Abstract
The BH3-interacting domain death agonist (Bid) is a pro-apoptotic member of the B-cell lymphoma-2 (Bcl-2) protein family. Previous studies have shown that stress reduces levels of Bcl-2 in brain regions implicated in the pathophysiology of mood disorders, whereas antidepressants and mood stabilizers increase Bcl-2 levels. The Bcl-2 protein family has an essential role in cellular resilience as well as synaptic and neuronal plasticity and may influence mood and affective behaviors. This study inhibited Bid in mice using two pharmacological antagonists (BI-11A7 and BI-2A7); the selective serotonin reuptake inhibitor citalopram was used as a positive control. These agents were studied in several well-known rodent models of depression—the forced swim test (FST), the tail suspension test (TST), and the learned helplessness (LH) paradigm—as well as in the female urine sniffing test (FUST), a measure of sex-related reward-seeking behavior. Citalopram and BI-11A7 both significantly reduced immobility time in the FST and TST and attenuated escape latencies in mice that underwent the LH paradigm. In the FUST, both agents significantly improved duration of female urine sniffing in mice that had developed helplessness. LH induction increased the activation of apoptosis-inducing factor (AIF), a caspase-independent cell death constituent activated by Bid, and mitochondrial AIF expression was attenuated by chronic BI-11A7 infusion. Taken together, the results suggest that functional perturbation of apoptotic proteins such as Bid and, alternatively, enhancement of Bcl-2 function, is a putative strategy for developing novel therapeutics for mood disorders.
Keywords: AIF, behavior, Bcl-2, Bid, depression, stress
Introduction
Mood disorders, including major depressive disorder and bipolar disorder (BPD), are chronic, severe and often highly disabling illnesses. Major depressive disorder is one of the leading causes of disability worldwide, and currently available therapeutics have limited efficacy, require chronic treatment and have undesirable side effects.1–4 Outcome is poor for many patients, who experience high rates of chronicity, residual symptoms, relapse, subsyndromes, cognitive and functional impairments, and psychosocial disability.2,5 Furthermore, the direct and indirect costs associated with disability and premature death in individuals suffering from mood disorders represent an economic burden of tens of billions of dollars annually in the United States alone.6 Not surprisingly, the World Health Organization’s Global Burden of Disease Study noted that mood disorders are among the leading causes of disability worldwide, with increasing disability likely in the coming years.7 In addition, mood disorders are associated with many other health-related consequences,8–10 as well as significantly elevated risk of suicide.11 Despite their prevalence, the pathophysiology of mood disorders is not as well understood as that of other common chronic and potentially fatal diseases.12 Therefore, the need to identify novel mechanisms for the development of new and more effective treatments is critical.
Although severe mood disorders have traditionally been conceptualized as neurochemical disorders, growing evidence from neuroimaging and postmortem studies suggests that these disorders are associated with increased cell death and changes in synaptic and neural plasticity.4,12–14 Thus, several, but not all, imaging studies report volume reductions in selected brain regions such as the hippocampus or subregions of the medial prefrontal cortex of individuals with mood disorders,4,12,13 and post-mortem studies have noted atrophy and loss of neurons and glial cells in selected brain regions.4,12,13
In the context of altered regulation of apoptotic signaling in mood disorders, it is worth noting that genome-wide screening approaches and independent validation methods have also found that B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated athanogene (BAG1) are upregulated by chronic treatment with mood stabilizers, the medications traditionally used to treat BPD (for a review see Hunsberger et al.15,16). The Bcl-2 family of proteins are prominent modulators of cell survival and apoptosis; members that promote cell death are tightly regulated and opposed by anti-apoptotic members such as Bcl-2.17,18 These proteins also play critical roles in calcium homeostasis,19 mitochondrial and endoplasmic reticulum function,19,20 neurogenesis,21 neuronal morphogenesis22–24 and—importantly—synaptic plasticity.25,26 In addition to increasing Bcl-2 levels, mood stabilizers also promote Bcl-2-related neuronal processes such as neurite outgrowth, adult hippocampal neuronal neurogenesis, and neuronal protection against a variety of insults (for a review see Hunsberger et al.15,16). Electroconvulsive shock27 and treatment with antidepressants from different classes—including amitriptyline, desipramine, imipramine, fluoxetine, reboxetine, tranylcypromine, and venlafaxine27–30—have also been shown to upregulate Bcl-2 expression in several brain regions. Studies have also found that behavioral stress in rodents, which can cause anhedonia-like behavior, decreases Bcl-2 levels in cortical, hippocampal and limbic brain structures, and that these decreases can be abolished by antidepressant treatment.27,31 Although behavioral studies examining the consequences of manipulating levels of Bcl-2-interacting proteins are in their infancy, it is notable that transgenic mice with neuronal BAG1 overexpression display accelerated recovery from helplessness in the learned helplessness (LH) paradigm, and from hyperactivity in the amphetamine-induced locomotion test.32 These data raise the intriguing possibility that members of the Bcl-2 protein family may mediate some of the behavioral effects of antidepressants and, ultimately, that pharmacological modulation of Bcl-2 function might produce antidepressant-like behavioral effects.
This study altered the functional balance of activity among the Bcl-2 family of proteins by inhibiting a protein that opposes Bcl-2 function in the apoptotic cascade. Specifically, we targeted the BH3-interacting domain death agonist (Bid) protein, a 22-kDa pro-apoptotic member of the Bcl-2 family. Intact Bid protein is localized to the cytoplasm and inhibited by Bcl-2, but upon cleavage by activated caspase-8, truncated Bid (tBid) translocates to the mitochondria.33,34 tBid binds with another pro-apoptotic protein (Bax), which can oligomerize with Bak to form pores that disrupt mitochondrial membrane integrity. These events precede the activation of two distinct apoptotic pathways: caspase-dependent and caspase-independent cell death pathways.35 Activation of the caspase-dependent pathway results in the release of pro-apoptotic stimuli such as SMAC (second mitochondria-derived activator of caspase) and cytochrome c.36 Cytochrome c, together with APAF-1 and caspase-9, forms the apoptosome complex, which results in the activation of caspase-3 and other effector caspases, ultimately causing cell death.37,38 Activation of the caspase-independent pathways results in the translocation of apoptosis-inducing factor (AIF) from the mitochondria to the nucleus, where it induces large-scale DNA fragmentation and apoptosis by an unknown, caspase-independent mechanism.39,40
We postulated that chemical inhibitors of Bid would exert antidepressant-like effects by blocking the activation of either caspase-dependent or caspase-independent signaling cascades. The Bid inhibitors used here—BI-11A7 and BI-2A7—are synthetic molecules that have previously been shown to block apoptosis signaling by preventing the cleavage of Bid protein (and thereby preventing its activation); biochemical assays found that BI-11A7 is more potent and effective than BI-2A7 at antagonizing Bid.41,42 This proof-of-principle study was conducted to examine the behavioral effects of BI-11A7 in a variety of preclinical behavioral paradigms related to depression. In addition, we measured levels of relevant cytosolic, mitochondrial, and nuclear proteins in the prefrontal cortex (PFC) of treated mice, in an attempt to determine the involvement of caspase-dependent and caspase-independent pathways in the therapeutic mechanism of Bid inhibitors.
