Abstract
Objective
To better understand physicians' views on factors of influence for the prescribing of antibiotics and on antibiotic resistance in the Berlin region, Germany.
Design
Qualitative study with focus groups.
Setting
Outpatient care and hospital care practice in the Berlin region, Germany.
Participants
7 General practitioners, two urologists, one paediatrician from outpatient care and eight internists, two paediatricians, two ear, nose and throat specialists and two urologists from hospital care.
Results
Physicians showed differential interest in topics related to antibiotic prescribing and antibiotic resistance. Outpatient care physicians were interested in topics around their own prescribing, such as being able to diagnose and prescribe precisely, and topics about patient demand and non-compliance. Hospital care physicians were interested in hygiene challenges, limited consult time and multi-resistant pathogens.
Conclusions
Physicians considered the development of resistance to be more in the domain of clinical treatment than that of the patient. Major challenges related to antibiotic resistance for this group of physicians are access to and clarity of treatment recommendations, implementation of hygienic measures, as well as increased outsourcing of laboratory services. Results raise questions about whether meeting physicians' expectations should be a focus when developing intervention that aims to influence antibiotic resistance in this and other areas of Germany.
Article summary
Article focus
Overuse of antibiotics across many specialities and in some of the most common diagnoses remains a driving force for antibiotic resistance.
While much attention has focused on limiting use and addressing clinical concerns like improving point-of-care diagnostic tests, prior literature has largely left out the consideration of socio-behavioural factors that influence physicians' decisions to prescribe antibiotics.
Focus group discussions were used to show physicians' views on factors that influence their prescribing of antibiotics and antibiotic resistance.
Key messages
Berlin area physicians are interested in receiving help to make informed decisions on the appropriate measures for mitigating patient discomfort and risk.
In this group, well-informed prescribing practice appears to be influenced by non-patient-oriented factors that are both structural (eg, overcrowding in hospitals) as well as non-structural in nature (eg, access to feedback from microbiologists or time allowed for patient consult).
Physicians desire intervention activities that address their own skills, like assessment of patient needs, time management for consult and navigation of pharmaceutical consulting.
Strengths and limitations of this study
Modern methodologies for focus group data analysis, including a comprehensive plan for ensuring validity in data-making and data reduction were used in the study.
Presented study methodology allows replication by other research groups.
The number of participating physicians was limited; however, they were recruited from diverse backgrounds with respect to age, sex, size of practice, care setting and number of years in practice.
Introduction
Antimicrobial use has remained a major concern in medicine and epidemiology over the last years. Surveillance initiatives have been implemented in order to monitor antimicrobial consumption and usage patterns and resistance data for selected pathogens in order to present trends over time and comparisons between countries and regions.1 2 The results provide evidence that antimicrobial resistance has continued to persist across all specialities and in some of the most common diagnoses. Efforts to combat resistance have focused on limiting antimicrobial use, providing patient education about appropriate use and developing better point-of-care tests. There are also other socio-behavioural factors of antibiotic use and resistance, which should also be a core part of campaigns that attempt to monitor resistance in both hospital and outpatient care settings.3–6
In 2007, the Robert Koch Institute, the federal public health institution in Germany, initiated a number of different studies to investigate factors to be considered when designing a national strategy to prevent the spread of antimicrobial resistance. The aim was to use different methodological approaches to describe factors of influence for antibiotic prescribing and antibiotic resistance in Germany. As a preliminary study, a literature review was conducted to identify previous work on factors of influence for antimicrobial prescribing and to guide further research. The aim of this study using focus groups was to elicit physicians' views on factors that influence their prescribing of antibiotics and antibiotic resistance. As a mixed-methods research approach can help to explore research findings in greater detail,7 8 a further aim was to generate exploratory information as the basis to develop a nationally representative cross-sectional survey on the same topic, conducted in 2008.9
Methods
Focus group conceptual structure
A conceptual structure was created to serve as the basis for the focus group discussions. Five conceptual areas encompassed influence factors for the following: (1) general impressions of antibiotic resistance (eg, How is the development of antibiotic-resistance perceived? How generally relevant is the topic of rising antibiotic resistance?), (2) prescribing in outpatient care (eg, Which influence factors are relevant for prescribing antibiotics? Which factors are relevant for prescribing in outpatient care?), (3) Prescribing in hospital care (eg, Which influence factors are relevant for prescribing antibiotics? Which factors are relevant for prescribing in hospital care?), (4) Information and knowledge about antibiotic treatment (eg, what are sources of knowledge about antibiotics? How are physicians generally informed about medical areas related to antibiotics?) and (5) Impressions on problematic areas of concern (eg, How are problem areas in antibiotics and antibiotic resistance addressed? Which factors should be addressed by potential interventions to combat antibiotic resistance?).
