in a romantic relationship were assigned a standardized score of zero (Frech and Williams, 2007). Eight items assessed community disorder and crime (Sampson, Raudenbush, & Earls, 1997); e.g. “How often do you have your child involved in supervised activities outside your neighborhood because you worry that your neighborhood is not safe?” Cronbach’s alpha was .78. Thirteen items assessed economic pressure (Conger & Elder, 1994); e.g. “During the past 12 months, how much difficulty have you had paying your bills?” Cronbach’s alpha was .77. Four items assessed positive life events (Conger and Elder, 1994); e.g., “Did you have a positive change in your employment situation in the past 12 months?” Two items assessed the neighborhood social network (Sampson and Graif, 2009) e.g., “How many friends do you have in your neighborhood?” Three items assessed positive aspects of the PCs job (Wickrama, Lorenz, Wallace, Peiris, Conger & Elder, 2001); e.g., “This job gives me opportunities for advancement.”). We reverse coded the positive environment measures, and then each component of compound chronic stress/support was standardized, and the six components were summed to form an overall index of this construct. Using Nunnally’s (1978) reliability formula for composite variables, the reliability for the overall index was .84.
To assess negative arousal at Waves 1, 4, and 5, we used the 10-item negative arousal scale from the Mini-Mood and Anxiety Symptom Questionnaire (Mini-MASQ; Casillas & Clark, 2000; Clark & Watson, 1997). All items indexed symptoms of anxious arousal during the past week; response options were 1 (not at all), 2 (somewhat), and 3 (extremely). Sample items include, “How much have you felt dizzy or lightheaded?” “How much have you been trembling or shaking?” and “How much have you had a very dry mouth?” Cronbach’s alpha was .90 at Wave 1, .80 at wave 4, and .80 at Wave 5.
Observational measures
We video recorded 20-minute parent-child interactions in the home at Waves 1 and 2. Field interviewers provided instructions, set up and started the video equipment, and then left the room so that they would not hear the parent-child discussion. Parent and child were seated facing the camera. To facilitate and standardize the interaction, they were given a set of 16 cards with trigger questions about topics such as enjoyable activities, parental rules and expectations, and the child’s biggest accomplishment or disappointment in the past year. They were asked to read the questions aloud and respond to them as they “normally would.” Dyads were encouraged to interact for the full 20 minutes. Parent and child behaviors were coded by the same coder but in separate passes through the tape; order was determined by flipping a coin. Observational scales included both negative (Hostility, Physical Attack, Neglecting/Distancing, Inconsistent Discipline, Poor Relationship Quality) and positive/engaged (Escalate Warmth, Endearment, Physical Affect, Positive Reinforcement, Inductive Reasoning) behaviors. As has been reported in detail elsewhere, intraclass correlations for observer ratings for individual scales ranged from .55 to .85 (Melby & Conger, 2001). All behaviors were rated using a molar 1 to 9 scale, with higher scores indicating greater intensity or frequency of the behavior. Positive scale ratings were reversed so that higher aggregate scores would indicate poorer functioning. Acceptable test-retest reliabilities have been obtained for composite measures of these behaviors (Ge, Best, Conger, & Simmons, 1996). In the current sample, using the individual scale scores as items, Cronbach’s alphas for the aggregate scale were .66 at Wave 1 and .62 at Wave 2.
Genotyping
Genotype at the DRD4 VNTR was determined as Lichter, Barr, Kennedy, Van Tol, Kidd and Livak (1993) described. This approach involved using the primers F-CGCGACTACGTGGTCTACTCG and RAGGACCCTCATGGCCTTG, standard Taq polymerase and buffer, standard dNTPs with the addition of 100 μM 7-deaza GTP and 10% DMSO. The resulting PCR products were electrophoresed on a 6% nondenaturing polyacrylamide gel and the products visualized using silver staining. Genotype was then called by two individuals blind to study hypotheses and other information about the participants.
Of the sample, 60.6% were homozygous for absence of the 7R+ allele, 34.2% were heterozygous, and 5.2% were homozygous for the presence of the 7R+ allele. The alleles were in Hardy-Weinberg equilibrium, suggesting no evidence of differential allele drop out, χ2= .063, df = 1, p = .80. Consistent with prior research, genotyping results were used to form two groups of participants (cf. Kluger, Siegfried, & Ebstein, 2002). In keeping with the demonstration by Ding, et al., (2002) that the origin of 2R to 6R alleles can be explained by a simple one-step recombination/mutation event, we combined all those homozygous for these high-activity alleles and contrasted them with those who had at least one low-activity allele (7 repeats or more).
