Abstract
The detection of growth hormone (GH) and its receptor in germinal regions of the mammalian brain prompted our investigation of GH and its role in the regulation of endogenous neural precursor cell activity. Here we report that the addition of exogenous GH significantly increased the expansion rate in long-term neurosphere cultures derived from wild-type mice, while neurospheres derived from GH null mice exhibited a reduced expansion rate. We also detected a doubling in the frequency of large (i.e. stem cell-derived) colonies for up to 120 days following a 7-day intracerebroventricular infusion of GH suggesting the activation of endogenous stem cells. Moreover, gamma irradiation induced the ablation of normally quiescent stem cells in GH-infused mice, resulting in a decline in olfactory bulb neurogenesis. These results suggest that GH activates populations of resident stem and progenitor cells, and therefore may represent a novel therapeutic target for age-related neurodegeneration and associated cognitive decline.
It is now clear that the adult mammalian brain contains populations of endogenous neural stem1,2 and progenitor cells (together termed precursor cells) that have the ability to replace lost populations of cells under normal conditions3 and can become activated after injury4,5. Stem cells are best defined by their ability to proliferate, self-renew over an extended period of time, and generate a large number of differentiated progeny6,7. While this functional definition is accurate, it unfortunately restricts investigators to a retrospective analysis. This degree of uncertainty has made the identification of neural stem cells (NSCs) a controversial area of research since their discovery1. Coincident with the investigation of NSC biology using functional assays, was the pioneering work of Alvarez-Buylla and colleagues8,9 which elegantly described the cytoarchitecture and cellular hierarchy of the adult subventricular zone (SVZ) of the lateral ventricle; one of two locations within the adult mammalian brain known to contain NSCs and their progeny. As recently reviewed by Kreigstein and Alvarez-Buylla10, NSCs in this region (termed Type B cells) proliferate to produce transient amplifying cells (Type C cells) that in turn generate migratory neuroblasts (Type A cells). It is these Type A cells that ultimately repopulate lost populations of interneurons in the olfactory bulb (OB) via the rostral migratory stream (RMS).
There is now a growing list of NSC markers that have been reported to localize with Type B cells such as glial fibrillary acidic protein (GFAP)11,12, Nestin13, CD13314 and platelet derived growth factor receptor alpha (PDGFRα)15,16. Unfortunately, as these markers are not found exclusively on NSCs, investigators continue to use a multifaceted approach, combining the use of these markers with functional studies to more confidently identify neural stem and progenitor cells in vivo17. Indeed, previous studies have used the neurosphere assay to assess stem and progenitor cell numbers and have shown that both type B and C cells have the ability to form neurospheres19,20 making it difficult to discriminate between the two populations. Fortunately, an in vivo culture technique, the neural colony forming cell assay (N-CFCA), has recently been developed whereby colony size enables the discrimination between NSC- and progenitor-derived colonies21. Studies employing this new technique have reinforced the hypothesis that Type C cells do not appear to possess the extensive self-renewal capabilities typically observed in populations of NSCs22,23,24,25.
In addition to phenotypic identification, understanding how NSCs and progenitor populations are activated is of considerable importance. While best known for its role in regulating somatic growth and metabolic processes, there is substantial evidence to suggest that growth hormone (GH) plays an important role in the development and repair of the mammalian central nervous system26,27,28,29. For example, growth hormone receptor GHR null (GHR−/−)30 and Suppressor of Cytokine Signaling-2 knockout mice, which represent loss and gain of GHR function respectively, display altered brain size, cortical architecture, and neuron and glial cell number31,32. Moreover, GH administration improves cognitive deficits in GH-deficient rodents33,34, and acts as a neuroprotective agent in aged animals35.
Prior work describing the widespread expression of GH and GHR in the perinatal and adult rat brain36,37,38,39, the ability of GH to cross the blood brain barrier40,41, and its ability to act as a neuroprotective agent when infused directly into the ventricles of rodents after stroke27 suggests GH acts directly on neural cells. Building on these findings, more recent in vitro studies have demonstrated that the addition of exogenous GH increases the frequency of both rodent-42 and human-derived neurospheres43. Moreover, neurospheres generated from GHR−/− mice appear smaller, contain fewer proliferating cells, and exhibit reduced self-renewal42. These results clearly demonstrate that GH is acting on neural precursors, but do not directly address whether a functional GHR is found on NSCs. Although, the detection of GH and GHR-immunoreactive (GHR+ve) cells in germinal regions of the adult brain highly enriched in precursor cells32,38 and our recent observation of an absence of exercise-dependent enhancement of NSC number in the SVZ of adult GHR−/− animals24 further suggests the GH/GHR pathway plays a direct role in activating endogenous NSCs. Accordingly, we sought to directly investigate whether a functional GHR is present on resident neural stem and progenitor cells in the adult mouse brain and determine whether it is able to regulate the activity of these cells.
Results
GHR+ve cells exhibiting stem cell properties are present in germinal regions of the mouse brain
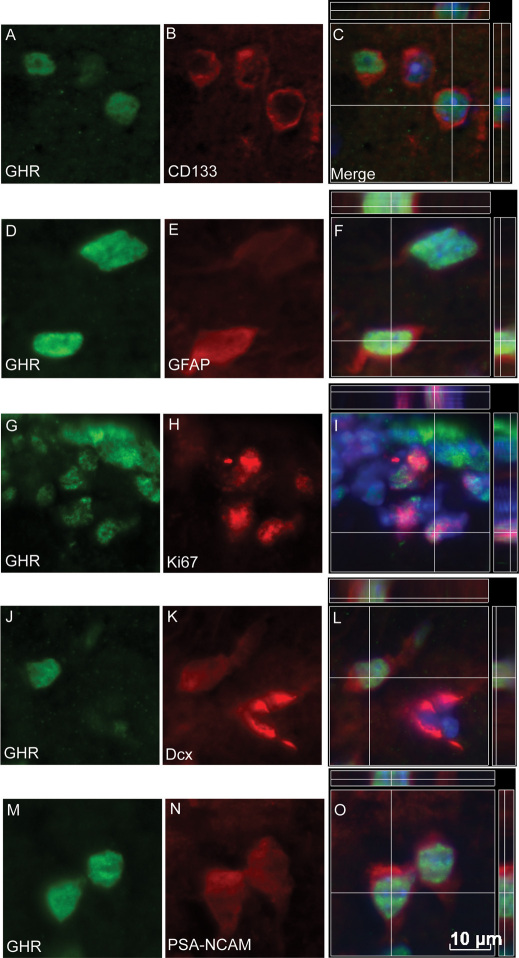
Consistent with prior studies in the rat using in situ hybridization38,39, GHR+ve cells (i.e. those immunoreactive for sc-20747Ab, which labels the intracellular portion of the receptor) were detected both in the adult SVZ surrounding the lateral ventricles and in the dorsolateral corner of the lateral ventricles. Using double-label immunocytochemistry we detected a population of GHR+ve cells within the SVZ that localized with markers typically found on endogenous stem/progenitor cells, namely CD13314,44 (Fig. 1A–C) and GFAP (Fig. 1D–F). GHR+ve cells also localized with the mitotic cell marker Ki6745 (Fig. 1G–I), the immature neuronal marker doublecortin46 (Dcx; Fig. 1J–L) and PSA-NCAM (polysialic acid-neuronal cell adhesion molecule, Fig. 1M–O), a known marker of migrating neuroblasts destined for the OB47.