Focus group participants
We recruited physicians from the Berlin region, Germany, with diverse backgrounds with respect to age, sex, specialty, practice type, the number of patients seen quarterly and location of practice. Physicians were offered monetary compensation of €200. We conducted four focus group sessions of five to seven physicians each: (1) outpatient setting, less experience; (2) outpatient setting, more experience and (3) hospital setting, less experience; (4) hospital setting, more experience (tables 1 and 2). A qualitative research agency drew the sample of physicians, and moderated and transcribed all focus group discussion sessions.10
Table 1.
Focus group participant details: outpatient care
| Focus group | Participant ID | Sex | Age | Specialty | Practice type | Years in practice | Location | Patients per quarter |
| 1 | 1 | Female | 46 | Paediatrics | Group | 12 | East | ∼900 |
| 2 | Female | 35 | General practitioner | Group | 5 | West | ∼200 | |
| 3 | Male | 48 | General practitioner | Single | 9 | East | ∼1000 | |
| 4 | Male | 54 | Urology | Single | 11 | West | ∼1200 | |
| 5 | Male | 40 | General practitioner | Group | 10 | West | ∼800 | |
| 2 | 1 | Male | 62 | General practitioner | Group | 25 | West | ∼2000 |
| 2 | Female | 53 | Urology | Group | 15 | West | ∼800–900 | |
| 3 | Female | 55 | General practitioner | Group | 16 | East | ∼150 | |
| 4 | Female | 42 | General practitioner | Group | 15 | East | ∼180 | |
| 5 | Male | 57 | General practitioner | Single | 15 | East | ∼800–900 |
Table 2.
Focus group participant details: hospital care
| Focus group | Participant ID | Sex | Age | Specialty/position | Beds (n) | Years in practice | Location | Patients per quarter |
| 3 | 1 | Female | 40 | Paediatrics/consultant | 1200 | 8 | West | ∼600–700 |
| 2 | Male | 34 | Internal/resident | 620 | 5 | West | ∼400 | |
| 3 | Male | 43 | Internal/consultant | 538 | 9 | East | ∼500 | |
| 4 | Male | 42 | Internal/resident | 626 | 4 | West | ∼300–400 | |
| 5 | Female | 34 | Internal/resident | 363 | 3.5 | West | ∼400 | |
| 6 | Male | 30 | ENT/resident | 1200 | 3 | East | ∼350 | |
| 7 | Male | 43 | Urology/consultant | 220 | 12 | West | ∼500 | |
| 4 | 1 | Male | 51 | Internal/consultant | 538 | 16 | West | ∼500 |
| 2 | Female | 40 | Internal/consultant | 1200 | 14 | East | ∼1000 | |
| 3 | Male | 56 | Internal/consultant | 276 | 31 | West | ∼500 | |
| 4 | Male | 48 | ENT/consultant | 1000 | 10 | West | ∼1400 | |
| 5 | Male | 41 | Internal/consultant | 1200 | 10 | West | ∼1000 | |
| 6 | Male | 44 | Paediatrics/consultant | 542 | 16 | West | ∼300–500 | |
| 7 | Female | 63 | Urology/consultant | 1200 | 37 | East | ∼4000 |
Interview methodology
The focus groups were held between 4 and 6 December 2007 in Berlin and were facilitated in four sessions of 2 h each. All sessions were held separately and conducted by a trained moderator. Moderators used a semistructured framework, a method which has been found to enable participants to share and confirm their views, or construct new views based on interactions in a peer context, and build knowledge together.11 For each discussion, the framework was based on the topics from the five conceptual areas but allowed participants in each group to explore topics differentially. Interviews were transcribed in real time, and each session was video recorded for later in-depth review. To check for accuracy of the text in each transcription, six random samples of 5–7 min were chosen from the video footage of each focus group and then checked against the corresponding text. Video footage was also later reviewed in greater detail in order to explore group dynamics.