Data Analysis
Because the presence of gene-environment correlations (rGE) can confound results in analyses of G×E interactions, and because rGEs have been found in prior work on family processes (Horwitz, Ganiban, Spotts, Lichtenstein, Reiss, & Neiderhiser2011; Reiss & Neiderhiser, 2011), we examined zero-order correlations between parent or child genotypes and outcomes to assess possible passive, active, or evocative rGE effects on selection into stressful environments or evocation of parent-child interaction patterns. To test the hypothesized relationships outlined in Figure 1, hierarchal regression models were run using Mplus 6.1 (Muthén & Muthén, 2010) statistical software to test for the main and interactive effects of stressors and genotypes on outcomes of interest. Main-effects-only models were conducted first to identify significant main effects and are reported in the Results section. In keeping with guidelines for susceptibility analyses (Belsky and Pluess, 2009), we analyzed predicted interaction effects in a second step regardless of whether there was a main effect of genetic polymorphism. The analysis with the G×E term included is reported in Table 1 for each stage of the model.
Table 1. Moderated Regression Analyses Examining DRD4 as a Moderator of Amplification Effects at Three Stages of the Context Amplification Process.
| Model 1 Unstandardized b [95% CI] |
Model 2 Unstandardized b [95% CI] |
Model 3 Unstandardized b [95% CI] |
|
|---|---|---|---|
| Intercept | 11.22** [9.52, 12.93] |
43.64** [34.79, 52.80] |
5.91** [4.33, 7.48] |
| Main Effect | |||
| Positive/negative environment (W1) |
.33 [-.10, .76] |
||
| Negative arousal (W1) | -.48 [-1.60, .65] |
||
| Positive/negative P-C relationship (W2) |
-.16 [-.44, .13] |
||
| DRD4 (7R+=1) | -.09 [-.75, .57] |
-.07 [-1.91, 1.77] |
.13 [-.33, .58] |
| Two-way Interaction | |||
| DRD4 × Positive/negative environment (W1) |
.69* [.04, 1.35] |
||
| DRD4 × Negative arousal (W1) |
2.00* [.12, 3.88] |
||
| DRD4 × Positive/negative P-C relationship (W2) |
.56* [.11, 1.01] |
||
| Time Effect | |||
| Positive/negative P-C relationship (W1) |
.42** [.32, .52] |
||
| Negative arousal (W4) | .48** [.38, .57] |
||
| Adjusted R2 | .043 | .210 | .254 |
|
R2 increase due to
interaction |
.012* | .013* | .013* |
Note. CI = confidence interval; W1 = Wave 1; W2 = Wave 2; W4 = Wave 4.
Education, family structure, age, and family poverty are controlled in the analyses.
p ≤ .05
p ≤ .01, two-tailed tests. N = 345.
We used multiple imputation techniques to estimate missing data at the item level for control variables to avoid loss of subjects. Rates of missing data ranged from 2% for Wave 4 negative arousal to 30.4% for income. We did not use imputed data for primary predictor and dependent variables (chronic stress, negative arousal at Waves 1 and 5, parent-child interaction at Wave 2). In all analyses, we controlled for demographic factors that have emerged in the past as predictors of family processes and symptomatic outcomes (education, single parenthood, poverty, parental age). Significant effects of control variables are noted in the Results section.
To explicate significant G×E interactions and to distinguish “for better” and “for worse” effects, we examined simple slopes and followed-up with the Johnson-Neyman (J-N) technique (Hayes & Matthes, 2009; Johnson & Neyman, 1936; Preacher, Curran & Bauer, 2006) to identify the regions of significance along the continuous distributions of the stressors where the impact of the stressors on outcomes became significantly different for carriers of low-activity versus high-activity DRD4 alleles. We report unstandardized (b) regression weights. All independent variables were standardized (mean of 0 and SD of 1) before the interaction terms were calculated, reducing multicollinearity and making the simple slope easier to test (Dawson & Richter, 2006). In the presence of a differential susceptibility effect, both “for better” and “for worse” regions of difference should emerge in graphical analysis of the G×E effects, reflecting significant differences for both positive and negative contexts.