Materials and methods
Animals
Eight-week-old 129S1/SVImJ mice (male, 20–25 g; Jackson Laboratories, Bar Harbor, ME, USA) were group housed in a facility with constant temperature (22±1 °C) and 12-h light/dark cycle (light—0600–1800 hours), and with access to food and water ad libitum. All animals underwent surgery to implant the intracerebroventricular infusion cannula and osmotic minipumps. All behavioral tests were conducted between the hours of 1000–1500 hours starting 12 days after the surgery in a separate room immediately adjacent to the facility. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Mental Health (NIMH) and were conducted according to National Institutes of Health guidelines.
Brain delivery of control and experimental agents
The ability of Bid inhibitors to penetrate the blood–brain barrier is largely unknown; therefore, we conducted this proof-of-principle study using direct intracerebroventricular infusion to deliver the control and experimental agents to the third ventricle of the brain, which is centralized and therefore only requires a single infusion cannula. The selective serotonin reuptake inhibitor citalopram was used as the positive control agent; the concentration of citalopram used for infusion was determined based on a previous study.43 Bid inhibitor concentrations were empirically selected based on the assumption that the infusion would result, over a 24-h period, in a whole brain concentration of the inhibitors similar to the concentration at which the agents produce significant chemical effects in cell culture.41 LL447951, an unrelated compound with a similar molecular weight to BI-11A7, was used as a negative control in the LH paradigm.
Infusion cannula and osmotic minipumps were implanted as described previously.44 Briefly, 129S1/SVImJ mice were anesthetized by inhalation of 3% isoflurane in oxygen and placed in a stereotaxic instrument. The scalp was incised and retracted laterally and the skull was leveled between lambda and bregma. A 2-mm opening was drilled through the skull in the bregma. Alzet osmotic minipumps, which are designed to infuse 0.25 µl h−1 for 14 days (model 1002; Alzet, Palo Alto, CA, USA), were placed subcutaneously; animals received 6 µl of treatment per day. Using a brain infusion kit (Brain kit 3; Alzet), a stainless-steel cannula was inserted to a depth of 3.0mm from the surface of the skull into the third ventricle. Pumps were filled with either sterile saline, citalopram (20 µm; Sigma Aldrich, St Louis, MO, USA), BI-2A7 (2 µm, Sanford-Burnham Medical Research Institute, La Jolla, CA, USA), BI-11A7 (2 µm, Sanford-Burnham Medical Research Institute) or L447951 (N-(4-((4-chlorohenyl) thio)phenyl)-4-ethoxybenzamide; C21H18ClNO2S, MW= 383.9, 2 µm; Sigma Aldrich). Filled minipumps were weighed before surgery and at the end of the experiments to ensure appropriate delivery.
Forced swim test
Transparent Plexiglas cylinders, 50 cm high with a diameter of 20 cm, were filled with tap water at 22–25 °C approximately 25cm high so that mice were unable to touch the floor or otherwise escape, as described previously.45 Normal overhead fluorescent lighting of approximately 400 lx was used during the test. Individual mice were placed in the water for a 6-min session, and their behavior was videotaped using the CaptureStar video recording software (Clever Systems Inc., Reston, VA, USA) for later analysis. At the end of each session, mice were dried with a paper towel and returned to their home cage. Water was replaced after each trial (n=10 in each group). Immobility time, defined as a lack of activity except movements needed to keep the nose above water, was scored during the last four minutes of each session using the ForcedSwimScan software (Clever Systems Inc.).
Tail suspension test
The tail suspension test (TST) was conducted as described previously.46 Briefly, one end of a 15cm long segment of masking tape was wrapped around the mid-section of the animal’s tail two times and the other end was adhered to a cross bar approximately 10cm from the floor. The mice were then suspended by the tail and videotaped for 6 min using the CaptureStar video recording software (Clever Systems Inc.). The videos were later analyzed using TailSuspScan software (Clever Systems Inc.) and scored for struggling and immobility behaviors for the entire duration of the test. Normal overhead fluorescent lighting of approximately 400 lx was used during the test (n = 10 in each group).
Open field test
Because general locomotor activity can confound the results of forced swim test (FST) and TST, we conducted the open field test (OFT) to determine the effect of the different treatments on this measure. A 35 × 35cm2 square arena was used for the OFT, as described previously.47 To study spontaneous locomotion, mice in the four different treatment groups (saline, citalopram, BI-2A7 or BI-11A7) were placed in one corner of the arena and their behavior was recorded for 30 min with the CaptureStar video recording software (Clever System Inc.). Following the taping, videos were scored for total distance traveled using Topscan video tracking system (Clever Systems Inc.). Lighting during the test was set at approximately 30 lx. The open field arena was wiped between trials with a 10% alcohol solution (n = 10 in each group).
LH paradigm
The LH paradigm was conducted using the Gemini Avoidance System (San Diego Instruments, San Diego, CA, USA) as described previously.47 Generally, there are three stages to the LH paradigm. First, the induction profile: 120 inescapable shocks (0.45 mA, 15-s duration, at random intervals with an average of 45 s); second, the screening profile: 30 trials (0.45 mA; 3-s duration for conditioned stimulus and 3-s duration for unconditioned stimulus, conducted at random intervals with a mean of 45 s); lastly, the active avoidance test: 30 trials (0.3 mA; 3-s duration for conditioned stimulus and 24-s for unconditioned stimulus, conducted at random intervals with a mean of 45 s). The number of escape failures and latency to escape were recorded for each mouse (n = 7–13 in each group). Mice were said to have achieved helplessness when they showed at least 20 failures to escape.47
Female urine sniffing test
The female urine sniffing test (FUST), a newly developed, non-operant test for measuring reward-seeking behavior in rodents based on interest in sniffing pheromonal odors from the opposite sex, was conducted as described previously.48 The predictive and face validity for measuring depressive-like symptoms in rodents using the FUST has been established.48,49 Rodents that have developed helplessness, as well as stressed mice, were found to spend significantly less time sniffing female urine compared to mice that did not undergo the LH paradigm or stress manipulation; citalopram treatment alleviated this attenuated time spent sniffing in these animals.
Briefly, 1 h before the test, mice from three different treatment groups (saline, citalopram, or BI-11A7) that had undergone the LH paradigm (and were classified as having achieved helplessness in the screening phase) were habituated to a sterile cotton-tipped applicator inserted into their home cage. For the test, animals were transferred into a dark room (~3 lx lighting). The test had three phases: (1) one exposure (3 min) to the cotton tip dipped in sterile water, during which sniffing duration of the applicator was measured; (2) an interval of 45 min during which no applicator was presented to the animal; and (3) one exposure (3 min) to a cotton tip infused with fresh urine collected from female mice of the same strain in estrus, during which time spent sniffing was measured (n = 12–13 in each group).