Data analysis
A semiquantitative approach was used to analyse the results of the focus group discussions. This first consisted of examining the data based on the five conceptual areas and the respective study questions. We were able to draw key relationships between conceptual areas, so called ‘code-categories’ under which were assigned individual topics arising from the content of the focus group discussions. The resulting framework was used to guide all subsequent data-making and analysis tasks.
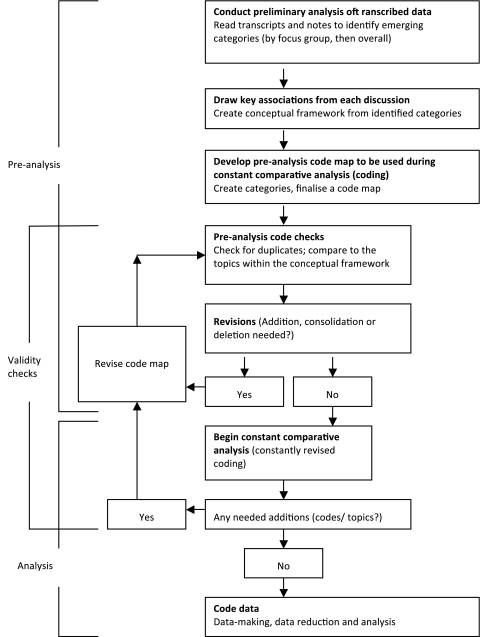
A preanalysis code map was developed from the framework, showing a hierarchy from the five code categories to each topic and subtopic. (online supplementary data table) The code map was then created, to be used later for constant comparative analysis—an iterative method of content analysis where each category is searched and constantly revised, popularly used to allow so called ‘emergent codes’ to be applied at all points in the analysis.12 13 Before beginning the analysis, we validated our code map by performing a code check, looking for duplicates and comparing codes to the topics within the aforementioned framework. Revisions were made and a resulting code map was used for subsequent data-making (figure 1).
Figure 1.
Plan for data-making, data reduction and analysis.
All text from transcripts was subjected to constant comparative analysis, and the frequencies of codes were used as a measure of significance. All data-making and content analyses were done using TAMS Analyser for Macintosh OS X (version 4.13), an open-source computer-assisted qualitative research tool.14
We extracted quotes from all transcripts when a specific topic involved multiple sentences, when the comment provided was observed to be provocative and/or when it generated lively discussion among more than two individuals. We extracted relevant quotes from each focus group interview in order to further establish an in-depth look at each topic. An epidemiologist who is fluent in German and a native English speaker completed German–English translations. We assigned each participant a quote identifier based on the focus group in which they belonged and their demographic information (shown in tables 1 and 2). The identifier is presented in the Results section as a two numbers (focus group number—ID number).
Results
Table 3 provides a detailed overview of the highest incident emergent codes and code categories from constant comparison analysis for all focus groups combined. Emergent codes served as a way to begin further critical analysis of the main insights reflected in this group of physicians, which we present in the following segments stratified by each focus group. Additional in-depth responses on several determinants of antibiotic prescribing and antibiotic resistance that cut across all focus groups, such as non-patient factors, hygiene, the pharmaceutical industry and antibiotic costs are also presented (table 4).
Table 3.
Top five highest incident emergent codes and categories from constant comparison analysis (all groups combined; total codes n=1035)
| Code-category | Five most frequent code topics | Frequency |
| General impressions on rising resistance | 401 | |
| Patient non-compliance | 15 | |
| Antibiotics development | 13 | |
| Hospital-specific issues, eg, hygiene, laboratories | 11 | |
| Antibiotic dosing | 10 | |
| Urinary tract infections | 10 | |
| Outpatient-specific influences on prescribing | 251 | |
| Patient history | 18 | |
| Patient demand | 18 | |
| Physician experience | 14 | |
| Patient self-education | 11 | |
| Patient compliance | 11 | |
| Sources of information on antibiotics | 234 | |
| Practice guidelines | 10 | |
| Continuing medical education | 8 | |
| Specialty journals | 8 | |
| Internet | 8 | |
| Quality of conferences | 7 | |
| Physician-oriented interventions | 84 | |
| Surveillance | 9 | |
| Laboratory feedback | 7 | |
| Information on local resistance situation | 7 | |
| Hospital | 4 | |
| Hygiene | 4 | |
| Hospital-specific influences on prescribing | 65 | |
| Up-to-date internal guidelines | 4 | |
| Laboratory/microbiologists exchange | 4 | |
| Specificity of internal guidelines | 4 | |
| Experience with infectious diseases | 3 | |
| Problematic diagnoses | 3 |
Table 4.