Results
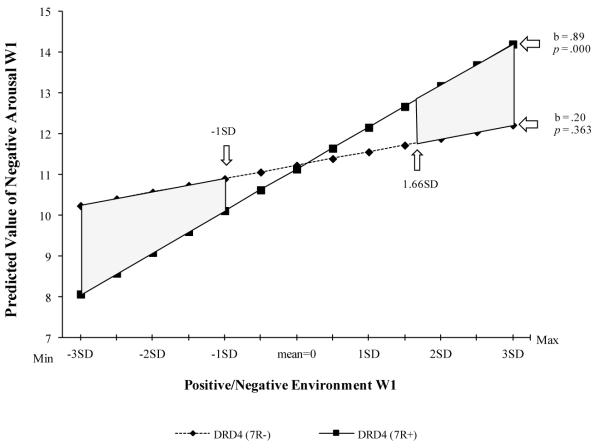
We first checked for evidence of rGE. All associations of parent or child genotype with parent-child interactions or environmental contexts were nonsignificant, ruling out potential confounding effects of passive, active, and evocative rGE attributable to DRD4 on observed interactions or selection into adverse or positive environments. Accordingly, we proceeded with our plan of analysis to examine DRD4 as a susceptibility gene at each of the three potential stages of contextual amplification illustrated in Figure 1. A significant main effect of compound chronic stressors/supports on negative arousal at Wave 1 emerged, b = .49, 95% CI [.16, .83], p = .004. No significant main effect of DRD4 emerged. An analysis of the interaction effect of compound chronic stress/support and DRD4 on Wave 1 negative arousal was conducted; results are presented in Table 1, Model 1. As predicted, a significant interaction effect emerged, b = .69, 95% CI [.04, 1.35], p = .038. No other effects in the model other than the intercept were significant, although a marginal effect (p = .079) emerged for the control variable of family income below the federal poverty line2. Examination of simple slopes (Aiken & West, 1991) indicated that the slope for the impact of context on negative arousal for respondents with DRD4 7R+ alleles was significantly different from zero, b = .89, 95% CI [.39, 1.40], t = 3.48, p = .001. In contrast, the slope for respondents homozygous for DRD4 7R− alleles was not significantly different from zero, b = .20, 95% CI [−.23, .63], NS. In Figure 2a, the graph of the interaction shows the cross-over pattern predicted by the differential susceptibility hypothesis; accordingly, the interaction was examined to identify “for better” and “for worse” effects.
Figure 2a.
Examination of the differential impact of compound chronic stressors/supports at Wave 1 on negative arousal at Wave 1 as a function of DRD4. Gray areas represent regions of significant difference (p < .05, one-tailed)
The J-N technique (Preacher, Curran, & Bauer, 2006) was used to assess regions of significant difference attributable to different DRD4 alleles. The shaded area in Figure 2a shows the regions of significant difference. As can be seen, DRD4 7R+ is associated with significantly greater (p = .049, one-tailed) negative arousal at Wave 1 when the context is more negative (i.e., compound chronic stressors/supports are greater than 1.66 SD above the mean for the sample). Conversely, DRD4 7R+ is associated with significantly lower (p = .048, one-tailed) negative arousal when the context is more positive (i.e., compound chronic stressors/supports are less than 1.00 SD below the sample mean). Thus, both “for better” and “for worse” effects were supported at stage 1 of the model. However, when positive and negative aspects of context were considered separately, the interaction of negative context with DRD4 was significant (b = .64, 95% CI [.002, 1.30], p = .047), whereas the interaction of positive context with DRD4 was not (b = −.36, 95% CI [−1.03, .31], NS).
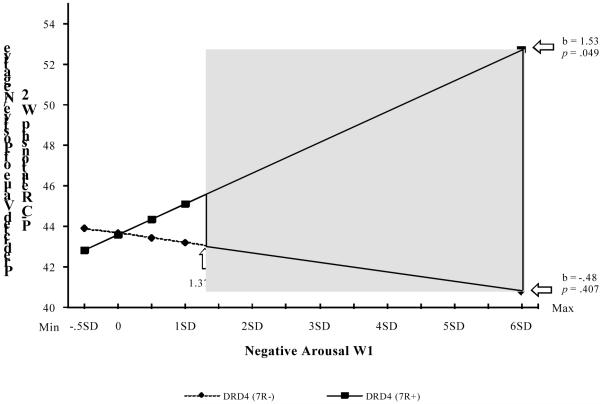
For stage 2 of the model, we examined the impact of negative arousal at Wave 1 on change in parent-child interaction from Wave 1 to Wave 2. Again, we first examined a main effect model, then examined the full model including the interaction term; results are presented in Table 1, Model 2. As expected, there was a significant effect of Wave 1 parent-child interaction on Wave 2 parent-child interaction, b = .42, 95% CI [.32, .52], p = .000, indicating stability in interaction patterns over time. A significant main effect also emerged for the control variable of mother’s education, b = −2.74, 95% CI [−4.78, −.70], p = .009. No other significant main effects emerged. We then examined the model that included the interaction term and identified a significant interaction of negative arousal with DRD4 in the prediction of Wave 2 parent-child interaction, b = 2.00, 95% CI [.12, 3.89], p = .038. Analysis using the simple slopes procedure (Aiken & West, 1991) indicated that the slope for the impact of negative arousal on quality of observed parent-child interaction for respondents with DRD4 7R+ alleles was significantly different from zero, b = 1.53, 95% CI [.00, 3.05], t = 1.97, p = .049. In contrast, the slope for respondents with DRD4 7R− alleles was not significantly different from zero, b = −.48, 95% CI [−1.61, .65], NS.