Figure 1. Distribution and phenotype of GHR+ve cells in the adult SVZ.
GHR+ve cells were detected scattered throughout the SVZ surrounding the lateral ventricle. These cells co-expressed the stem cell markers CD133 (A–C) and GFAP (D–F), as well as the cell proliferation marker Ki67 (G–I). GHR+ve cells were also detected in the dorsolateral corner of the lateral ventricle localized with Dcx (J–L) and PSA-NCAM (M–O). DAPI was used to label the nucleus (Blue in merged images) Omission of primary antibody resulted in loss of immunoreaction. Scale bar: = 10 μm.
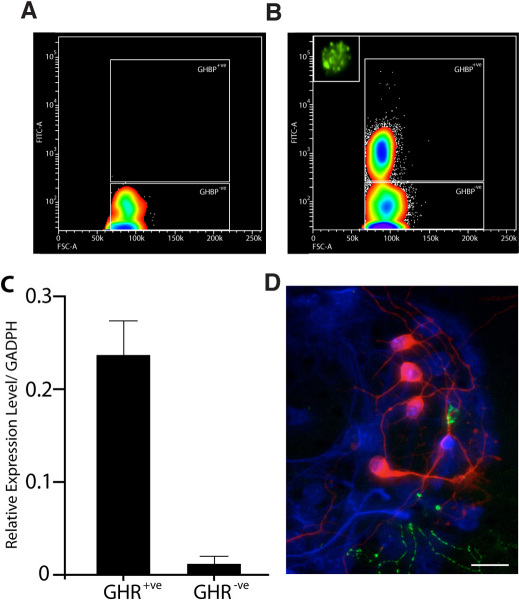
As these results suggest that a GHR is found on a subpopulation of neural precursor cells, we next employed two functional assays; the neurosphere assay1 and N-CFCA21 to confirm that GHR was indeed located on neural stem and progenitor cells. Accordingly, the periventricular region (PVR), which we define as tissue including the ependymal cell layer, SVZ, and the immediately surrounding parenchyma, was harvested (bilaterally), enzymatically brought to a single cell suspension, immunostained for GHR, and then sorted directly into 35 mm dishes containing complete neurosphere medium. As sc-20747Ab labels the intracellular portion of the receptor, a second antibody against the extracellular portion48 was used for all subsequent experiments. Consistent with our detection of GHR+ve cells in the adult SVZ, flow cytometric analysis of tissue harvested from the same region revealed a distinct population of viable growth hormone binding protein (GHBP)+ve cells (Fig. 2A and B). When plated in neurosphere-generating conditions, 1 in every 112±23.0 (mean±SEM, N = 3) cells from the GHBP+ve population was able to generate a primary neurosphere. As an extra confirmation step that the sorted population of GHBP+ve cells obtained possessed GHR, transcripts for the receptor by PCR were compared between the positive and negative cell populations (Note: the relative expression level was to the internal reference gene GADPH). Markedly increased expression levels relative to sorted GHBP−ve cells were observed (Fig. 2C) confirming the reliability of the antibody.
Figure 2. GHR-IR cells represent a distinct population of cells that exhibit extensive proliferation and self-renewal in vitro.
Contour plot of cells harvested from the PVR of adult mice incubated with secondary alone (PE conjugated goat-anti-rabbit IgG) (A) or anti-mouse GHBP (B) reveals a distinct population of viable GHBP+ve cells comprising 0.29±0.03% (mean±SEM, n = 9) of the total population. High power magnification of a typical GHBP-IR cell illustrates the punctate nature of labeling (Inset, B). (C) Relative expression levels of GHR transcript to GADPH in GHR+ve and GHR−ve sort populations harvested from adult SVZ (n = 3 independent experiments, mean ± SEM). (D) Individual clonally derived neurospheres from sorted GHR+ve cells are multipotent, generating neurons (red, β-III-tubulin), astrocytes (blue, GFAP), and oligodendrocytes (green, O4). Scale bar = 10 μm.
As primary neurosphere formation does not demonstrate the presence of a NSC per se20,21, individual clonally-derived neurospheres generated from GHR+ve sort population were enzymatically dissociated and serially passaged every 7 days to demonstrate their self-renewal potential. To demonstrate multipotency, neurospheres were harvested at the 5th passage and transferred to differentiating conditions for a period of 7 days. These differentiated spheres were then fixed and processed for triple-antigen immunoreactivity against βIII-tubulin (neurons), GFAP (astrocytes), and O4 (oligodendrocytes). All three cell types were simultaneously detected in 91% of the 188 GHR+ve cell-derived spheres examined (Fig. 2D). Taken together, these data demonstrate that a subpopulation of GHR+ve cells harvested from the adult SVZ exhibits all of the cardinal functional properties of a NSC in vitro, suggesting that GH-responsive NSCs are present in the adult SVZ.
Addition of GH increases stem cell frequency in vitro
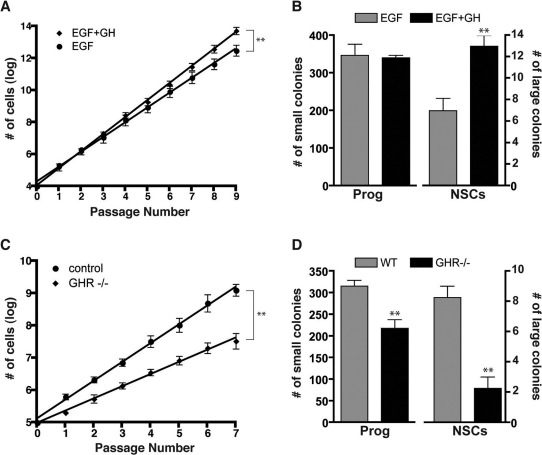
To determine whether exogenous GH alters the proliferative activity of NSCs in vitro, we generated neurosphere cultures from wild-type (WT) adult PVR tissue and serially subcultured the cells in the continued presence of epidermal growth factor (EGF) or EGF supplemented with GH (EGF+GH). The number of cells generated at each passage was recorded, and the rate of expansion calculated. By log transforming the cumulative number of cells produced as a function of time for each of the cultures and applying linear regression analysis, we found that the addition of GH resulted in a significantly (p<0.01) increased slope as compared to that generated by cells cultured in EGF alone (Fig. 3A). Given that changes to the rate of expansion over long-term culture can only be attributed to a self-renewing population49,50, these results suggest GH-supplemented cultures are characterized by a greater NSC frequency than those cultured in EGF alone.
Figure 3. Addition of exogenous GH increases NSC numbers, while deletion of GHR reduces NSC frequency in long-term neurosphere cultures.
(A) Linear regression analysis of the total number of cells generated after each passage in adult SVZ-derived long-term neurosphere cultures grown in EGF (20 ng/ml) or EGF supplemented with GH (EGF+GH; 100 ng/ml) reveals that the addition of GH results in a significantly increased rate of expansion (i.e. slope) as compared to neurospheres cultured in EGF alone (p = 0.008; n = 3, Student's t-test). (B) When transferred to the N-CFCA, neurosphere-derived cells passaged in EGF+GH generated significantly more NSC-derived colonies as compared to those passaged in EGF alone (13.0±1.0 vs. 7±1.2, n = 3, p = 0.011, Student's t-test), without affecting the overall number of progenitor cell-derived (<2.0 mm) colonies. (C) In contrast, linear regression analysis of the number of cells generated after each passage in long-term neurosphere cultures generated from the PVZ demonstrates a significantly reduced rate of expansion in adult GHR−/− (p<0.0001, n = 4) as compared to control. (D) When transferred to the N-CFCA, neurosphere-derived cells from GHR−/− mice generated significantly fewer large colonies, as compared to those derived from controls (2.25±0.75 vs. 8.25±0.75, n = 4, p = 0.0013, Student's t-test). The number of progenitor cell-derived colonies (218.3±20.3 vs. 315.8±13.7, n = 4, p = 0.0072) were also significantly reduced in GHR−/− mice as compared to littermate controls. *p<0.05, **p<0.01.