Selected in-depth responses from focus group discussions
| Category | Quotes |
| Hygiene |
|
| Laboratory and resistance data |
|
| Pharmaceutical industry |
|
| Cost |
|
| |
| Other non-patient determinants |
|
Focus group 1: outpatient care physicians with fewer years of practice experience
Physicians focused on themes that are related to prescribing in the outpatient care setting (frequency: 146). Discussion focused on general impressions of rising resistance (115), sources of information on antibiotics (64) and physician-oriented interventions (17). They expressed concern about difficulties dealing with complicated patient histories (12), patient compliance (9) and patient perception of treatment (8). Participants frequently discussed the development of antibiotic substances (11) and about responsibility in their own practice (4). Participants also focused on specific diagnoses that are perceived to be driving resistance, with major discussion occurring around the topic of uncomplicated urinary tract infections (UTIs) (5). Cost was also discussed as a factor influencing antimicrobial prescribing, specifically, the effects of health regulations on the accessibility of medications.
Conferences (9) and pharmaceutical companies (4) were discussed most when it came to common sources of information on antibiotics. A large amount of time was spent discussing pharmaceutical representatives, whom participants found to be persistent and aggressive:
“They come often and always have antibiotics on hand. You get a bag of them every day. And high doses of drugs. It all stacks up in the cabinet. For me there are 4 to 5 representatives each day” (Participant 1–3: tables 1 and 2).
“I notice that they approach me, too. But I do not accept them all. I would estimate that there are about 5–7 every day, and they do bring whole bags full (of giveaways).” (1-2)
“The representatives come into my practice. And you do listen to them. You even take the information they offer, even if with a critical eye. But you do learn something as well.” (1-5)
“The pharmaceutical industry is very aggressive.” (1-3)
“For urology I cannot remember in recent months receiving a visit on this issue. But that is certainly very different than in the primary care sector.” (1-4)
There was no single participant dominating the discussion, and comments readily came from each; however, the paediatrician did mention that there is less pharmaceutical presence in her practice. This group most frequently saw feedback on their resistance situation and cooperation with laboratories (5) as ways to address the problem of rising antibiotic resistance.
Focus group 2: outpatient care physicians with more years of practice experience
Unlike the first outpatient group, this group veered away from a dominant focus on outpatient-specific topics and discussed most frequently those topics within the category of general impressions on rising resistance (150). The group was also concerned with having adequate sources of information on antibiotics (126), outpatient-specific influences on prescribing (105) and other physician-oriented interventions (28).
Physicians frequently discussed the effectiveness of antibiotic substances and drug development (6). As in the previous outpatient care group, cost was seen as a factor of influence on antimicrobial prescribing. In this group, participants agreed that they are less wary of the cost of antibiotics because the nature of predominantly short treatments makes it affordable compared with longer-term treatments, like those prescribed for high blood pressure. This group also talked about social factors that may be driving the situation, like increased foreign travel (6), over-the-counter availability of drugs abroad (4) and migration (4). The topic of UTIs arose as a specific concern driving resistance.