Again, we used the J-N technique (Preacher, Curran, & Bauer, 2006) to identify regions of significant difference attributable to different alleles of DRD4. The shaded area in Figure 2b shows that DRD4 7R+ was associated with significantly greater (p < .05, one-tailed) increases in potentially problematic, negative parent-child interaction when negative arousal was 1.37 SD or more above the sample mean. Despite an apparent intersection of the lines at very low levels of negative arousal, however, there was no significant difference in positive vs. negative parent-child interactions when negative arousal was low for parents with a DRD4 7R+ allele compared to those without this allele. Thus, we did not find evidence of a “for better” effect at this stage of the model.
Figure 2b.
The effect of negative arousal at Wave 1 on positive/negative parent-child interaction at Wave 2, moderated by DRD4 with Johnson-Neyman confidence bands indicating significant confidence regions (p < .05, one-tailed).
At stage 3, to test the expectation that shifts in interpersonal context could carry effects forward over long periods of time, we determined whether self-generated interpersonal context in the form of positive vs. negative parent-child interactions would influence parental negative arousal at Wave 5, 10 years after the collection of the initial baseline data and 8 years after collection of the Wave 2 parent-child interaction data. In the main effects only model, we found a significant effect of Wave 4 negative arousal, b = .48, 95% CI [.38, .58], p = .000; a significant effect of poverty, b = .53, 95% CI [.01, 1.06], p = .049; and a marginal effect of education, b = −.48, 95% CI [−.98, .02], p = .059. We next examined the model including the interaction term, Model 3 in Table 1. A significant interaction emerged for quality of parent-child interaction at Wave 2 with DRD4 in the prediction of Wave 5 negative arousal, b = .56, 95% CI [.11, 1.01], p = .015. No other effects in the model than the intercept and main effect of Wave 4 negative arousal were significant.
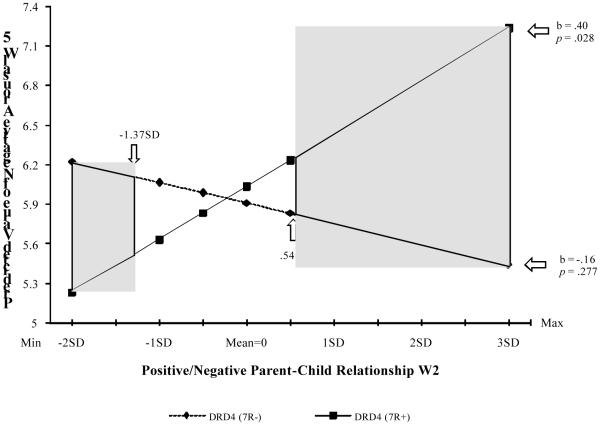
Analysis using the simple slope procedure (Aiken & West, 1991) indicated that the slope of impact of parent-child interaction at Wave 2 on negative arousal at Wave 5 for respondents with at least one DRD4 7R+ allele was significantly different from zero, b = .40, 95% CI [.04, .76], t = 2.21, p = .028. In contrast, the slope for respondents with no DRD4 7R+ alleles was not significantly different from zero, b = −.16, 95% CI [−.44, .13]. Again, we used the J-N technique (Preacher, Curran, & Bauer, 2006) to assess the regions in which significant differences emerged. The graph presented in Figure 2c shows the crossover pattern predicted in the differential susceptibility hypothesis, with both “for better” and “for worse” effects. When problematic parent-child interaction was more pronounced, (i.e., .54 SD or more above the mean), individuals who carried the DRD4 7R+ allele experienced greater increases in negative arousal from Wave 4 to Wave 5. When problematic parent-child interaction was infrequent or positive interaction common (i.e., 1.37 SD or more below the mean), however, those with at least one DRD4 7R+ allele reported significantly less negative arousal at Wave 5.
Figure 2c.
The Effect of Parent-Child interaction at Wave 2 on PC’s negative arousal at Wave 5, moderated by DRD4 with Johnson-Neyman confidence bands indicating significant confidence regions (p < .05, one-tailed).