Sequence of behavioral paradigms
We evaluated the effects of Bid antagonists in depression-related behavioral paradigms using four animal cohorts in the following order:
First cohort
At 12 days after the surgery for minipump implantation (Day 12), the OFT was conducted. On Day 13, the TST was conducted; on Day 14, the FST was conducted.
Second cohort
Two days before minipump implantation, the induction phase of the LH paradigm was conducted. One day before surgery (24 h later), mice were screened for LH behavior; only mice that had developed helplessness in the screening phase were chosen for surgery. At 12 days after surgery, animals were again screened for LH behavior in the active avoidance test.
Third cohort
Two days before minipump implantation, the induction phase of the LH paradigm was conducted. One day before surgery (24 h later), mice were screened for LH behavior; only mice that had developed helplessness (in the screening phase) were chosen for surgery. At 12 days after minipump implantation (Day 12), the FUST was conducted. On Day 13, animals were killed and brain tissue was rapidly dissected and snap frozen for protein expression work.
Fourth cohort
For experiments measuring protein expression following LH screening, mice were inducted and screened using the same parameters described above. Control animals, however, did not undergo the induction phase; control animals were placed into LH chambers for an equal amount of time. Both inducted and control mice were screened for LH 24 h later using the parameters outlined above. One day after screening, all LH and control (non-LH) animals were killed and brain tissue was rapidly collected and snap frozen in methylbutane.
Immunoblot detection of Bid-interacting proteins
Mitochondrial and cytosolic fractions were isolated from freshly dissected PFC tissue as described above. Crude nuclear pellets were lysed in radioimmuno-precipitation assay buffer containing 150 mm NaCl, 1.0% Triton X-100 (Sigma Aldrich), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and 50 mm Tris (pH 8.0) to extract nuclear proteins. Protein concentrations were determined using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). To obtain semiquantitative results, immunoblotting was performed using protein concentrations demonstrated to be within the linear range for analysis. Equal amounts of protein were loaded onto 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis criterion gels (Bio-Rad, Hercules, CA), separated by electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes using the iBlot transfer system (Invitrogen, Carlsbad, CA, USA).
After the transfer, polyvinylidene difluoride membranes were washed in Tris-buffered saline Tween-20 and blocked in Tris-buffered saline Tween-20 containing 5% milk. Membranes containing mitochondrial samples were cut and immunoblotted with the primary antibodies against tBid (1:1000, no. AB10002; Millipore Waltham, MA, USA), AIF (1:1000, #04-430; Millipore) and SMAC (1:1000, #AB3609; Millipore) in Tris-buffered saline blocking buffer. Membranes were stripped using Re-Blot Plus (Millipore) and probed with anti-porin antibody (1:3500, #ab15895; Abcam, Cambridge, MA, USA) as a loading control. Cytosolic samples were probed with Bid (1:1000, #2003; Cell Signaling, Danvers, MA, USA), Bcl-2 (1:1000, #2870; Cell Signalling), pro/active caspase-9 (1:1000, #9504; Cell Signaling Technologies) and reprobed for β-actin (1:1000, #4967; Cell Signaling Technologies) as a loading control. Nuclear samples were probed for AIF and subsequently reprobed for β-actin (loading control). Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G was used as a secondary antibody (1:2000; GE Healthcare, Piscataway, NJ, USA), followed by visualization using ECL Plus enhanced chemiluminescent signal detection (GE Healthcare) and exposure to Kodak Biolight film (Rochester, NY, USA). Proteins of interest were normalized to loading controls and subsequently analyzed by densitometric film analysis using the AlphaImager software (Alpha Innotech, San Leandro, CA, USA).
Cytochrome c enzyme-linked immunosorbent assay
Cytosolic, mitochondrial, and nuclear proteins were isolated from freshly dissected PFC tissue using the Qproteome Mitochondria Isolation Kit (Qiagen USA, Valencia, CA, USA). The manufacturer’s instructions were followed to isolate and purify the cytosolic and mitochondrial fractions. Protein concentrations were determined using BCA assay (Thermo Fisher Scientific, Waltham, MA, USA) and samples were diluted to a concentration that was within the standard curve’s linear range. Cytosolic cytochrome c was measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Data analysis
Statistical analysis was performed using Prism Version 4 (GraphPad Software, La Jolla, CA, USA). Statistical analyses used either one-way analysis of variance (ANOVA), repeated measures for two-way ANOVA, or independent t-tests. Scheffe post hoc tests were used to compare significant ANOVA results. Data are reported as mean±s.e.m. Significance was evaluated at P < 0.05, two-tailed.
Results
Effect of Bid inhibitors and citalopram on immobility tests
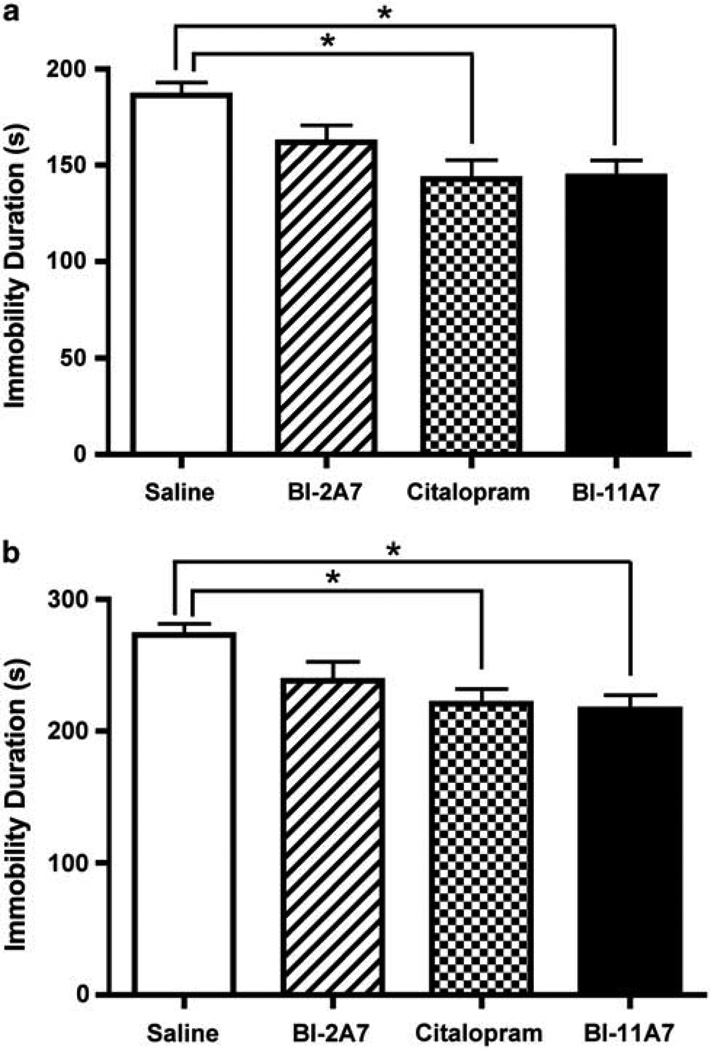
BI-11A7 and BI-2A7 are chemical inhibitors of Bid that were generated using a nuclear magnetic resonance (NMR)-based chemical fragment linking strategy. BI-11A7 has approximately fivefold more binding potential to purified Bid protein in vitro than BI-2A7.41,42 In the FST (Figure 1a), duration of immobility was significantly affected by treatment type (one-way ANOVA; F(3, 36) = 5.606; P < 0.01). Although no significant differences were noted between mice receiving saline and those treated with BI-2A7, mice treated with either citalopram or BI-11A7 were immobile for significantly less time than mice treated with saline (Scheffe post hoc tests; P < 0.05).