However, to confirm that a greater number of stem cells were present in GH-stimulated cultures, neurosphere-derived cells were harvested at passage five, and cultured in the N-CFCA in the presence of EGF alone. Unlike the neurosphere assay, which does not allow for discrimination between stem and more restricted progenitor cells20,21,23,24, NSC-derived colonies can be identified from non-NSC-derived colonies in the N-CFCA on the basis of size. Only cells comprising the largest colonies (i.e. those >2.0 mm in diameter, demonstrating the highest replicative potential) exhibit the cardinal properties of bona fide NSCs in vitro21. Thus, by enumerating large colonies >2.0 mm, as compared to those <2.0 mm, a measure of stem versus progenitor cell numbers, respectively, can be obtained19,25.
Consistent with the long-term expansion data (Fig. 3A), after 3 weeks in vitro, those neurosphere-derived cells passaged in EGF+GH generated a significantly greater number of large (i.e. NSC-derived) colonies, as compared to those passaged in EGF alone (Fig. 3B; p ≤ 0.01). Importantly, no significant difference was detected in the number of progenitor cell-derived colonies in EGF versus EGF+GH conditions, suggesting that GH acts directly on NSCs. It should be noted that exogenous bFGF was absent from all culture conditions either in the original media or during the course of the culture period.
Deletion of GHR in vivo reduces the number of NSCs in vitro
As GHR stimulation increased the number of NSCs in vitro, we next determined whether deletion of the receptor in vivo resulted in the generation of fewer NSCs. Accordingly, long-term neurosphere cultures were generated from adult GHR−/− mice (which lack a functional GHR due to the targeted deletion of the mouse GHR/GHBP gene30), and littermate controls. GHR deletion resulted in significantly decreased slope in the neurosphere assay as compared to cultures from littermate controls (Fig. 3C; p<0.01). Furthermore, when cells from these mice were harvested at passage 5 and cultured in the N-CFCA, significantly fewer NSC-derived colonies were detected from GHR−/− cultures compared to controls (Fig. 3D; p<0.01). Of note, neurosphere-derived cells harvested from GHR−/− mice also generated significantly fewer progenitor cell-derived colonies as compared to littermate controls (p<0.01).
Fewer endogenous stem and progenitor cells are present in GHR−/− mice
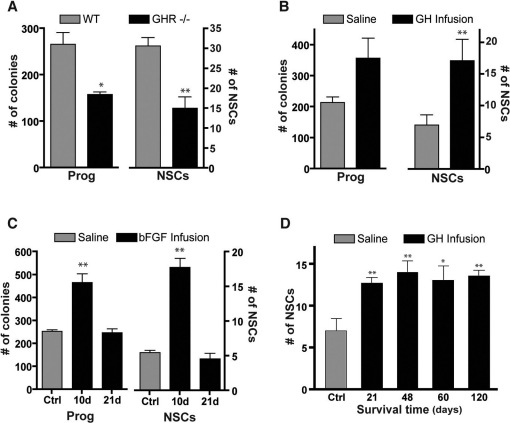
To determine whether GHR−/− mice have fewer endogenous stem and progenitor cells, we next cultured cells harvested cells from the PVR of adult GHR−/− mice and cultured them directly in the N-CFCA without an intermediate neurosphere culture step. We found that significantly fewer NSC-derived (p<0.01) and progenitor cell-derived (p<0.05) colonies were generated from GHR−/− mice than from littermate controls (Fig. 4A). A significant reduction in the number of progenitor cell-derived colonies was not unexpected here, given our previous observation of a reduction in the number of primary neurospheres from adult GHR−/− mice compared to controls.
Figure 4. Fewer NSCs are detected in the PVR of adult GHR−/− mice, while an acute ICV infusion of GH into wild-type mice results in a long-term increase in endogenous NSCs.
(A) PVR cells harvested from adult GHR−/− mice generate significantly fewer NSC-derived colonies (15.0±2.9 vs. 30.6±2.1, n = 4, p = 0.0066) and progenitor cell-derived colonies, as compared to littermate controls (158.3±5.5 vs. 266.3±25.7, n = 4, p = 0.017). (B) Cells harvested from the PVR of GH-infused mice 10 days from the onset of infusion generate a significantly greater number of stem cell-derived colonies compared to vehicle-infused control mice (17.1±3.0 vs. 7.1±1.4, n = 9, p = 0.004), with no associated change in the number of progenitor cell-derived colonies (311.6±49.5 vs. 228.8±13.8, n = 9, p = 0.127). (C) Ten days after bFGF infusion, the number of progenitor (253.9±7.2 vs. 467±37.9, n = 5, p = 0.002) and NSC-derived colonies (5.5±0.33 vs. 17.8±1.3, n = 5, p = 0.0001) was significantly increased relative to saline-infused controls. After 21 days of the bFGF infusion, however, the number of NSC-derived (4.6±0.8, p = 0.643, n = 4) and progenitor cell-derived (247±17.1, n = 4, p = 0.060) colonies returned to levels similar to that of saline-infused controls. (D) The number of NSC-derived colonies remained significantly increased at 21 days (12.8±0.7, n = 6, p = 0.009), 48 days (14.1±1.4, n = 6, p = 0.003), 60 days (13.1±1.8, n = 6, p = 0.021) and 120 days (13.6±0.7, n = 6, p = 0.004) following the onset of GH infusion as compared to vehicle-infused controls (7.1±1.4, n = 9, p = 0.0038). *p<0.05, **p<0.01, Student's t-test.
Acute infusion of GH results in a long-term increase in NSC number
To complement the loss-of-function data, we next infused GH (5 ng/hour) directly into the lumen of the lateral ventricle of WT mice for 7 days, and then harvested PVR tissue 3 days after completion of the infusion, and cultured the resulting single cell suspension directly in the N-CFCA. Consistent with the in vitro actions of exogenous GH (Fig. 3A and B), GH infusion resulted in a significant increase in the number of NSC-derived colonies (p<0.01) with no significant change in the number of progenitor cell-derived colonies (p>0.05) as compared to saline-infused controls (Fig. 4B).
To determine whether the GH-dependent activation of endogenous precursor cells was transient, as observed with the ICV infusion of basic fibroblast growth factor (bFGF) (Fig. 4C), we harvested and cultured PVR tissue from GH-infused mice over increasing survival periods following the 7-day infusion. Surprisingly, we observed a significantly greater number of large colonies at 21 (p<0.01), 48 (p<0.01), 60 (p<0.05), and 120 days (p<0.01) after pump placement (compared to saline-infused controls), demonstrating a unique long-term effect of GH in the activation of endogenous NSCs (Fig. 4D).