This group discussed the category of hospital-specific influences on prescribing (8), like multi-resistant pathogens (6). The topic of hospital hygiene arose in each of the two outpatient focus groups, which agreed that antibiotic resistance was largely a problem of the hospital setting, “In hospitals resistance plays a bigger role because there one finds hospital specific germs.” (1–4) Incidentally, the topic of resistance was often quickly averted when brought up, instead being commented as a problem specific to the hospital care setting:
“I think the development of resistance is more the domain of clinical treatment and not the patient.” (2-5)
“Exactly.” (2-3)
“Yeah, especially in intensive care.” (2-4)
Participants discussed most frequently that patient demand (11) is a major driver for prescribing in the outpatient setting, followed by doctor experience (9) and specific diagnoses (6). The role of the patient, including patient non-compliance and self-medication, also emerged. Physicians discussed two types of patients: those concerned with getting an antibiotic and those concerned with avoiding what they think is harmful:
“Pressure from patients is not insignificant…the worst are the mothers where the children are really very sick and the mothers say: I don't want any chemotherapy. The lymph nodes are thick with pus, almost hanging out, and then the mother says no, no antibiotics for us. That's bad.” (2-1)
Physicians in this group valued information that is concise and available to them in a way that complements their work without taking up too much time:
“Is there a new antibiotic? What is the resistance situation? Which organisms are being affected? What are the indications, what are the side effects? The interactions with other drugs? Are there alternatives? If this information could be given to us in a short and sweet way, then we would be happy. Something like this is not currently available to us.” (2-1)
Participants in focus group 2 found treatment guidelines (8), pharmaceutical-based materials (5) and conferences (4) to be main sources of information on antibiotics. This group found information from pharmaceuticals to be concise and readily available:
“There's been a big change from the expertise of representatives who come in. These are all clinicians and they do not give a bad impression at all. They bring me a lot of information although, of course, you have to make sense of it all. But I do admit that I feel as though I am getting good consulting. Because I don't have the time to do my own research nor to sit down on the Internet every evening. I am very grateful for the very specific information they offer me.” (2-2)
As evidenced above, most other comments about the pharmaceutical industry also remained positive in this group. There were comments that patient outreach is not needed in Germany (2), and this focused largely on the belief that the patient population is well informed and, if at all, opposed to antibiotics, sometimes opting for alternative therapies.
They discussed the need to have more access to surveillance of their local resistance situation: “I think we need what there was in (the former) East Germany, a short, independent information sheet that shows the current epidemiological situation in the country or the region where I live.” (2-5) The group seems to have agreed since they mostly discussed interest the following intervention options: increased surveillance (9), including information on their regional resistance situation (5), constraints on their patient consult time (3) and consulting (2).
Focus group 3: hospital physicians with fewer years of experience
Physicians most frequently discussed their general impressions on rising resistance (70), hospital-specific influences on prescribing (40), sources of information on antibiotics (15) and physician-oriented interventions (12). Patient non-compliance (8), correct prescribing and antibiotic dosing (5), hospital care (3) and hygiene (3) were the most frequently addressed topics.
The internet (3), pharmaceutical advertising (2) and conferences (2) were listed as the most frequent physician-oriented interventions mentioned by this group. The visibility of pharmaceutical advertising was also discussed, and this group found it easy to access and useful for learning. Participants were in agreement about how pharmaceutical advertising is more accessible than other traditional forms of information dissemination, such as medical journals.
Participants overwhelmingly stayed with the topic of hospital workplace concerns, like hygiene (7) and time for patient consult (4) as the most needed intervention to combat resistance in their setting. They discussed non-structural demands on the hospital, such as advances in treatment possibilities for more complex indications, which might necessitate more antibiotics consumption in the hospital setting, which may in turn itself be a driver for resistance.
The hospital itself was viewed as having structural aspects that might contribute to increased antibiotic use and resistance (7). One such aspect, maintaining hygiene, was a perceived danger of interrelated issues of increased patient load (3), patient–patient contact (1) and infectiousness (2). One physician noted that the pressure to treat more patients has led to a related need for a faster consult time, which may put strain on the thoroughness of hospital hygiene measures. Hospital physicians also pointed out that they would prefer to pursue intervention through new programmes for hygiene, although they also recognise it to be a challenging method of improvement. Participants also discussed the benefits of transparency and feedback on antibiotic consumption, costs and trends in the hospital setting.
Focus group 4: hospital physicians with more years of experience
Participants discussed most frequently about their general impressions on rising resistance (66), followed by hospital-specific influences on prescribing (29), sources of information on antibiotics (27) and physician-oriented interventions (21). The most frequent topics brought up by this group were diagnostics possibilities (5), patient history/epidemiology (increasingly acute cases in care) (4) and social factors like ageing (4). When talking about the influence on prescribing in hospital care, the following topics were most frequent: indication and disease (2), risk assessment in acute cases (2), specificity of guidelines (2) and time constraints during patient consultation (2). This group of physicians made relatively long commentaries at a higher level of detail than was observed in participants during the other focus group sessions. The group spoke at such detail about non-patient factors of antibiotic prescribing and antibiotic resistance, including patient stays in non-intensive wards of hospitals as increasing risk and minimised hygiene routines in hospital due to increased patient intake.