Figure 1.
Immobility tests—chronic treatment. Male 129S1/SVImJ mice were chronically infused with saline, BI-2A7, citalopram, or BI-11A7. The behavioral effects of the treatments were evaluated using the forced swim test (FST) (a) and the tail suspension test (TST) (b). Significant differences were detected between treatment groups. Data are mean±s.e.m. *P < 0.05.
In the TST (Figure 1b), treatment had a significant overall effect on immobility (one-way ANOVA, F(3, 35) = 5.06; P < 0.01). As in the FST, no significant differences were noted between mice receiving saline and those treated with BI-2A7. In contrast, mice treated with citalopram or BI-11A7 were immobile for significantly less time than mice treated with saline (Scheffe post hoc tests; P < 0.05).
Effect of Bid inhibitors and citalopram on the OFT
We measured open field activity to evaluate any changes in motor activity following chronic treatment with either citalopram or Bid inhibitors. None of the treatment groups (saline, citalopram, BI-11A7, or BI-2A7) differed on measures of locomotor activity as assessed by total distance traveled (in centimeters) in the OFT (one-way ANOVA, F(3, 35) = 0.68; P = 0.57; data not shown).
Effect of Bid inhibitors and citalopram on the LH paradigm
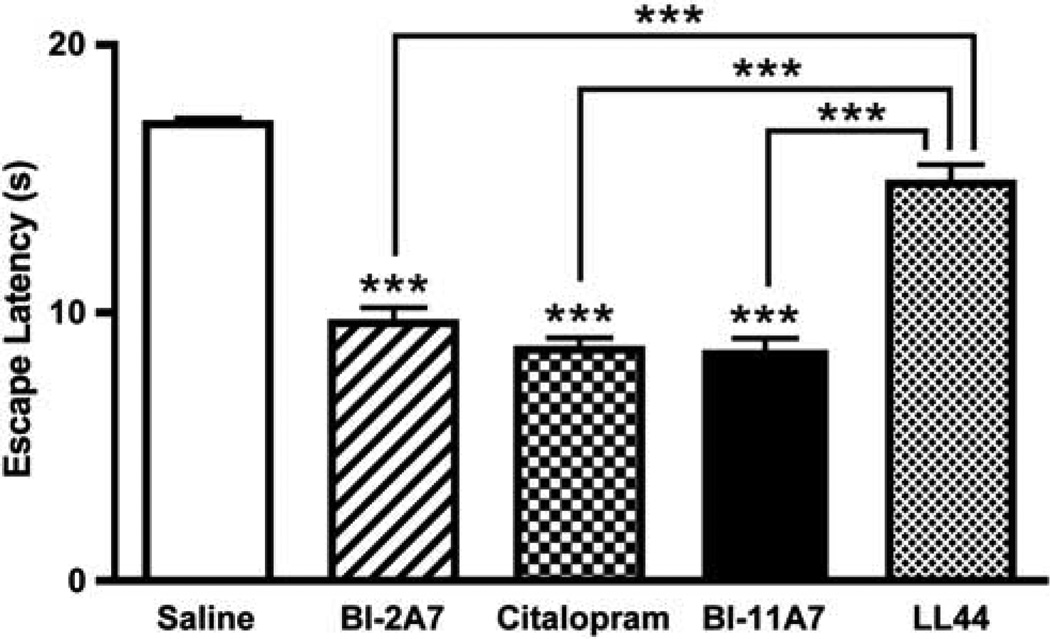
A cohort of mice underwent helplessness induction in the LH paradigm. After induction, mice were screened for LH, as determined by their failure to escape in at least 20 of 30 trials on the day of screening. Mice that had developed helplessness then underwent inhibitor infusion surgery, and treatment effects were examined 12 days after surgery. In the active avoidance test portion of the LH paradigm, neither the number of trials nor the interaction between the trials and the treatments affected overall outcome; however, treatment had a significant effect on escape latency duration (Figure 2) (two-way ANOVA treatment: F(4, 1200) = 31.72; P < 0.01; trials: F(29, 1200) = 0.59; P = 0.96; interaction: F(116, 200) = 0.21; P = 1.00). Scheffe post hoc tests found no significant differences between mice receiving saline and those treated with LL447951 (P = 0.14), but mice treated with either citalopram, BI-2A7, or BI-11A7 exhibited significantly shorter escape latencies than mice treated with either saline (P < 0.0001)or LL447951 (P < 0.0001).
Figure 2.
Learned helplessness paradigm—chronic treatment. Male 129S1/SVImJ mice underwent the learned helplessness (LH) paradigm. Mice that developed helplessness were then chronically infused with saline, BI-2A7, citalopram, BI-11A7, or LL44795. Significant effects were noted in escape latencies between treatment groups (BI-2A7, citalopram, BI-11A7) and saline (asterisks alone), as well as treatment groups vs the control compound LL44795 (bars with asterisks). Data are mean±s.e.m. ***P < 0.0001.