Endogenous NSCs are activated in the SVZ following GH infusion
It is possible that the long-term increase in the number of large (i.e. NSC-derived) colonies following GH infusion reflects a peculiarity of the N-CFCA, and not a bona fide increase in the number of endogenous NSCs. We therefore adopted an in vivo ablation/regeneration approach to distinguish between these two possibilities. Ablation of the dividing cells within the SVZ can be achieved either by the infusion of anti-mitotic agents directly into the ventricle51 or by exposing the brain to ionizing or x-irradiation52,53. Given their quiescent nature, NSCs are considered to be largely spared by this process2 as evidenced by efficient regeneration of the region post-irradiation. We reasoned that if GH infusion was activating normally quiescent NSCs to divide, then exposure to ionizing radiation after GH infusion would ablate the GH-responsive dividing NSCs, resulting in an incomplete repopulation of the region.
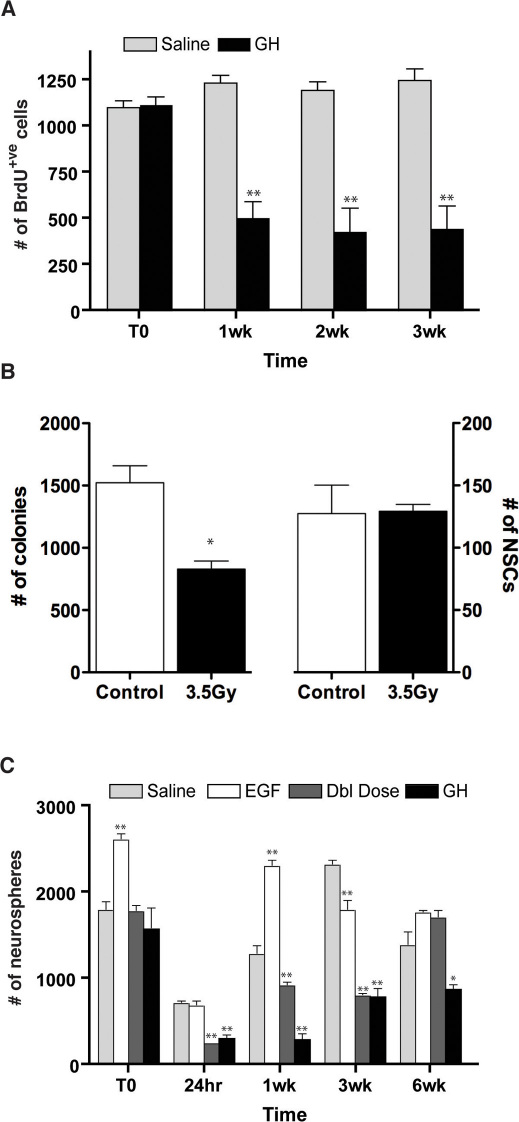
Accordingly, GH- and saline-infused mice were exposed to a single dose of ionizing radiation (3.5 Gy) 14 days after pump removal, and then sacrificed at increasing chase periods. We tracked the repopulation of all dividing cells in the region of the ventricles harvested in a typical SVZ dissection (i.e. +2.54 to +0.94 in relation to Bregma) by administering a single injection of BrdU (45 mg/kg body weight) to GH- and saline-infused mice 2 hours prior to sacrifice. Compared to saline-infused controls, significantly fewer BrdU-immunoreactive (BrdU+ve) cells were detected in the SVZ of GH-infused mice at 1-, 2- and 3-week (p<0.01) survival time points (Fig. 5A), reflecting an incomplete repopulation of the region, presumably due to the irradiation-induced loss of NSCs activated in response to GH infusion2,53.
Figure 5. Repopulation of the PVR is significantly compromised in GH-infused mice.
(A) Significantly fewer BrdU+ve cells were detected in the PVR of GH-infused mice as compared to saline-infused controls at 1 (p = 0.0016), 2 (p = 0.0047) and 3 weeks (p = 0.0044) after a single does of irradiation (n = 3 for all treatments). (B) There was a decrease in the total number of colonies from PVR harvested from wild-type non-irradiated compared to wild-type 3.5Gy irradiated animals five hours post-irradiation (1523±136.8 vs. 829±64.9, n = 3, p = 0.01), however, there was no change in the number of NSC-derived colonies (127.3±22.9 vs. 129.3±5.5, n = 3). (C) Number of neurospheres generated from adult PVR tissue harvested from saline- (░), EGF- (□), GH-infused (█), and “double-dose” (▒) mice immediately prior to irradiation (T0), and at increasing survival times following irradiation. As compared to control (1815.8±67, n = 6), only EGF-infused mice generated significantly more neurospheres at T0 (2672±79, p = 0.002). At 24 hours post-irradiation, PVR tissue from GH-infused (300±44, p = 0.002) and Double (Dbl) dose (236±9, p = 0.0002) mice generated significantly fewer neurospheres than controls. By 1 week post-irradiation the number of neurospheres generated from both GH and Dbl dose mice remained significantly reduced (p = 0.0004 and 0.007, respectively) as compared to control (1318±56). In contrast, EGF-infused mice generated significantly more neurospheres compared to controls at both T0 (p = 0.003) or 1 week post-irradiation (2358±72, p<0.001). By 6 weeks, the number of neurospheres generated from EGF-infused (1797±29) and Dbl dose mice (1746±85) did not differ from controls (1416±157), while GH-infused mice continued to generate significantly fewer neurospheres (888±66, p<0.05). Significance relative to saline control. N = 3 unless otherwise stated. *p<0.05, **p<0.01.
To more directly demonstrate that a single 3.5 Gy dose of irradiation spared the NSC population, a cohort of wild-type animals was irradiated 5 hours prior to sacrifice and compared to non-irradiated animals. When PVR tissue was harvested from these mice and cultured directly in the N-CFCA, we observed a significant (p<0.01) decrease in the number of progenitor cell-derived colonies (Fig. 5B). However, consistent with the sparing of a relatively quiescent population of NSCs, we failed to observe a change in the number of NSC-derived colonies between control and irradiated animals (Fig. 5B; 127.3±22.9 vs. 129.3±5.5, respectively).
Therefore, to complement our in vivo finding of an incomplete repopulation of BrdU+ve cells in the SVZ of GH-infused mice (Fig. 5A), we next repeated the ablation/repopulation approach using the reappearance of neurosphere-forming cells as an additional in vitro readout of SVZ repopulation. We included two additional cohorts of mice to provide support for our hypothesis that the incomplete repopulation effect was due to the GH-induced activation (and subsequent radiation-induced ablation) of endogenous NSCs. One cohort received a 7-day ICV infusion of EGF (20 ng/ml; a treatment we previously demonstrated stimulates endogenous progenitor cell proliferation, leaving NSC number unchanged54) 14 days prior to irradiation to demonstrate that ablation of mitotically-active progenitor cells does not have the same detrimental effect on SVZ repopulation. A second cohort of mice received an additional dose of irradiation (3.5 Gy) 2 days prior to normal irradiation. These “double-dose” mice were employed based on the observation by Morshead and colleagues that normally quiescent NSCs (which were activated in response to the initial ablation of dividing cells) could be ablated by subjecting the mice to a second “kill” 2 days following the initial ablation2. Having demonstrated (Fig. 5A) that a single dose of ionizing radiation was sufficient to ablate dividing cells, largely sparing and then activating quiescent NSCs (as evidenced by the previously reported efficient repopulation of the region2,51 and our work here, see Fig. 5B), we reasoned that the “double-dose” approach of NSC ablation would mimic GH-infused animals, where a substantial proportion of NSCs were GH-activated prior to irradiation.