Physicians frequently consulted specialty journals (9), clinical handbooks (3) and the internet (3) as sources of information on antibiotics. Discussion points on hospital feedback on the resistance situation (5) and continuing education (2), especially in the area of hygiene (2) and infectious diseases (2) emerged most frequently in discussions regarding intervention for antibiotic resistance.
Collegial exchange with microbiologists/laboratories (5) emerged as the most frequent topic under the category of hospital-specific influences on prescribing, something that was also observed in focus group 3. Physicians in this group spoke about opportunities to closely collaborate with laboratories and microbiologists, which they saw as helpful in navigating antibiotic treatments:
The microbiologists that we have are top. We mostly get reports via the doctor calling us before anything is published on our intranet. It is then also discussed, what underlying disease does the patient have, which antibiotic was given, and the provisional findings will be communicated first. Short, quick ways; you have to communicate well with people. (4-1)
The topic of outsourcing of laboratories arose throughout this discussion. Physicians perceived this as prohibiting close communication and producing too much bureaucracy, “For us, it is unfortunately not the case. The laboratory has been outsourced. A service provider is at the other end of town; they can't communicate with us much.” (4-5) Other emerging themes were the role of the hospital pharmacist in influencing prescribing choices (4), followed by how often and appropriately internal/hospital antibiotic treatment guidelines are updated (4) and subsequently by multi-resistant pathogens (3).
Discussion
Past research has underlined the importance of patient-oriented factors of influence for prescribing, and the focus has primarily been on patient demand and non-compliance.15–17 This is consistent with the historical data on the subject showing that antibiotics are more likely to be prescribed when the patient expects them and that they may be even more likely to be prescribed when the doctor may perceive that the patient wants a prescription, when in fact the demands of patient are unclear.18 Responses from physicians in these groups indicated something different: an overwhelming interest in non-patient factors that influence antibiotic prescribing and resistance.
A major topic in both groups of participating physicians from outpatient care was their experience of increasingly difficult diagnoses that are complicated by resistance patterns. A good example is the increasing prevalence of antibiotic-resistant UTIs. Many participants are involved in the management of UTIs, a finding supported by the cross-sectional study component of this research (survey).9 Indeed, the trends in many European studies of antimicrobial resistance show UTIs to be accountable for a large amount of antibiotics consumption.1 Many of the common pathogens leading to UTIs, such as Escherichia coli, Proteus mirabilis and Klebsiella pneumoniae, are increasingly becoming resistant to standard treatments, which affects antibiotic treatment choices19 20; however, physicians showed differential interest topics related to their antibiotics prescribing and resistance, based on their care setting.
Outpatient care physicians found resistance primarily a problem of the hospital care setting, related to the presence of different multi-resistant pathogens and challenges with hygiene. This was also a major topic discussed by hospital physicians. The increasing prevalence of multiresistant pathogens is of particular concern, especially given the views that the hospital ward is increasingly faced with more patients at any single time and that patients—many of whom are carrying more complex indications—are also seen during shorter consult times.21 22 In fact, data from the survey identified that status as a hospital physician was a predictor for deciding to start antimicrobial therapy on a patient.9 This could be attributed to the fact that, generally, hospital physicians attend more acute cases than their outpatient care counterparts.
Hospital care physicians were accustomed to regular and easy collaboration with microbiologists when discussing indications and possibilities for therapy. This was also found in the study sample of the survey, which showed that hospital physicians found it either important or very important that they receive data on regional antimicrobial resistance and appropriate feedback for prescribing.9 This opinion was also shared in the focus group discussions among physicians, who want laboratories to provide feedback on the resistance situation for their hospitals. Participants expressed frustration and concern around outsourcing of laboratories. It was a matter of having less contact with helpful microbiologists and described a need: that even in a hospital setting with outsourced laboratory services, it is important to offer chances to dialogue with microbiologists. While this finding does seem to match the views shown by the national survey, more qualitative research on other groups could help to show whether or not there is a need to enhance access to their local resistance situation in the hospital setting in other areas of Germany.