Effect of BI-11A7 and citalopram on the FUST
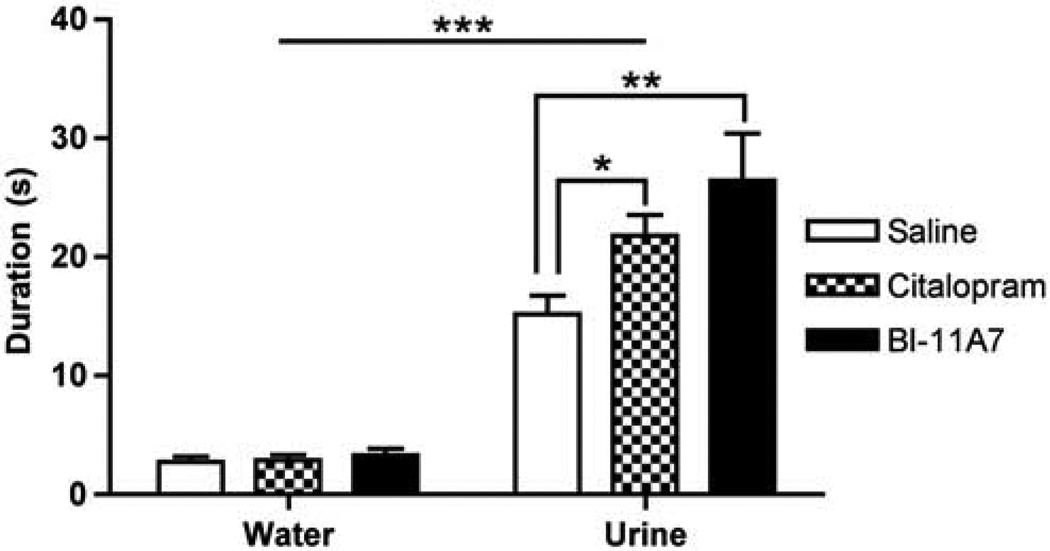
We used the FUST to assess the effects of citalopram and the most potent Bid inhibitor (BI-11A7) on reward-seeking activity in mice that had developed helplessness in the LH paradigm (Figure 3). As expected, male mice from all groups spent more time sniffing urine than water (repeated measures two-way ANOVA for odor: water vs urine: F(1, 19) = 144.3; P < 0.0001), suggesting that sniffing female urine is a preferred activity. Furthermore, significant differences were noted in sniffing duration between the mice in the different treatment groups. LH mice treated with BI-11A7 or citalopram spent more time sniffing the cotton tip applicator dipped in female urine than mice that had developed helplessness that were treated with saline (repeated measures two-way ANOVA for treatment: F(2, 19) = 4.702; P = 0.0219). The interaction (repeated measures two-way ANOVA; F(2, 19) = 4.066; P = 0.0399) revealed that while treatment type had no significant effect on sniffing duration for the water-dipped applicator, mice treated with BI-11A7 (t = 4.119, P < 0.01) or citalopram (t = 2.499, P < 0.05) spent significantly more time sniffing the applicator dipped in female urine than mice treated with saline.
Figure 3.
Mean sniffing duration in the female urine sniffing test (FUST). The FUST was used to assess the effects of treatment with saline, citalopram, or BI-11A7 on time spent sniffing water or female urine in male 129S1/SVImJ mice that had developed helplessness during the learned helplessness (LH) paradigm. Mice of all groups spent more time sniffing urine than water. In mice that had developed helplessness, treatment with BI-11A7 or citalopram resulted in significantly more time spent sniffing urine than saline. Data are mean±s.e.m. *P < 0.05; **P < 0.01; ***P < 0.0001.
Effect of treatments on levels of Bid-related proteins
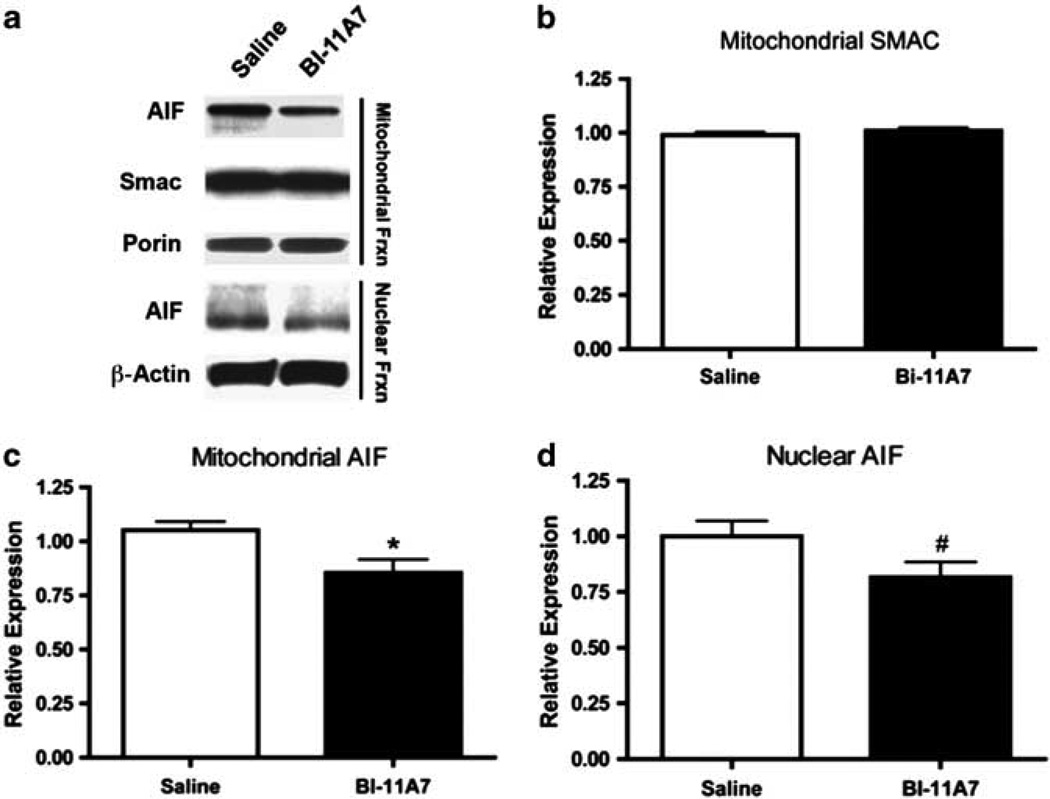
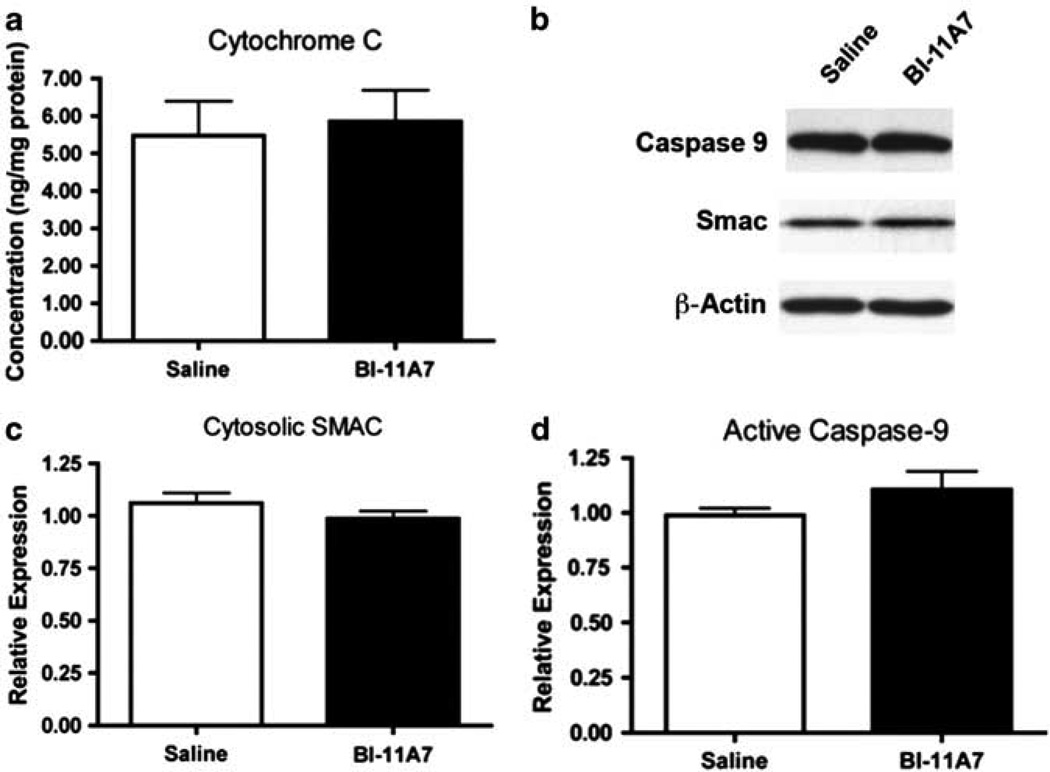
Next, we examined whether chronic inhibition of Bid would affect apoptotic signaling at the molecular level. Immunoblotting was also used to evaluate apoptosis-related proteins found in the mitochondrial and nuclear fractions (Figure 4a). tBid expression was not detected in the mitochondria (data not shown). Levels of mitochondrial SMAC were not significantly altered in any of the treatment groups (t(10) = 1.05; P = 0.31; Figure 4b). Mitochondrial levels of AIF, however, were significantly lower in samples from mice treated with BI-11A7 compared to the saline-treated mice (t(18) = 2.68; P < 0.05; Figure 4c). A trend toward lower AIF expression in the nuclear fraction of mice receiving BI-11A7 was also observed (t(18) = 1.887; P = 0.0754; Figure 4d).