We disaggregated positive and negative parent-child interactions to examine whether both positive and negative interaction contexts exerted an effect on parent’s later negative arousal. All model effects are presented in Table 2. We found a marginally significant interaction between positive parent-child interaction at Wave 2 and DRD4 in the prediction of Wave 5 negative arousal, b = −.42, 95% CI [−.87, .03], p = .066, as well as a significant interaction between negative parent-child interaction at Wave 2 and DRD4 in the prediction of Wave 5 negative arousal,. b = .55, 95% CI [.10, 1.00], p = .017. Both effects are in the direction predicted, with both reverse coded positive interactions and negative interactions associated with greater parental negative arousal at long-term follow-up.
Table 2. Moderated Regression Analyses Examining DRD4 as a Moderator of Amplification of the Effect of Positive (Model 1) and Negative (Model 2) Parent-child relationship on Parents’ Negative Arousal at Wave 5.
| Model 1 Unstandardized b [95% CI] |
Model 2 Unstandardized b [95% CI] |
|
|---|---|---|
| Intercept | 5.73** [4.16, 7.30] |
5.91** [4.34, 7.48] |
| Main Effect | ||
| Positive P-C relationship (W2) |
.16 [−.13, .44] |
|
| Negative P-C relationship (W2) |
−.08 [−.35, .20] |
|
| DRD4 (7R+=1) | .13 [−.32, .58] |
.10 [−.35, .55] |
| Two-way Interaction | ||
| DRD4 × Positive P-C relationship (W2) |
−.42† [−.87, .03] |
|
| DRD4 × Negative P-C relationship (W2) |
.55* [.10, 1.00] |
|
| Time Effect | ||
| Negative arousal (W4) | .48** [.39, .58] |
.47** [.37, .57] |
| Adjusted R2 | .248 | .256 |
|
R2 increase due to
interaction |
.008† | .012* |
Note. CI = confidence interval; W1 = Wave 1; W2 = Wave 2; W4 = Wave 4.
Education, family structure, age, and family poverty are controlled in the analyses.
p ≤ .05
p ≤ .01, two-tailed tests. N = 345.
Discussion
As predicted by the contextual amplification model in Figure 1, chronic contextual stressors and supports were associated with level of negative arousal at baseline, but the impact of context on negative arousal was significantly greater for those with at least one 7R+ allele at DRD4. This association emerged as a susceptibility effect, with both “for better” and “for worse” effects evident. Moreover, as predicted in the second stage of the contextual amplification model, negative arousal in parents was associated with a shift toward more negative and less positive parent-child interaction only among those with at least one 7R+ allele at DRD4. In this case, significant effects were confined to the “for worse” portion of the interaction. Completing the hypothesized process, level of self-generated positive vs. negative parent-child interactions were associated with change in negative arousal from Wave 4 to Wave 5, again, only for parents with the DRD4 7R+ allele, and again demonstrating a “for better or for worse” pattern. Thus, as predicted by the contextual amplification model, the role of the 7R+ allele of DRD4 was apparent at each of three separate stages of context amplification. For the outcomes related to level of negative arousal, DRD4 acted as a susceptibility gene (Belsky & Pluess, 2009). For the effect of negative arousal on parenting, however, DRD4 acted as a vulnerability factor (Shanahan & Hofer, 2005), with significant differences only in the “for worse” direction. No evidence emerged for a parallel “for better” process in which very low levels of negative arousal led to significantly more positive and less negative parent-child interaction among those with the DRD4 7R+ allele. Together, the results help to explicate better the influence of chronic stressors on negative arousal and parent-child interactions. The results also suggest considerable potential for a combination of a stress-generation framework with the genetic susceptibility perspective to account for the substantial burden that exposure to compound chronic stressors may impose and the way in which this burden may be transformed into a persisting source of interpersonal stress with long-term impact on negative arousal.
We also examined a different aspect of “for better or for worse” effects by disaggregating positive and negative contexts to examine whether each contributed independently to the greater susceptibility of those with the DRD4 7R+ allele. In this regard the evidence was more equivocal, with consistent significant effects only for negative context and marginal or non-significant effects for positive context considered separately. However, effects of positive context were in the expected direction, suggesting the possibility that “for better” effects in response to positive contexts may have a small effect size and so require larger samples for reliable detection.
Prior to examining G×E interaction effects, we looked for evidence of rGE. It is important to examine data for potential rGE because, when present, rGE suggests the possibility that observed interaction effects are the result of passive selection of individuals with different genotypes into different environments or perhaps the presence of active processes that creates differential exposure to environments for those with different genotypes. In family research this issue is particularly pertinent because rGE effects have been identified using behavior analytic methods, and effect sizes controlling for genetic confounds tend to be reduced (Turkheimer & Waldren, 2000). Genetically informed family studies have also demonstrated that broadly based genetic influences on aggressive personality may account for variance in family conflict (e.g. Horwitz, et al., 2011). Accordingly, examination of rGE remains an important consideration in testing any G×E hypothesis involving family context and family interaction. We found no associations, however, of parental DRD4 with our measure of context, indicating no active rGE at stage 1. Likewise, there was no effect for child DRD4, indicating no passive rGE among children. Nor did we find any association of either parental or child DRD4 with either positive or negative aspects of parent-child interaction, indicating that there were no evocative rGE effects at stages 2 or 3. We conclude that positing rGE effects involving DRD4 is not supported in the current investigation.