There was differential discussion about treatment guidelines, which may also be an important influence factor on physician prescribing practice. Participants from the outpatient care setting found clinical recommendations to be difficult to access quickly and use. For the hospital setting, this was significantly different. There was more discussion about whether guidelines are up to date and about their relevance, specificity and availability in clinical practice. There are many guidelines with varying degrees of quality available to physicians. Hospital care physicians have an array of inhouse developed guidelines, differentially taking into account local resistance data.23 But, as also evidenced by other studies, availability is differential and may warrant addressing this separately for each practice setting.4 24
The pharmaceutical industry was often a major topic of discussion, but it remains unclear how large the current influence of the pharmaceutical industry is on physicians in Germany. Physicians indicated that the pharmaceutical industry plays a large role in outpatient care practice. Visits to doctors' offices by the industry and free samples of antibiotics are ubiquitous; their informational materials are generally perceived as attractive. This may have to do with the fact that information from the industry presents information in ways that are more convenient than scientific literature on the same topics.25 These important findings about the presence of the pharmaceutical industry also showed up among the participants of the survey: despite some caution about the persistence of the industry, most outpatient care physicians welcome their assistance and view them as another resource among many other sources of information on antibiotics. Results from these focus groups and the survey indicate that the pharmaceutical industry has a large presence among physicians in Germany.
Conclusions
Our findings suggest that physicians in Berlin are interested in topics around their own prescribing, like physician sensitivity to patient need, time management for patient consult, access to guidelines and their perception of the pharmaceutical industry. These non-patient determinants, when coupled with intervention ideas for the hospital care setting (eg, improving hygiene measures, easing diagnostics and cooperation with laboratories), are different from factors of antibiotic prescribing and resistance that have been previously observed in similar contexts: they are physician oriented. Furthermore, focus group discussions provided more details about some of the determinants that were also found relevant by physicians participating in the survey component of this research. Together, these study components raise questions about whether targeting other physicians may be a better approach for intervention that aims to influence antibiotic resistance in this and other areas of Germany. This could be a remarkable finding for Germany: in other countries, intervention to reduce antimicrobial resistance has often been targeted at the patient directly, but more qualitative research and similar focus groups in other areas of Germany could show whether or not this trend is nationally relevant.
Study limitations
Participants were all from the Berlin region and included physicians from diverse backgrounds with respect to age, sex, size of practice, care setting and number of years in practice. Additionally, we recruited physicians from the former east and west areas of Berlin and from outer city areas to reflect greater diversity specific to this setting in Germany. We used a relatively small, purposive convenience sample of physicians from specialties known to prescribe most often; thus, there may have been some degree of representational bias. Although many findings from the focus groups align well with findings from our nationally representative survey, which was conducted to further explore influence factors on this topic, other focus groups in other regions or large metropolitan areas in Germany could strengthen these results and are critical before determining national relevance.
The same moderator conducted all focus group discussions based on a conceptual framework drawn before the sessions, so there could be issues of reliability due to its application to four different groups of physicians. But, since we intended for the moderator to allow for participants in each group to explore topics differentially around this framework, so that any new or previously unanticipated topics could come up, we believe that this provided a strength that is unique to this qualitative approach.
Supplementary Material
Acknowledgments
We thank the following individuals for cooperating on this research effort: Werner Espelage, Kirsten Heckenbach, Jürgen Hoffmann, Michael Kramer and Ines Noll.
Footnotes
To cite: Velasco E, Ziegelmann A, Eckmanns T, et al. Eliciting views on antibiotic prescribing and resistance among hospital and outpatient care physicians in Berlin, Germany: results of a qualitative study. BMJ Open 2012;2:e000398. doi:10.1136/bmjopen-2011-000398
Funding: This research was funded by a departmental grant from the Federal Ministry of Health. The study sponsors had no role in the study design or in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study was approved by the institutional commission for data protection of the Robert Koch Institute. All participants gave informed consent before taking part in focus group discussions.