Figure 4.
Prefrontal cortex (PFC) mitochondrial and nuclear fractions of BI-11A7- and saline-treated mice. Male 129S1/SVImJ mice were treated with saline or BI-11A7 for 14 days before PFC mitochondrial and nuclear fractions were isolated. Immunoblotting (a) was used to evaluate expression of mitochondrial levels of SMAC (b) and apoptosis-inducing factor (AIF) (c). Expression of nuclear AIF (d) was also examined. Data are mean±s.e.m. *P < 0.05; #P < 0.10.
In the PFC cytosolic fraction, levels of cytochrome c measured using enzyme-linked immunosorbent assay did not differ among the treatment groups (t(18) = 0.30; P = 0.77; Figure 5a). Immunoblotting was used to measure cytosolic proteins related to Bid-mediated signaling (Figure 5b). No significant differences were found between saline- and BI-11A7-treated mice in cytosolic levels of Bid (data not shown), Bcl-2 (data not shown), SMAC (t(12) = 1.32; P = 0.23; Figure 5c), pro-caspase-9 (data not shown), or active caspase-9 (t(10) = 1.26; P = 0.24; Figure 5d).
Figure 5.
Prefrontal cortex (PFC) cytosolic fraction of BI-11A7- and saline-treated mice. Male 129S1/SVImJ mice were treated with saline or BI-11A7 for 14 days. Prefrontal cortical levels of cytochrome c were measured in the cytosol (a) using enzyme-linked immunosorbent assay (ELISA). Immunoblotting of cytosolic proteins (b) was used to evaluate SMAC release (c) and caspase-9 activation (d). Data are mean±s.e.m.
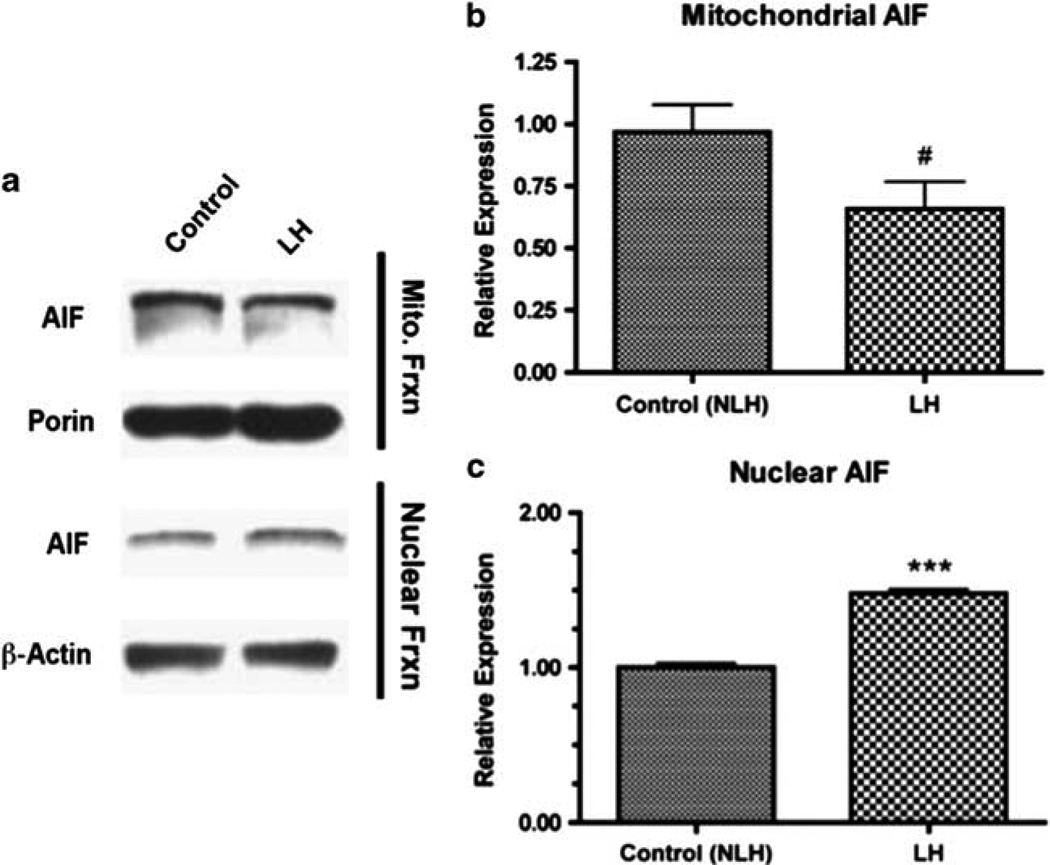
AIF expression in the LH paradigm
Lastly, we used immunoblotting to examine how LH induction affected AIF expression (Figure 6a). In the mitochondrial fraction, a trend toward reduced expression of AIF in LH vs control mice was noted (t(14) = 2.009, P = 0.0675; Figure 6b). In the nuclear fraction, AIF expression was significantly increased in LH mice (t(14) = 13.16, P < 0.0001; Figure 6b).
Figure 6.
Protein expression changes following learned helplessness (LH) induction. Immunoblotting (a) was used to evaluate changes in protein expression following learned helplessness (LH) induction and screening. Apoptosis-inducing factor (AIF) levels in the mitochondria (b) and in the nucleus (c) were measured. Data are mean±s.e.m. ***P < 0.001; #P < 0.10.