As expected from previous reports52,53 the number of neurosphere-forming cells in saline-infused mice was reduced to approximately 60% of normal (i.e. the number of cells present in non-irradiated (T0) animals), 24 hours following irradiation, rebounding to supra-control levels in the period following ablation (Fig. 5C). Interestingly, although EGF-infused mice generated significantly more neurospheres immediately prior to irradiation (T0, p<0.01), and displayed a more rapid SVZ repopulation post-irradiation compared to controls (1 vs. 3 weeks, respectively), no significant difference in the number of neurospheres generated by 6 weeks post-irradiation was observed in the EGF-infused mice compared to the controls, reflecting complete repopulation of the region. In contrast, the repopulation of the SVZ in both “double-dose” and GH-infused mice was severely compromised, with sphere-forming cells remaining significantly reduced in these cohorts, as compared to saline controls 3 weeks post-ablation (p<0.01) and pre-irradiation levels (p<0.01). Indeed, repopulation of GH-infused mice remained static between 3 and 6 weeks post-irradiation.
GH-induced increase in SVZ NSCs augments adult neurogenesis
Considering the known contribution of SVZ stem and progenitor cells to OB neurogenesis, we next investigated how GH infusion affected the frequency of GH-responsive cell types in the SVZ, and whether the GH-dependent increase in SVZ NSCs increased OB neurogenesis. Accordingly, brains were removed from GH-infused mice 10 and 21 days after the onset of a 7-day ICV infusion, sectioned (14 μm), and processed for double-label immunocytochemisty to detect GHR+ve cells from the onset of the lateral ventricles to the level of the anterior commissure (i.e. +1.42 to 0.14 mm rostral to Bregma). We failed to detect any change in the overall frequency of GHR+ve cells in the PVR surrounding the lateral ventricles or the dorsolateral corner of the ventricle at either 10- (1282±56) or 21-day (1084±17) survival times, compared to naïve mice (1098±47). However, the frequencies of four subsets of GHR+ve cells (those expressing putative NSC antigens) did increase transiently at 10 days, returning to basal levels by 21 days. These included GHR+ve/Ki67+ve (72±6 to 111±6, p = 0.01, n = 3), GHR+ve/Nestin+ve (62±5 to 100±7, p<0.01, n = 3), and GHR+ve/CD133+ve (184±15 to 219±9, p<0.05, n = 3) cells in the PVR surrounding the ventricle, and GHR+ve/PSA-NCAM+ve (474±12 to 594±40, p<0.05, n = 3) cells in the dorsolateral corner of the ventricle.
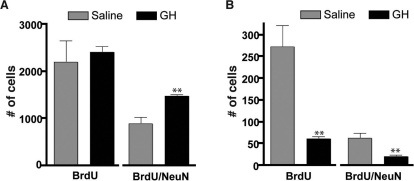
Although these results suggest that GH infusion increases the frequency of migrating neuroblasts; to more accurately determine whether the GH infusion alters the number of new neurons being generated in the OB, and whether this is a NSC-related effect, two final experiments were performed. In the first experiment, GH-infused mice were given a single i.p. injection of BrdU (45 mg/kg body weight) 7 days after GH pump placement and sacrificed after a 28-day chase period, providing sufficient time for newly generated cells to migrate to the OB and differentiate into neurons. The number of newly generated cells reaching the OB (i.e. BrdU+ve) and newly differentiated neurons (i.e. BrdU+ve/NeuN+ve) was determined by examining tissue sections through the entire OB. While GH did not alter the overall number of migrating cells reaching the OB (BrdU, Fig. 6A), it did significantly increase the number of new neurons (BrdU/NeuN, p<0.01, n = 3).
Figure 6. GH-dependent alteration of olfactory bulb neurogenesis.
(A) No significant difference was apparent in the number of BrdU+ve cells detected in the OB 28 days after the 7-day ICV infusion of either GH or saline. In contrast, a significantly greater number of BrdU+ve/NeuN+ve cells was detected in the OB of GH- versus saline-infused mice (1455±37 vs. 869±140, p = 0.008, n = 3). (B) Significantly fewer BrdU+ve cells were detected in the OB of GH-infused mice following irradiation as compared to saline-infused controls (62±5 vs. 273±50, p = 0.007, n = 3). Likewise, the number of BrdU+ve/NeuN+ve cells detected in the OB of GH- versus saline-infused controls was also significantly reduced (21±4 vs. 63±12, p = 0.014, n = 3). **p<0.01.
In the second experiment to determine whether radiation ablation of GH-activated NSCs would also result in a significant decline in OB neurogenesis, a cohort of mice received a 7-day ICV infusion of GH, followed by a single dose (3.5 Gy) of irradiation 7 days later. Twelve days after the end of the infusion, these mice were administered one i.p. injection of BrdU every 2 hours until five injections were given. Multiple injections of BrdU were employed to ensure a meaningful number could be detected following the irradiation-induced ablation of the majority of dividing cells. Twenty-eight days after the irradiation, the mice were sacrificed. As illustrated in Figure 6B, and consistent with our observation of a significant increase in GHR+ve/PSA-NCAM+ve cells in the RMS following GH infusion, the number of BrdU+ve cells reaching the OB was significantly reduced (p<0.01, n = 3) as compared to saline-infused controls. Moreover, the number of newly generated neurons was also significantly reduced in GH-infused mice (p<0.05, n = 3). Taken together, these results demonstrate that in addition to its NSC-stimulatory effect, GH also regulates the number of new neurons destined for the OB in adult mice.
Discussion
In light of its importance in neural development26, its role in the exercise-induced increase in NSCs24 and our detection of GHR+ve cells in the PVR of the adult brain32,38, we hypothesized that a functional GHR is present on a population of NSCs, where it regulates the activity of these cells both in vitro and in vivo. Consistent with this hypothesis is our recent demonstration that while insufficient to generate neurospheres, GH functions as an autocrine mitogen, augmenting the proliferation of adult neurosphere cultures42. However, given only a minority of neurospheres are NSC-derived, these results do not address whether GHRs are present on NSCs, or whether they regulate NSC activity directly.
In contrast, our demonstration here of a reduced rate of expansion of long-term neurosphere cultures generated from GHR−/− mice and reduced numbers of NSC-derived colonies (as compared to WT littermates), together with our observation that GH increases the rate of expansion and the number of NSC-derived colonies generated from long-term neurosphere cultures, now provides direct evidence of a regulatory role for GH/GHR on adult NSCs in vitro. Furthermore, we have shown that GHR+ve cells in the PVR of adult mice localize with the NSC markers GFAP11,12 and CD133 (which is coexpressed with musashi and SOX-1/-244), and when sorted directly into neurosphere generating conditions, GHR+ve cells from the PVR exhibit the cardinal stem cell attributes of proliferation, self-renewal and the ability to generate differentiated progeny (Fig. 2). Taken together, these results demonstrate the presence of GHR on a population of cells in vivo, whose phenotypic and functional attributes are consistent with those of NSCs.
The inability of BrdU-label retention to discriminate NSCs from more restricted proliferative progenitors19, the absence of selective positive markers and sorting strategies to generate pure populations of viable NSC55, and the difficulty of apply ultrastructural approaches dictated our approach to next demonstrate a regulatory role for GH/GHR on endogenous NSCs. We began by showing that deletion of the receptor results in fewer NSC-derived colonies being detected (Fig. 4A), while the infusion of GH results in the activation and associated increase in the number of endogenous NSCs (Fig. 4B). Interestingly, as part of our infusion studies we unexpectedly found that in contrast to similar infusions of the mitogens EGF54 or bFGF (Fig. 4C), the short-term infusion of GH resulted in a significant and sustained elevation in the number of endogenous NSCs a response not previously observed with any growth factor. While outside of the scope of this study, further study is warranted to determine whether these unexpected results reflect an alteration in the phenotype and/or function of these GH-responsive cells, and the physiological consequences of such a change.
