Abstract
Succinic acid is considered as an important platform chemical. Succinic acid fermentation with Actinobacillus succinogenes strain BE-1 was optimized by central composite design (CCD) using a response surface methodology (RSM). The optimized production of succinic acid was predicted and the interactive effects between glucose, yeast extract, and magnesium carbonate were investigated. As a result, a model for predicting the concentration of succinic acid production was developed. The accuracy of the model was confirmed by the analysis of variance (ANOVA), and the validity was further proved by verification experiments showing that percentage errors between actual and predicted values varied from 3.02% to 6.38%. In addition, it was observed that the interactive effect between yeast extract and magnesium carbonate was statistically significant. In conclusion, RSM is an effective and useful method for optimizing the medium components and investigating the interactive effects, and can provide valuable information for succinic acid scale-up fermentation using A. succinogenes strain BE-1.
Keywords: Succinic acid, Response surface methodology (RSM), Optimization, Actinobacillus succinogenes
1. Introduction
Succinic acid is an intermediate product of the tricarboxylic acid cycle (TCA), as well as one of fermentation products of anaerobic metabolism. It is identified as one of the most important platform chemicals based on the ease of its biotechnological production (Bechthold et al., 2008). It has wide industrial applications, such as food, pharmaceuticals, resins, and agriculture. Traditionally, succinic acid has been acquired from petrochemicals, which is costly and may cause pollution. Compared with traditional synthetic methods by fossil fuels, the production of succinic acid from a naturally-derived biomass would alleviate the dependence on oil supply in the future. Recently, microbial conversion of biomass into succinic acid has been attracting increasing interest as an eco-friendly and energy-saving process (Li et al., 2010).
Microorganisms intensively investigated for succinic acid fermentation include Actinobacillus succinogenes (Zheng et al., 2009), recombinant Escherichia coli (Wu et al., 2009), Anaerobiospirillum succiniciproducens (Lee et al., 2003), and Mannheimia succiniciproducens (Lee et al., 2002). Among them, A. succinogenes is able to accumulate higher succinic acid in the bioreactor, and is more resistant to succinic acid than any other previously reported succinic acid producers (Bechthold et al., 2008). Therefore, A. succinogenes is considered to be the most promising strain.
The theoretical yield is 2 mol succinic acid per mol glucose. However, the succinic acid yield (0.49 g/g glucose) in fermentation is not high enough for industrial production. Given the immense potential of A. succinogenes, it is worthwhile to optimize the production of succinic acid for maximum yields. The traditional method for succinic acid fermentation medium component optimization is carried out by changing one parameter at a time and keeping the others at a constant level (viz. ‘one-at-a-time’ method). This technique not only is time-consuming, but also lacks factor interaction analysis. Therefore, an alternate strategy involving a statistical approach, e.g., response surface methodology (RSM), was adopted to investigate the combined effects between the main factors (Sharma et al., 2009). RSM is a collection of statistical and mathematical techniques for an optimizing process, which can be used to evaluate the interactions between a set of independent experimental factors and observed responses. This methodology can also reduce the replicated experimental units required to determine optimal conditions (Ghosh and Hallenbeck, 2010). Furthermore, it has been used for optimizing the fermentation medium and culture conditions (Mohana et al., 2008; Yuan et al., 2008; Açıkel et al., 2010).
Recently, several studies have described the use of RSM for the fermentation process parameters in succinic acid production. Isar et al. (2006) reported on the concentration of succinic acid in fermentation with E. coli and interactive effects of five factors (sucrose, tryptone, magnesium carbonate, inoculum size, and incubation period). The concentration of succinic acid was increased from 7.00 to 14.30 g/L. In another project, the production of succinic acid obtained from the fermentation of Bacteroides fragilis was improved from 5.40 to 12.50 g/L under the process optimization with RSM (Isar et al., 2007). However, no literature reported the optimization of the medium components in the production of succinic acid with A. succinogenes strain BE-1 by RSM. In this work, central composite design (CCD), the standard RSM, is used for the study of optimization of succinic acid fermentation media with A. succinogenes strain BE-1 as well as the interactive effects between the main components.
2. Materials and methods
2.1. Microorganism
A. succinogenes strain BE-1, a strain that produces a high concentration of succinic acid, was isolated from bovine rumen and collected in China General Microbiological Culture Collection Center (No. CGMCC2650).
2.2. Culture media and succinic acid fermentation
Inoculum was prepared in an aerobic flask containing 50 ml of 3% tryptic soy broth (TSB) medium (0.03 g/ml; pancreatic digest of casein 17.00 g/L, soy peptone 3.00 g/L, glucose 3.00 g/L, NaCl 5.00 g/L, and K2HPO4 2.50 g/L). The seed cultures were grown in a rotary shaker at 37 °C and 180 r/min for 5 h. The fermentation was conducted in 100 ml flask with 50 ml of the medium containing: glucose 30.00 g/L, yeast extract 10.00 g/L, K2HPO4 1.37 g/L, KH2PO4 1.53 g/L, NaCl 1.50 g/L, MnCl2 0.07 g/L, CaCl2 0.38 g/L, and MgCO3 30.00 g/L. All chemicals used in this study were of analytical level and were purchased from either OXOID (England) or Sinopharm Chemical Reagent Beijing Co., Ltd. (China) unless otherwise described. Glucose was separately sterilized at 115 °C for 20 min and added to the medium to maintain the initial concentration of 30.00 g/L. The initial pH of the sterilized medium was adjusted to 7.00 by H3PO4. The fermentation media were inoculated with 5% of the seeds prepared and grown in the rotary shaker at 37 °C and 40 r/min for 12 h.