Discussion
This study examined the targeting of Bid, a proapoptotic member of the Bcl-2 family, as a novel strategy for antidepressant development. We studied Bid inhibitors in several well-known rodent models of depression, as well as the recently developed FUST.48 We found that the Bid inhibitor BI-11A7 significantly reduced immobility time in the FST and TST, but did not affect locomotor activity, attenuated escape latencies in the LH paradigm, and significantly improved the duration of female urine sniffing in the FUST. Taken together, our results suggest that pharmacologically targeting Bcl-2 pro-apoptotic family proteins is a viable strategy for developing mechanistically novel antidepressants.
Both the FST and the TST (for a review see Cryan and Mombereau50 and Cryan et al.51) are based on the same principle—animals under acute stress in an inescapable condition (for instance, a cylinder filled with water in the FST or suspended by their tail in the TST) will eventually become immobile.51 Both of these tests have been used to examine a wide variety of clinically effective antidepressant drugs52 as well as novel and unique compounds with potentially antidepressant-like activity, regardless of whether the agents are administrated acutely or chronically.53,54 However, these tests have some substantial and important differences: (a) in contrast to the FST, which causes a hypothermic response, the TST involves a hyperthermic response;55 (b) the FST involves swimming ability, which differs between rodent strains56 or during certain developmental phases;57,58 (c) in contrast to the FST, in the TST rodents can become and remain immobile earlier in the paradigm;51 and (d) different effects can be exhibited in one test but not the other; for instance, either between compounds or between different strains of transgenic mice.59,60 Therefore, the use of both of these paradigms is both appropriate and relevant when assessing compounds with potential antidepressant activity.
In this study, mice treated with citalopram or BI-11A7 (which binds with greater affinity than BI-2A7) remained immobile for significantly less time than mice treated with saline in both the TST and the FST. We also conducted the OFT to explore the effect of Bid antagonists and citalopram on general locomotor activity. Changes in motor function can confound the results of both the FST and TST; however, neither treatment (citalopram or BI-11A7) significantly affected general locomotor activity.
Rodents were also studied using the LH paradigm,51,61 which is based on the principle that after a series of exposures to uncontrollable and unpredictable electroshock stressors, 50–80% of rodents develop an escape coping deficit, termed ‘helplessness’.61,62 This coping deficit can persist for weeks, but can be reduced in rodents by chronic treatment with antidepressants;63 selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, monoamine oxidase inhibitors and tricyclic antidepressants, as well as ketamine,47 have all been shown to reverse and prevent LH in rodents.64
In the LH paradigm, treatment with citalopram and both Bid inhibitors (BI-11A7 or BI-2A7) significantly reduced escape latencies compared with those mice treated with either saline or LL44795. As noted above, BI-11A7 has approximately fivefold more binding potential to purified Bid protein in vitro than BI-2A7;41,42 nevertheless, BI-2A7 is still an active compound producing significant chemical effects in cell culture.41 Therefore, the fact that BI-2A7 significantly affected certain behaviors is not surprising. LL44795 is an inert compound similar in size to BI-11A7, and LL44795-infused mice were included as a second negative control group in the LH cohort. Taken together, these data demonstrate the antidepressant-like effects of Bid inhibitors as assessed by well-known and well-validated animal paradigms of depression.
Anhedonia is a core symptom of depression65 that is distinct from the despair-related measures often evaluated using the FST and TST.12,66,67 We thus decided to use the FUST to assess reward-seeking activity in these mice. The FUST is based on the observation that female urine sniffing by male mice is a preferred activity, resulting in ultrasonic vocalizations, and accompanied by elevated dopamine levels in the nucleus accumbens.48 Previous studies from our laboratory found that mice that had developed helplessness during the LH paradigm spent less time sniffing female urine than those that had not developed helplessness.48 In this study, LH mice treated with BI-11A7 or citalopram spent significantly more time sniffing female urine than those treated with saline, suggesting that these treatments reverse some of the pleasure-seeking deficits typically observed in LH mice.
In an attempt to investigate the potential mechanisms underlying the behavioral effects of the BI-11A7 Bid inhibitor, we also monitored levels of apoptosis-signaling constituents related to Bid function in various subcellular compartments. We evaluated both caspase-dependent and caspase-independent signaling pathways downstream of Bid, and measured proteins—such as cytochrome c, SMAC and AIF—that are commonly released when mitochondrial membrane integrity is jeopardized, a hallmark feature of apoptosis. Bid and Bcl-2 expression were unaltered, and no significant change was observed in caspase-9 (both the pro-isoform and active isoform) expression, which is a direct downstream target of Bid. In addition, no significant difference was observed in cytosolic SMAC or cytochrome c expression; both are released from the mitochondria and are downstream of tBid. These findings suggest that chronic infusion with BI-11A7 in animals exposed to the stress associated with the FST, TST, and LH paradigm do not exhibit significant alterations in prefrontal cortical levels of caspase-dependent apoptosis signaling, nor are there any significant global disruptions in mitochondrial membrane permeability.
Interestingly, we did find that mice infused with BI-11A7 had significantly lower AIF levels in the mitochondrial fraction than saline-treated mice. AIF is a mitochondrial protein that translocates to the nucleus in response to specific death signals—that is, oxygen or glucose deprivation—and precedes morphological signs of cell death such as DNA fragmentation and nuclear pyknosis.40,68 AIF is part of a caspase-independent apoptotic signaling cascade,40,69 is known to be downstream of Bid,70 and was previously implicated as having altered activity following BI-11A7 treatment in vitro.41 Although lower mitochondrial expression of AIF could suggest increased accumulation in the nucleus, we instead found that BI-11A7-treated animals also had a trend toward reduced expression of nuclear AIF. These findings suggest that chronic blockade of Bid translocation reduces overall mitochondrial AIF expression, thereby limiting the amount available for nuclear translocation. We further demonstrated that mice undergoing LH induction had significantly elevated expression of nuclear AIF (with a concomitant trend toward decreased levels of mitochondrial AIF) compared with mice that only underwent the active avoidance screening component of the paradigm; this implies that elevated nuclear AIF levels may be involved in depression. It thus appears that the therapeutic response underlying chronic BI-11A7 treatment is mediated by reducing the expression of basal levels of caspase-independent cell death signaling cascade members like AIF. More work is needed to clarify whether the acute stress paradigms used in this study induce cell death, disrupt neuroplasticity, or promote dendritic remodeling in PFC circuits that can be blocked via inhibition of caspase-independent pathways.
In summary, this study obtained a comprehensive set of data demonstrating that chemical inhibition of Bid produced antidepressant-like effects in the FST, TST, and LH paradigm and alleviated the loss of pleasure-seeking deficits associated with helplessness in the FUST. These data support the hypothesis and recent experimental studies showing that chemically targeting Bcl-2 family proteins may be a novel strategy for developing mechanistically unique antidepressants.3,71,72 It remains to be seen whether Bid antagonists are suitable for long-term systemic use and whether Bid inhibition or enhancement of Bcl-2 function can alleviate depressive symptoms in human beings. Given the ubiquitous nature of the Bcl-2 family’s expression, it will be important to consider the potential side effects of targeting apoptosis signaling in other areas of the body beyond the brain. More preclinical work is also essential to gain a richer understanding of the complex interactions that exist within these dynamic and elaborate apoptotic signaling cascades and their potential side effects. Although these studies are still in their earliest stages, the data presented here are an important addition to the important research question of whether Bcl-2 family protein dysfunction contributes to impairments of neural plasticity and the mechanisms underlying mood vulnerability, as well as the extent to which targeting these proteins (instead of, for instance, neurotransmitter release) for novel drug development may ultimately result in mechanistically unique and effective therapeutics.