To provide additional in vivo evidence of a functional role for GH in the regulation of endogenous NSCs we undertook a series of SVZ ablation/regeneration studies, using both in vitro (i.e. neurosphere and N-CFCA assays) and in vivo assays (immunocytochemistry) as readouts of stem and progenitor cell activity. Based on earlier studies2,52,53, we predicted that if GH was activating normally quiescent NSCs, these activated NSCs would be ablated by subsequent irradiation, resulting in fewer NSC-derived colonies, and a diminished regenerative response. Indeed, this was the case. Consistent with previous reports2,51, those animals whose endogenous stem cells were not activated (i.e. saline and EGF-infused mice) prior to irradiation exhibited a complete repopulation (to pre-irradiation levels) of the BrdU+ve (Fig. 5A) and neurosphere forming (Fig 5C) cells in the SVZ by 3 weeks post-irradiation. However, those animals in which quiescent NSCs were activated prior to irradiation (GH-infused, and “double dose” mice, failed to repopulate either BrdU+ve or neurosphere forming cells to pre-irradiation levels by 3 weeks. Of interest, the repopulation of “double dose” mice reached pre-irradiation levels, but took approximately double the time to complete, demonstrating that the radiation-induced depletion of the PVR in itself is not sufficient cause incomplete repopulation.
Finally, we examined the effect GH infusion would have on OB neurogenesis. This approach was based on the finding that SVZ NSCs (Type B cells) represent the ultimate source of newly-generated OB interneurons10. Consistent with GH activating Type B cells, we found that an acute ICV infusion of GH increased the number of new neurons in the OB, while the irradiation-induced ablation of GH-activated NSCs resulted in a significant reduction both in the number of BrdU+ve cells and newly generated neurons in the OB.
Taken together, these results suggest that GHRs are present on a population of endogenous NSCs, where they regulate the activity of these cells.
It has become apparent that stem cell numbers in a variety of tissues decline as part of the aging process, and are unable to maintain tissue homeostasis at youthful levels24,56. It is plausible that activating a cohort of NSCs by GH infusion could lead to a long-term temporal extension of functional tissue homeostasis. This could be a powerful therapeutic in countering age-related decline in regenerative capacity and cognitive function, as this progressive loss also correlates with the age-dependent decline in GH secretion in rodents and humans33. Independent of its potential therapeutic value, investigation into the mechanism by which NSC numbers are altered will undoubtedly increase our understanding of how the brain responds to its environment.
Methods
Tissue processing
Animals were treated in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and all experiments were approved by The University of Queensland Animal Ethics Committee. Adult (6–8 weeks old) male and female C57BL/6J mice were deeply anesthetized with sodium pentobarbitone (260 mg/kg), and then transcardially perfused with ice-cold 0.9% saline followed by 4.0% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were harvested, post-fixed, and cryoprotected, as previously described57. Serial frontal sections (14 μm) were cut with a MICROM cryostat, mounted on SuperFrost Plus slides (SuperFrost Plus), dried at room temperature (RT) and stored at −20°C until processed. Every second section was collected from the rostral tip of the OB to Bregma (+2.58 mm) for examination of the OB, and from the onset of the lateral ventricle (+1.42 mm) to the joining of the anterior commissure (+0.14 mm) for examination of the lateral ventricles.
In all cases, tissue sections were initially rinsed in PBS (3×5 minutes) before incubation in blocking solution (5% fetal bovine serum (JRH Biosciences, USA) plus 5% normal goat serum (Sigma-Aldrich, USA) in 0.1 M PBS + 0.2% Triton (Sigma-Aldrich)) for 60 minutes at RT.
Tissue sections were processed for the detection of bromodeoxyuridine (BrdU) or dual-label BrdU/anti-neuronal nuclei (NeuN, 1∶60; Chemicon, USA, MAB377. GHR+ve cells were detected on fixed tissue using anti-GHR (1∶300; Santa Cruz, USA, SC-20747 which labels an intracellular portion of the GHR). Note: specificity of the anti-GHR antibody was verified by an absence of immunoreactivity on tissue sections harvested from GHR−/− mice. For all GHR dual-label immunocytochemistry, sections were incubated with anti-GHR (SC-20747) and anti-Ki67 (1∶60; Novacastra, UK, NCL-L-Ki67-MM1), anti-glial fibrillary acidic protein (GFAP, 1∶300; Chemicon, MAB360), anti-NeuN (1∶100; Chemicon, MB377), anti-Nestin (1∶500; Abcam, AB6142), anti-PDGFRα (1∶300, Abcam, AB69506) anti-PSA-NCAM (1∶200; Chemicon, MAB5324), and anti-CD133 (1∶100; Abcam, AB27699), diluted in blocking solution and incubated overnight at 4°C. Sections were washed three times with PBS and then incubated for 60 minutes (RT) with the appropriate secondary antibodies (1∶300; Alexa Fluor goat anti-rabbit-488 for anti-GHR and 1∶1000; Alexa Fluor goat anti-mouse-568 for remaining antibodies; Molecular Probes, USA) plus 4′,6-diamidino-2-phenylindole (DAPI, 1∶1000, Sigma-Aldrich). Sections were then washed three times with PBS and once in dH20, before being coverslipped with fluorescent mounting medium (DakoCytomation, USA). Images were captured using a Zeiss Axioimager Z1 with Apotome and Axiocam MRm software (Zeiss, Germany).
Intracerebroventricular (ICV) infusions
Twelve hours prior to surgery, osmotic mini pumps (Alzet, #1007D; 7 day infusion at 0.5 μl/hour) were loaded with GH (10 μg/ml, recombinant rat, GroPep Australia), bFGF (20 μg/ml, human recombinant, Stem Cell Technologies, Canada), EGF (40 μg/ml, Stem Cell Technologies) or vehicle solution (0.9% sterile physiological saline, Sigma-Aldrich), and attached to the infusion cannula. Although the physiological concentration of GH in the cerebral spinal fluid of mice is unknown, a previous study27 carried out a single ICV infusion in rats of 20 μg/ml, thus we reasoned that a conservative dose of 5 ng/hour of GH should mimic endogenous levels in the mouse. Details of the infusion protocol are given in Blackmore et al. 2009. A separate cohort of GH- and vehicle-infused mice received a single 150 μl intraperitoneal (i.p.) injection of BrdU (9 mg/ml, 45 mg/kg body weight), dissolved in 0.07 N NaOH in 0.9% NaCl; Sigma-Aldrich) 2 hours prior to the completion of the infusion period, and were subsequently sacrificed 28 days later.