Contributors: EV completed all analysis and drafted the manuscript. AZ, TE and GK conceived of the study and obtained funding. All authors contributed to the study design, the carrying out of the study and provided critical feedback to the manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Ferech M, Coenen S, Malhotra-Kumar S, et al. European surveillance of antimicrobial consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother 2006;58:401–7 [DOI] [PubMed] [Google Scholar]
- 2.Vander Stichele RH, Elseviers MM, Ferech M, et al. Hospital consumption of antibiotics in 15 European countries: results of the ESAC Retrospective Data Collection (1997-2002). J Antimicrob Chemother 2006;58:159–67 [DOI] [PubMed] [Google Scholar]
- 3.Cotter M, Daly L. Antibiotic prescription practices of general practitioners. Ir Med J 2007;100:598–601 [PubMed] [Google Scholar]
- 4.Guerra CM, Pereira CA, Neves Neto AR, et al. Physicians' perceptions, beliefs, attitudes, and knowledge concerning antimicrobial resistance in a Brazilian teaching hospital. Infect Control Hosp Epidemiol 2007;28:1411–14 [DOI] [PubMed] [Google Scholar]
- 5.Hulscher ME, Grol RP, Van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis 2010;10:167–75 [DOI] [PubMed] [Google Scholar]
- 6.Harbarth S, Monnet DL. Cultural and socioeconomic determinants of antibiotic use. In: Gould IM, van der Meer J, eds. Antibiotic Policies—Fighting Resistance. Berlin: Springer, 2007:29–40 [Google Scholar]
- 7.O'Donnel A, Luftey K, Marceau L, et al. Using focus groups to improve the validity of cross-national survey research: a study of physician decision making. Qual Manag Health Care 2007;17:971–81 [DOI] [PubMed] [Google Scholar]
- 8.Kuper A, Reeves S, Levinson W. An introduction to reading and appraising qualitative research. BMJ 2008;337:a288. [DOI] [PubMed] [Google Scholar]
- 9.Velasco E, Espelage W, Faber M, et al. A national cross-sectional study on socio-behavioural factors that influence physicians' decisions to begin antimicrobial therapy. Infection 2011;39:289–97 [DOI] [PubMed] [Google Scholar]
- 10.H, T, P, Concept Website. http://www.inspirationformarketing.com/
- 11.Lehoux P, Poland B, Daudelin G. Focus group research and “the patient's view”. Soc Sci Med 2006;63:2091–104 [DOI] [PubMed] [Google Scholar]
- 12.Leech N, Onwuegbuzie A. An array of qualitative data analysis tools: a call for data analysis triangulation. Sch Psychol Q 2007;22:557–84 [Google Scholar]
- 13.Pope C, Van Royen P, Baker R. Qualitative methods in research on healthcare quality. Qual Saf Health Care 2002;11:148–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TAMS website. http://tamsys.sourceforge.net/
- 15.Butler CC, Rollnick S, Pill R, et al. Understanding the culture of prescribing: qualitative study of general practitioners' and patients' perceptions of antibiotics for sore throats. BMJ 1998;317:637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockburn J, Pit S. Prescribing behaviour in clinical practice: patients' expectations and doctors' perceptions of patients' expectations–a questionnaire study. BMJ 1997;315:520–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg Infect Dis 2002;8:1460–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faber MS, Heckenbach K, Velasco E, et al. Antibiotics for the common cold: expectations of Germany's general population. Euro Surveill 2010;15:19655. [PubMed] [Google Scholar]
- 19.Vasquez GA, Siu HR, Luna EM, et al. Risk factors for Quinolone-resistant Escherichia coli urinary tract infection. Infect Dis Clin Pract 2011;17:309–13 [Google Scholar]
- 20.Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis 2001;7:337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern WV, Nink K, Steib-Bauert M, et al. Regional variation in outpatient antibiotic prescribing in Germany. Infection 2006;34:269–73 [DOI] [PubMed] [Google Scholar]
- 22.Goossens H, Ferech M, Vander SR, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579–87 [DOI] [PubMed] [Google Scholar]
- 23.Deja M, Nachtigall I, Halle E, et al. Antibiotikatherapie—Strategien für die Verordnung von Antibiotika in der Intensivmedizin [Strategies in the treatment of infections with antibiotics in intensive care medicine]. Anasthesiol Intensivmed Notfallmed Schmerzther 2007;42:108–15 [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan A, Song X, Richards A, et al. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med 2004;164:1451–6 [DOI] [PubMed] [Google Scholar]
- 25.Avorn J, Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacologic basis of therapeutics. Ann Intern Med 2000;133:128–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.