Limitations of the current study include loss of sample due to attrition and to nonparticipation in the parent-child observational interaction or in genotyping. In addition, our focus on negative arousal to the exclusion of positive affect may have limited our ability to detect “for better” effects when examining the influence of DRD4 on parent-child interactions in stage 2 of the model. Specifically, it may be that some positive states may have interacted with DRD4 to predict more positive parent-child interactions. Accordingly, it would be premature to conclude that there are no “for better” effects involving DRD4 on parent-child interaction. This possibility should be a focus of continuing research, perhaps using alternative approaches with greater flexibility to examine specific hypotheses in an experimental context (e.g., Howe, Beach, & Brody, 2010). Likewise, the time lags that characterize the current investigation may not have been ideal for capturing some “for better” or “for worse” effects. These effects may be observed more clearly over a shorter time frame or through the use of genetically informed experimental designs (e.g. Howe et al., 2010); future research should also focus on these possibilities.
Using conceptual tools from recent theories on genetic effects in family contexts (Belsky & Pluess, 2009) and the stress generation framework for understanding the transformation and accumulation of stress over time in family contexts (Beach & Whisman, 2011; Hammen, 2006), it appears possible to construct a coherent contextual amplification model. DRD4 effects observed in the current investigation suggest that the dopaminergic system may have a range of effects relevant to understanding family dynamics and their interplay with stressful environments. In particular, it appears that 7R+ alleles of DRD4 may amplify the influence of chronic contextual stressors and supports. Under negative circumstances, such amplification can create worse outcomes by prolonging stress effects and generating new family stressors; under more benign circumstances, it can create better outcomes in the form of lower negative arousal. However, in the current data set the interaction of variation in negative contexts with DRD4 appeared more robust than did the interaction of variation in positive contexts with DRD4. This suggests that DRD4 may play an important role in the modeling of feedback loops that characterize the impact of chronic contextual stressors on family relationships and perhaps on a range of stress-related health outcomes.
It is worth noting that, in the current analysis, negative arousal and not depressive symptoms drove the contextual amplification process. This finding extends a growing line of research suggesting that processes other than depression may be important in the stress generation process, with differences among processes in the ways in which they drive stress generation. Consistent with a traditional stress-generation framework, the current findings suggest that chronic stress may accumulate over time for some individuals, in part by creating problems in close relationships, potentially creating feedback loops and vicious cycles over time (cf. Hammen, 2006; Joiner, 2000). Because “for better” effects were observed, however, the data also suggest that those with the 7R+ allele may be more likely to experience “beneficial” cycles in certain contexts.
Although implications should be considered tentative pending replication, the current findings suggest that family interventions can play an important role in interrupting stress generation or promoting resilience, particularly among parents most likely to respond to contextual influences. More broadly, the results support continued investigation of particular G×E pathways that may contribute to accumulation of stress or growth in resilience over time. Because the current sample is African American, the results also suggest a possible mechanism through which racial disparities in environmental adversity may contribute to disparities in health and family functioning. The stress generation framework suggests that multiple opportunities may be available to interrupt the iterative processes that result in heightened strain and morbidity for some individuals.
Exposure to chronic stressors may be inevitable for some African American parents, particularly those living in rural areas with limited economic opportunities; however, family interventions may have the potential to interrupt stress generation processes (Beach & Whisman, 2011) for some parents. Prior to investing resources in targeted preventive efforts, it will be important to determine whether family intervention is more beneficial when parents or children have the 7R+ allele at DRD4 (e.g., Beach et al., 2010). Likewise, because parenting is central to the transmission of problems to offspring (Goodman, 2007), it will be important to determine whether interventions and prevention programs that enhance parenting pay dividends both now, in terms of improvements in parents’ mental and physical health, and for future generations, in terms of reductions in problems among youths and young adults.