Acknowledgments
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Health (NIMH: OM, TT, DRA, IDH, GC and HKM) and NIH Grant (R01 HL082574) to JCR. Adithya Simha provided invaluable technical assistance.
Footnotes
Conflict of interest
There are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Drs Chen and Manji are now at Johnson & Johnson Pharmaceutical Research and Development; this work was initiated and largely undertaken while they were employees of the NIMH.
References
- 1.Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialog Clin Neurosci. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 3.Hunsberger J, Austin DR, Henter ID, Chen G. The neurotrophic and neuroprotective effects of psychotropic agents. Dialog Clin Neurosci. 2009;11:333–348. doi: 10.31887/DCNS.2009.11.3/jhunsberger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 5.Goldman LS, Nielsen NH, Champion HC. Awareness, diagnosis, and treatment of depression. J Gen Intern Med. 1999;14:569–580. doi: 10.1046/j.1525-1497.1999.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. [cited 1 October 2009];The Global Burden of Disease (GBD) 2004 Update. 2008 available from http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 8.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 9.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Hiroeh U, Appleby L, Mortensen PB, Dunn G. Death by homicide, suicide, and other unnatural causes in people with mental illness: a population-based study. Lancet. 2001;358:2110–2112. doi: 10.1016/S0140-6736(01)07216-6. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders. 2nd edn. New York: Oxford University Press; 2007. [Google Scholar]
- 12.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 14.Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, A Gray N, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 15.Hunsberger JG, Austin DR, Chen G, Manji HK. Cellular mechanisms underlying affective resiliency: the role of glucocorticoid receptor- and mitochondrially-mediated plasticity. Brain Res. 2009;1293:76–84. doi: 10.1016/j.brainres.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunsberger JG, Austin DR, Chen G, Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromol Med. 2009;11:173–182. doi: 10.1007/s12017-009-8070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed JC. Apoptosis mechanisms: implications for cancer drug discovery. Oncology (Williston Park) 2004;13(Suppl 10):11–20. [PubMed] [Google Scholar]
- 18.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 19.Hetz C, Glimcher L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol. 2008;18:38–44. doi: 10.1016/j.tcb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Ray R, Dubik D, Shi L, Cizeau J, Bleackley RC, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186:1975–1983. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan Pe, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan P, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 25.Jonas E. BCL-xL regulates synaptic plasticity. Mol Interv. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 27.Kosten TA, Galloway MP, Duman RS, Russell DS, D’Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology. 2007;33:1545–1558. doi: 10.1038/sj.npp.1301527. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, Peng CH, Yang YP, Wu CC, Hsu WM, Wang HJ, et al. Desipramine activated Bcl-2 expression and inhibited lipopolysaccharide-induced apoptosis in hippocampus-derived adult neural stem cells. J Pharmacol Sci. 2007;104:61–72. doi: 10.1254/jphs.fp0061255. [DOI] [PubMed] [Google Scholar]
- 29.Murray F, Hutson PH. Hippocampal Bcl-2 expression is selectively increased following chronic but not acute treatment with antidepressants, 5-HT(1A) or 5-HT(2C/2B) receptor antagonists. Eur J Pharmacol. 2007;13:41–47. doi: 10.1016/j.ejphar.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 31.Bravo JA, Diaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, et al. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol. 2009;20:273–285. doi: 10.1097/FBP.0b013e32832c70d9. [DOI] [PubMed] [Google Scholar]
- 32.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both maniclike and depression-like behavioral impairments. Proc Natl Acad Sci USA. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- 34.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 36.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 37.Zou H, Li Y, Liu X, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 38.Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, et al. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becattini B, Culmsee C, Leone M, Zhai D, Zhang X, Crowell KJ, et al. Structure-activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting Bid. Proc Natl Acad Sci USA. 2006;103:12602–12606. doi: 10.1073/pnas.0603460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC, et al. Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol. 2004;11:1107–1117. doi: 10.1016/j.chembiol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, et al. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–789. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- 44.Xu B, Pu S, Kalra PS, Hyde JF, Crowley WR, Kalra SP. An interactive physiological role of neuropeptide Y and galanin in pulsatile pituitary luteinizing hormone secretion. Endocrinology. 1996;137:5297–5302. doi: 10.1210/endo.137.12.8940349. [DOI] [PubMed] [Google Scholar]
- 45.Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainite receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 48.Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry. 2010;67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–245. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 51.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 53.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 54.Foreman MM, Hanania T, Stratton SC, Wilcox KS, White HS, Stables JP, et al. In vivo pharmacological effects of JZP-4, a novel anticonvulsant, in models for anticonvulsant, antimania and antidepressant activity. Pharmacol Biochem Behav. 2008;89:523–534. doi: 10.1016/j.pbb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res. 2003;37:249–259. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 56.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 57.Abel EL. Ontogeny of immobility and response to alarm substance in the forced swim test. Physiol Behav. 1993;54:713–716. doi: 10.1016/0031-9384(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 58.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 59.Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 60.Porsolt R, Lenegre A. Behavioral models of depresion. In: Elliot J, Heal D, Marsden C, editors. Experimental Approaches to Anxiety and Depression. London: Wiley and Sons; 1992. pp. 73–85. [Google Scholar]
- 61.Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Henn FA, Edwards E. Muneyyirci. Animal models of depression. Clin Neurosci. 1993;1:152–156. [Google Scholar]
- 63.Gambarana C, Scheggi S, Tagliamonte A, Tolu P, DeMontis MG. Animal models for the study of antidepressant activity. Brain Res Brain Res Protoc. 2001;7:11–20. doi: 10.1016/s1385-299x(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 64.Henkel V, Bussfeld P, Moller HJ, Hegerl U. Cognitive-behavioural theories of helplessness/hopelessness: valid models of depression? Eur Arch Psychiatry Clin Neurosci. 2002;252:240–249. doi: 10.1007/s00406-002-0389-y. [DOI] [PubMed] [Google Scholar]
- 65.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 66.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 68.Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:458–466. doi: 10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci. 2005;25:1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, et al. Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ. 2008;15:1553–1563. doi: 10.1038/cdd.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKernan DP, Dinan TG, Cryan JF. ‘Killing the Blues’: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–263. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Drzyzga LR, Marcinowska A, Obuchowicz E. Antiapoptotic and neurotrophic effects of antidepressants: a review of clinical and experimental studies. Brain Res Bull. 2009;79:248–257. doi: 10.1016/j.brainresbull.2009.03.009. [DOI] [PubMed] [Google Scholar]