Irradiation
Mice were restrained within a plastic chamber and placed in a lead-shielded container leaving only the head exposed for irradiation. Single or “double-dose” irradiation was induced by exposure to a Co60 source in a Gamma Cell 200 irradiator until a 3.5 Gy dose had been given. The PVR was harvested from GH-, EGF- and vehicle-infused animals at 24 hours, 1-, 3- or 6-weeks post-irradiation and cultured in the neurosphere assay (described below). In addition, a second cohort of GH- and vehicle-infused mice received a single injection of BrdU (as above) 2 hours prior to sacrifice, after which the brain was removed, sectioned, and processed for the detection of BrdU+ve cells. For OB experiments, owing to the irradiation-induced loss of dividing cells, cohorts of GH- and vehicle-infused mice received a total of five BrdU injections (as above) over a period of 10 hours, 14 days post-infusion. These mice were irradiated 48-hours after the last BrdU injection and sacrificed 28 days post-irradiation.
Primary neurosphere cultures
Adult (6–8 week old) mice (C57Bl/6J or GHR−/− on this background) were sacrificed by cervical dislocation, their brains removed immediately, and transferred to Petri dishes containing HEPES-buffered minimum essential medium (HEM). Full details of the protocol are given in Blackmore et al. 2009.
Neurosphere passaging and differentiation markers
Adult neurospheres were collected by centrifugation (5 minutes at 100 g rcf), after which they were resuspended and incubated in 0.1% trypsin-EDTA for 4 minutes before being washed with trypsin inhibitor in neurosphere media (NS media). Adult neurosphere cultures were passaged every 7 days in vitro (DIV) in EGF (20 ng/ml) containing complete medium either with or without rat GH (100 ng/ml). For differentiation, neurospheres were plated at time of passage onto poly-L-ornithine (Sigma-Aldrich) coated glass coverslips in NeuroCult™ NSC Basal Medium plus Proliferation Supplements (Stem Cell Technologies) without 1% fetal calf serum for 7 days. The differentiated neurospheres were then fixed with 4% paraformaldehyde in 0.1 M PBS at RT for 15 minutes, rinsed three times with PBS, and incubated with anti-O4 (Clone 81, IgM, Boehringer Mannheim, Germany) diluted 1∶50 with PBS/10% normal goat serum for 2 hours at 37°C. Cells were then rinsed three times with PBS and incubated with FITC-conjugated goat-anti-mouse IgM (1∶200, Molecular Probes) for 30 minutes at 37°C. Cells were again rinsed in PBS (3×5 minutes) before incubation with anti-βIII-tubulin (mouse monoclonal IgG, 1∶1500, Chemicon) + anti-GFAP (rabbit polyclonal, 1∶500, DakoCytomation) diluted in PBS/0.3% Triton/10% normal goat serum for 120 minutes at 37°C. Again, cells were rinsed in PBS (3×5 minutes) and then incubated with goat-anti-mouse-CY3 and goat-anti-rabbit-AMCA (both 1∶200, Molecular Probes) diluted in MTPBS/10% NGS at 37°C for 30 minutes. Finally, cells were rinsed (3×5 minutes in PBS) and mounted using Fluorsave (DakoCytomation).
Neural colony forming cell assay
Primary tissue was dissociated and the number of viable cells determined as per the primary neurosphere cultures (see above). Neurosphere-derived cells were cultured in 35 mm cell culture dishes with a 2 grid (Nunc, USA) at a density recommended for the mouse NeuroCult™ Neural Colony Forming Cell Assay (Stem Cell Technologies). Cells were incubated for 21 DIV in 5% CO2 with appropriate growth factors (either EGF alone or EGF +GH) being added every 7 days. After 21 DIV, the total number of colonies and the diameter of each colony was determined using an eyepiece graticule on an inverted Leica light microscope with phase contrast.
Flow cytometry
Single cell suspensions were generated from tissue and sorted for surface growth hormone binding protein (GHBP) expression using an anti-GHBP antibody (BETO 8041)48. Murine GHBP consists of a ligand-binding domain that is identical to the extracellular portion of the GHR, together with a short C-terminal sequence coded by an alternate exon. For immunostaining, cell suspensions were incubated in NS basal medium with anti-GHBP48 (1∶100) for 15 minutes at 4°C. Cell suspensions were rinsed via centrifugation (7 minutes at 100 g rcf), and then incubated in blocking solution containing Alexa Fluor 488 conjugated goat-anti-rabbit IgG antibody (1∶1000; Molecular Probes) for 30 minutes at RT. The cell suspension(s) were then rinsed in PBS + propidium iodide (100 μg/ml; Molecular Probes) before cell sorting. Cells were sorted using a FACS Vantage SE DiVA (Becton, Dickinson, USA) with data analysis performed using FlowJo 6 (TreeStar Inc., USA) software. Cell to event ratios were determined for each population by seeding 500 events into individual wells of a 96-well plate, then visually scoring cells <1 hour after plating. All cells were collected directly into 35 mm dishes containing NSA+EGF (and GH where appropriate) and incubated at 37°C in a 5% CO2 incubator. Images of fixed GHR-IR cells were captured using an AMNIS Image Stream Analyzer (AMNIS, USA).
qRT-PCR
RNA was extracted using TRIZOL® (Invitrogen, USA) and cDNA was made using Superscript III® (Invitrogen). TaqMAN qRT PCR was undertaken with specific GHR primers for the cytoplasmic domain provided from Applied Biosystems TaqMan® Gene Expression Assays (ID #Mm00439093-m1). cDNA was diluted 1∶5 and samples were normalized to 18S rRNA.
Statistical Analysis
Factorial design analysis of variance (ANOVA) or Student's two-tailed unpaired t-tests were used to analyze data as appropriate. Significant ANOVA values were followed by post hoc comparisons of individual means using the Tukey multiple comparisons test where appropriate. All values are expressed as mean ± SEM unless otherwise indicated with significance for all comparisons p<0.05.
Author Contributions
LH and RA contributed study material, while BL and MG contributed to the collection and assembly of data. All of the remaining authors contributed to experimental design, collection and assembly of data, data analysis and interpretation. Composition of the manuscript was performed by DB, MW and RR.
Acknowledgments
This study was supported by the National Health and Medical Research Council (NHMRC; Australia) project grants 301134 and 511200 and a Pfizer Australia Senior Research Fellowship awarded to RLR, as well as by a NHMRC project grant (401668) and an Australian Research Council Discovery (DP0985145) to MJW. We thank Geoff Osborne and Virginia Nink for their assistance with the flow cytometry, Rowan Tweedale and Ashley Cooper for their assistance in the preparation of this manuscript, and the staff at the University of Queensland Biological Resources for maintaining the animals used in this study.
References
- Reynolds B. A. & Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992). [DOI] [PubMed] [Google Scholar]
- Morshead C. M. et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13, 1071–1082 (1994). [DOI] [PubMed] [Google Scholar]
- Ming G. L. & Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28, 223–250 (2005). [DOI] [PubMed] [Google Scholar]
- Emsley J. G., Mitchell B. D., Kempermann G. & Macklis J. D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Progress in neurobiology 75, 321–341 (2005). [DOI] [PubMed] [Google Scholar]
- Thored P. et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24, 739–747 (2006). [DOI] [PubMed] [Google Scholar]
- Potten C. S. & Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110, 1001–1020 (1990). [DOI] [PubMed] [Google Scholar]
- Till J. E. & Mc C. E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research 14, 213–222 (1961). [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M. & Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17, 5046–5061 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M. & Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999). [DOI] [PubMed] [Google Scholar]
- Kriegstein A. & Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience 32, 149–184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Tramontin A. D., Garcia-Verdugo J. M. & Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A 101, 17528–17532 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D., Doan N. B., Imura T., Bush T. G. & Sofroniew M. V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7, 1233–1241 (2004). [DOI] [PubMed] [Google Scholar]
- Mignone J. L., Kukekov V., Chiang A. S., Steindler D. & Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 469, 311–324 (2004). [DOI] [PubMed] [Google Scholar]
- Coskun V. et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A 105, 1026–1031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. L. et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51, 187–199 (2006). [DOI] [PubMed] [Google Scholar]
- Chojnacki A., Mak G. & Weiss S. PDGFRalpha expression distinguishes GFAP-expressing neural stem cells from PDGF-responsive neural precursors in the adult periventricular area. J Neurosci 31, 9503–9512 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore D. G. & Rietze R. L. in Heart Development and Regeneration Vol. 2 (eds R. P. Harvey & N. Rosenthal) Ch. 13.1, .857–877 (Academic Press, 2010).