Acknowledgments
This research was supported by the National Institute of Mental Health MH080898 awarded to Robert A. Philibert, 2 R01 MH062666-06 awarded to Carolyn Cutrona, and NIDA 1P30DA027827-01 awarded to Gene Brody. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
To examine whether the context amplification process began in adolescence while children were still at home, we examined the interaction of DRD4 and parent-child interaction in the prediction of wave 3 negative arousal. There was a significant interaction b = .47, 95% CI [.09, .85], p < .05. When problematic parent-child interaction was more pronounced, parents who carried the DRD4 7R+ allele experienced greater increases in negative arousal from Wave 2 to Wave 3. There was no evidence of a “for better effect.” Accordingly, it appears that the context amplification process was already under way when offspring were adolescents living at home.
Table 1, displaying results of all regressions, with and without interaction terms, and displaying all b-values for control variables (Education, Single family status, Age, Family below poverty level) is available from the first author.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, factchecking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/fam
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48(5):406–409. doi: 10.1002/dev.20152. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Lei MK, Philibert RA. Differential susceptibility to parenting among African American youth: Testing the DRD4 hypothesis. Journal of Family Psychology. 2010;24:513–521. doi: 10.1037/a0020835. doi:10.1037/a0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Whisman MA. Affective disorders. Journal of Marital and Family Therapy. 2011 doi: 10.1111/j.1752-0606.2011.00243.x. published online 2 Sept 2011. doi: 10.1111/j.1752-0606.2011.00243.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Booth A, Johnson DR, Edwards JN. Measuring marital instability. Journal of Marriage and the Family. 1983;45:387–394. doi: 10.2307/351516. [Google Scholar]
- Bryant CM, Wickrama KAS, Bolland J, Bryant BM, Cutrona CE, Stanik CE. Race matters, even in marriage: Identifying factors linked to marital outcomes for African Americans. Journal of Family Theory & Review. 2010;2:157–174. [Google Scholar]
- Casillas A, Clark LA. The Mini Mood and Anxiety Symptom Questionnaire (Mini-MASQ); Poster presented at the 72nd Annual Meeting of the Midwestern Psychological Association; Chicago, IL. 2000.May, [Google Scholar]
- Clark LA, Watson D. The Mini Mood and Anxiety Symptom Questionnaire (Mini-MASQ) Unpublished manuscript, University of Iowa; Iowa City: 1997. [Google Scholar]
- Conger RD, Elder GH., Jr. Families in troubled times: The Iowa Youth and Families Project. In: Conger RD, Elder GH Jr., editors. Families in troubled times: Adapting to change in rural America. Aldine de Gruyter; Hawthorne, NY: 1994. pp. 57–78. [Google Scholar]
- Cutrona CE, Russell DW, Hessling RM, Brown PA, Murry V. Direct and moderating effects of community context on on the psychological well-being of African American women. Journal of Personality and Social Psychology. 2000;79:1088–1101. doi: 10.1037//0022-3514.79.6.1088. doi: 10.1037/0022-3514.79.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Abraham WT, Gardner KA, Melby JN, Bryant C, et al. Neighborhood context and financial strain as predictors of marital interaction and marital quality in African American couples. Personal Relationships. 2003;10:389–409. doi: 10.1111/1475-6811.00056. doi: 10.1111/1475-6811.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Clarifying the role of personality dispositions in risk for increased gambling behavior. Personality and Individual Differences. 2008;45:503–508. doi: 10.1016/j.paid.2008.06.002. doi: 10.1016/j.paid.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JE, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. Journal of Applied Psychology. 2006;91:917–926. doi: 10.1037/0021-9010.91.4.917. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences. 2002;99:309–314. doi: 10.1073/pnas.012464099. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B,J, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. European Journal of Neuroscience. 2002;15:158–164. doi: 10.1046/j.0953-816x.2001.01842.x. doi: 10.1046/j.0953-816x.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and posttraumatic stress disorder in the general population. Acta Psychiatrica Scandinavica. 2005;111:280–291. doi: 10.1111/j.1600-0447.2004.00463.x. doi: 10.1111/j.1600-0447.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- Frech A, Williams K. Depression and the psychological benefits of entering marriage. Journal of Health and Social Behavior. 2007;48:149–163. doi: 10.1177/002214650704800204. doi: 10.1177/002214650704800204. [DOI] [PubMed] [Google Scholar]
- Ge X, Best KM, Conger RD, Simons RL. Parenting behaviors and the occurrence and co-occurrence of adolescent depressive symptoms and conduct problems. Developmental Psychology. 1996;32:717–731. doi: 10.1037/0012-1649.32.4.717. [Google Scholar]
- Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody GH. Perceived discrimination and substance use in African American parents and their children: A panel study. Journal of Personality and Social Psychology. 2004;86:517–529. doi: 10.1037/0022-3514.86.4.517. doi: 10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Hamer D, Sirota L. Beware the chopsticks gene. Molecular Psychiatry. 2000;5:11–13. doi: 10.1038/sj.mp.4000662. doi: 10.1038/sj.mp.4000662. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology. 2006;62:1065–1082. doi: 10.1002/jclp.20293. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavioral Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Horwitz BN, Ganiban JM, Spotts EL, Lichtenstein P, Reiss D, Neiderhiser JM. The role of aggressive personality and family relationships in explaining family conflict. Journal of Family Psychology. 2011;25:174–183. doi: 10.1037/a0023049. doi: 10.1037/a0023049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GW, Beach SRH, Brody GH. Microtrial methods for translating gene-environment interaction into preventive interventions. Prevention Science. 2010;11:343–354. doi: 10.1007/s11121-010-0177-2. doi: 10.1007/s11121-010-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, Neyman J. Tests of certain linear hypotheses and their applications to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Joiner TE. Depression’s vicious scree: Self-propogating and erosive processes in depression chronicity. Clinical Psychology: Science and Practice. 2000;7:203–218. doi: 10.1093/clipsy.7.2.203. [Google Scholar]
- Jouriles EN, Pfiffner LJ, O’Leary SG. Marital conflict, parenting, and toddler conduct problems. Journal of Abnormal Child Psychology. 1988;16:197–206. doi: 10.1007/BF00913595. doi: 10.1007/BF00913595. [DOI] [PubMed] [Google Scholar]
- Kluger AN, Siegfried Z, Ebstein RP. A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Molecular Psychiatry. doi: 10.1038/sj.mp.4001082. 2002;7:712–717. doi: 10.1038/sj.mp.4001082. [DOI] [PubMed] [Google Scholar]
- Lichter JB, Barr CL, Kennedy JL, Van Tol HHM, Kidd KK, Livak KJ. A hyper variable segment in the human dopamine receptor D4 (DRD4) gene. Human Molecular Genetics. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- Melby JN, Conger RD. The Iowa Family Interaction Rating Scales: Instrument summary. In: Kerig P, Lindahl K, editors. Family observational coding systems: Resources for systematic research. Erlbaum; Mahwah, NJ: 2001. pp. 33–58. [Google Scholar]
- Muthén LK, Muthén BO. Mplus 6.0 user’s guide. CA: Authors; Los Angeles: 2010. [Google Scholar]
- Nunnally JC. Psychometric theory. McGraw-Hill; New York: 1978. [Google Scholar]
- Overbeek G, Vollebergh W, de Graaf R, Scholte R, de Kemp R, Engels R. Longitudinal associations of marital quality and marital dissolution with the incidence of DSM-III-R disorders. Journal of Family Psychology. 2006;20:284–291. doi: 10.1037/0893-3200.20.2.284. doi: 10.1037/0893-3200.20.2.284. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. doi: 10.3102/10769986031004437. [Google Scholar]
- Reiss D, Neiderhiser JM. Marital dynamics and child proaction: Genetics takes a second look at developmental theory. In: Kenneth A. Dodge, Michael Rutter., editors. Gene-Environment Interactions in Developmental Psychopathology. Guilford Press; New York: 2011. pp. 105–120. [Google Scholar]
- Roebuck BJ, Brown S. Race-ethnic differences in marital quality and divorce. Social Service Review. 2007;36:945–967. [Google Scholar]
- Sampson RJ, Graif C. Neighborhood social capital as differential social organization: Resident and leadership dimensions. American Behavioral Scientist. 2009;52:1579–1605. [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: Retrospect and prospect. Journals of Gerontology Series B: Psychological Sciences and Social Science. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. doi: 10.1093/geronb/60.Special_Issue_1.65. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics Journal. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. doi:10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Seeger G, Schloss P, Schmidt MH, Ruter-Jungfleisch A, Henn FA. Gene-environment interaction in hyperkinetic conduct disorder (HD + CD) as indicated by season of birth variations in dopamine receptor (DRD4) gene polymorphism. Neuroscience Letters. 2004;366(3):282–286. doi: 10.1016/j.neulet.2004.05.049. doi: 10.1016/j.neulet.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, et al. Dopamine genes and ADHD. Neuroscience & Biobehavioral Reviews. 2000;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. doi:10.1016/S0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Waldron M. Nonshared environment: A theoretical, methodological, and quantitative review. Psychological Bulletin. 2000;126:78–108. doi: 10.1037/0033-2909.126.1.78. doi: 10.1037/0033-2909.126.1.78. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. Journal of Neuroscience. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama KAS, Lorenz FO, Wallace LE, Peiris L, Conger RD, Elder GH. Family influence on physical health during the middle years: The case of onset of hypertension. Journal of Marriage and Family. 2001;63:527–539. doi: 10.1111/j.1741-3737.2001.00527.x. [Google Scholar]