- Blau H. M., Brazelton T. R. & Weimann J. M. The evolving concept of a stem cell: entity or function? Cell 105, 829–841 (2001). [DOI] [PubMed] [Google Scholar]
- Golmohammadi M. G. et al. Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells 26, 979–987 (2008). [DOI] [PubMed] [Google Scholar]
- Reynolds B. A. & Rietze R. L. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods 2, 333–336 (2005). [DOI] [PubMed] [Google Scholar]
- Louis S. A. et al. Enumeration of neural stem and progenitor cells in the neural colony forming cell assay. Stem Cells 26, 988–996 (2008). [DOI] [PubMed] [Google Scholar]
- Pluchino S. et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain : a journal of neurology 131, 2564–2578 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari H., Louis S. A., Sharififar S., Vedam-Mai V. & Reynolds B. A. Neural-colony forming cell assay: an assay to discriminate bona fide neural stem cells from neural progenitor cells. J Vis Exp, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore D. G., Golmohammadi M. G., Large B., Waters M. J. & Rietze R. L. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells 27, 2044–2052 (2009). [DOI] [PubMed] [Google Scholar]
- Young K. M., Fogarty M., Kessaris N. & Richardson W. D. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci 27, 8286–8296 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajo R., Cacicedo L., Navarro C. & Sanchez-Franco F. Growth hormone action on proliferation and differentiation of cerebral cortical cells from fetal rat. Endocrinology 144, 1086–1097 (2003). [DOI] [PubMed] [Google Scholar]
- Scheepens A. et al. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 104, 677–687 (2001). [DOI] [PubMed] [Google Scholar]
- Noguchi T. Effects of growth hormone on cerebral development: morphological studies. Horm Res 45, 5–17 (1996). [DOI] [PubMed] [Google Scholar]
- Morisawa K., Sugisaki T., Kanamatsu T., Aoki T. & Noguchi T. Factors contributing to cerebral hypomyelination in the growth hormone-deficient little mouse. Neurochem Res 14, 173–177 (1989). [DOI] [PubMed] [Google Scholar]
- Zhou Y. et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A 94, 13215–13220 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome M. I., Goldshmit Y., Bartlett P. F., Waters M. J. & Turnley A. M. Comparative analysis of CNS populations in knockout mice with altered growth hormone responsiveness. Eur J Neurosci 19, 2069–2079 (2004). [DOI] [PubMed] [Google Scholar]
- Turnley A. M., Faux C. H., Rietze R. L., Coonan J. R. & Bartlett P. F. Suppressor of cytokine signaling 2 regulates neuronal differentiation by inhibiting growth hormone signaling. Nat Neurosci 5, 1155–1162 (2002). [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E. et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol 204, 31–36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey M. M., Weiner J. L., Moore T. P., Carter C. S. & Sonntag W. E. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience 129, 119–127 (2004). [DOI] [PubMed] [Google Scholar]
- Azcoitia I. et al. Growth hormone prevents neuronal loss in the aged rat hippocampus. Neurobiology of aging 26, 697–703 (2005). [DOI] [PubMed] [Google Scholar]
- Hojvat S. et al. Growth hormone (GH), thyroid-stimulating hormone (TSH), and luteinizing hormone (LH)-like peptides in the rodent brain: non-parallel ontogenetic development with pituitary counterparts. Brain Res 256, 427–434 (1982). [DOI] [PubMed] [Google Scholar]
- Gossard F., Dihl F., Pelletier G., Dubois P. M. & Morel G. In situ hybridization to rat brain and pituitary gland of growth hormone cDNA. Neurosci Lett 79, 251–256 (1987). [DOI] [PubMed] [Google Scholar]
- Lobie P. E. et al. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Res Dev Brain Res 74, 225–233 (1993). [DOI] [PubMed] [Google Scholar]
- Zhai Q., Lai Z., Roos P. & Nyberg F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr Suppl 406, 92–95 (1994). [DOI] [PubMed] [Google Scholar]
- Nyberg F. Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol 21, 330–348 (2000). [DOI] [PubMed] [Google Scholar]
- Pan W. et al. Permeation of growth hormone across the blood-brain barrier. Endocrinology 146, 4898–4904 (2005). [DOI] [PubMed] [Google Scholar]
- McLenachan S., Lum M. G., Waters M. J. & Turnley A. M. Growth hormone promotes proliferation of adult neurosphere cultures. Growth Horm IGF Res 19, 212–218 (2009). [DOI] [PubMed] [Google Scholar]
- Pathipati P. et al. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience 190, 409–427 (2011). [DOI] [PubMed] [Google Scholar]
- Corti S. et al. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol 205, 547–562 (2007). [DOI] [PubMed] [Google Scholar]
- Kee N., Sivalingam S., Boonstra R. & Wojtowicz J. M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115, 97–105 (2002). [DOI] [PubMed] [Google Scholar]
- Brown J. P. et al. Transient expression of doublecortin during adult neurogenesis. The Journal of comparative neurology 467, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- Gascon E., Vutskits L., Jenny B., Durbec P. & Kiss J. Z. PSA-NCAM in postnatally generated immature neurons of the olfactory bulb: a crucial role in regulating p75 expression and cell survival. Development 134, 1181–1190 (2007). [DOI] [PubMed] [Google Scholar]
- Aguilar R. M. et al. MAP dendrimer elicits antibodies for detecting rat and mouse GH-binding proteins. J Pept Sci 15, 78–88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo S. G. et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444, 761–765 (2006). [DOI] [PubMed] [Google Scholar]
- Deleyrolle L. P. et al. Determination of somatic and cancer stem cell self-renewing symmetric division rate using sphere assays. PLoS One 6, e15844 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M. & Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A 96, 11619–11624 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G. P. 2nd, Scott E. W., Zheng T., Laywell E. D. & Steindler D. A. Ionizing radiation enhances the engraftment of transplanted in vitro-derived multipotent astrocytic stem cells. Stem Cells 23, 1276–1285 (2005). [DOI] [PubMed] [Google Scholar]
- Tada E., Yang C., Gobbel G. T., Lamborn K. R. & Fike J. R. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol 160, 66–77 (1999). [DOI] [PubMed] [Google Scholar]
- Craig C. G. et al. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci 16, 2649–2658 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum H. I. & Geschwind D. H. Molecular markers in CNS stem cell research: hitting a moving target. Nature reviews. Neuroscience 2, 843–846 (2001). [DOI] [PubMed] [Google Scholar]
- Rando T. A. Stem cells, ageing and the quest for immortality. Nature 441, 1080–1086 (2006). [DOI] [PubMed] [Google Scholar]
- Rietze R., Poulin P. & Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424, 397–408 (2000). [PubMed] [Google Scholar]