2.3. Analysis methods
The culture broth was centrifuged at 10 000×g for 10 min, filtered, and 10 μl of each test sample was run on high-performance liquid chromatography (HPLC; Agilent 1200, USA) equipped with an Agilent model DAD UV-Vis detector and ZORBAX SB-Aq column (25 cm×4.6 mm, 5 μm; Agilent). The column was operated at 25 °C. The mobile phase was 20 mmol/L KH2PO4/H3PO4 buffer of the pH 2.70 at a flow rate of 1.0 ml/min. And the glucose used in the fermentation was analyzed by SBA-40D Biosensor Analyzer (Biology Institute of Shandong Academy of Sciences, China).
2.4. Key medium components screened for succinic acid production
Experiments were carried out by the conventional ‘one-at-a-time’ method to select the suitable factors for maximum succinic acid production. The medium components (carbon, nitrogen, and metal ions) were selected while the temperature, pH, medium volume, inoculum size, and fermentation time were examined as the conditions of the fermentation. The optimal concentrations of the selected key factors were further determined to obtain a higher production of succinic acid.
2.5. RSM: CCD as the experimental design
In order to evaluate the effect of factors on the response surface in the region of investigation, a three-factor-five-level CCD was performed (Aktaş et al., 2006). Based on the best results of the one-at-a-time method, the ranges and levels of three variables viz. glucose (A), yeast extract (B), and magnesium carbonate (C) are listed in Table 1. The test variables were coded according to the following equation (Kılıç et al., 2002):
| xi=(Xi−Xi*)/∆Xi, | (1) |
where xi is the coded value of the ith independent variable, Xi is the uncoded value of the ith independent variable, Xi * is the uncoded ith independent variable at the centre point, and ∆Xi is the step change value.
Table 1.
Experimental ranges and levels of the three independent variables used in RSM in terms of actual and coded factors
| Coded value | Actual value (g/L) |
||
| Glucose | Yeast extract | MgCO3 | |
| −1.68 | 9.89 | 3.30 | 9.89 |
| −1 | 15.00 | 5.00 | 15.00 |
| 0 | 22.50 | 7.50 | 22.50 |
| +1 | 30.00 | 10.00 | 30.00 |
| +1.68 | 35.11 | 11.70 | 35.11 |
The statistical software package ‘Design-Expert 7.0 (trial version)’ was used to analyze the experimental design. The total number of experiments with three factors was 20 (2k+2k+6, when k=3, where k is the number of factors). The design matrix with three variables (glucose, yeast extract, and magnesium carbonate)×five levels (−1.68, −1, 0, +1, +1.68) is presented in Table 2. All the variables were taken at the coded values. In order to control the error bar, 20 runs were performed in a random order in which there were six replications at the center points to evaluate the pure error. Process performance was evaluated by analyzing the concentration of succinic acid produced after 12 h in the fermentation medium. In optimization, the response can be related to chosen factors by linear or quadratic models. A quadratic model, which also includes a linear model, is given as follows:
| Y=β0+β1A+β2B+β3C+β12AB+β13AC+β23BC+β11A2+β22B2+β33C2, | (2) |
where Y is the predicted response; β 0, intercept; β 1, β 2, β 3, linear coefficients; β 12, β 13, β 23, interaction coefficients; β 11, β 22, β 33, squared coefficients. Data were processed for Eq. (2) using the Design-Expert 7.0 program including analysis of variance (ANOVA) to obtain the interactive effects between the process variables and the response. The quality of fit of the polynomial model was expressed by the coefficient of determination R 2, and its statistical significance was checked by the F-test in the same program.
Table 2.
Design matrix of centered central composite design (CCD) for succinic acid production
| Run | Glucose | Yeast extract | MgCO3 | Succinic acid (g/L) |
|
| Observed | Predicted | ||||
| 1 | −1 | −1 | −1 | 7.884 | 6.543 |
| 2 | +1 | −1 | −1 | 10.403 | 9.609 |
| 3 | −1 | +1 | −1 | 11.692 | 10.267 |
| 4 | +1 | +1 | −1 | 15.127 | 15.441 |
| 5 | −1 | −1 | +1 | 3.260 | 3.138 |
| 6 | +1 | −1 | +1 | 6.223 | 7.840 |
| 7 | −1 | +1 | +1 | 12.601 | 13.589 |
| 8 | +1 | +1 | +1 | 18.863 | 20.399 |
| 9 | −1.68 | 0 | 0 | 6.782 | 8.021 |
| 10 | +1.68 | 0 | 0 | 17.804 | 16.326 |
| 11 | 0 | −1.68 | 0 | 0.104 | 0.586 |
| 12 | 0 | +1.68 | 0 | 14.998 | 14.278 |
| 13 | 0 | 0 | −1.68 | 11.226 | 13.257 |
| 14 | 0 | 0 | +1.68 | 16.831 | 14.562 |
| 15 | 0 | 0 | 0 | 14.447 | 16.421 |
| 16 | 0 | 0 | 0 | 15.435 | 16.421 |
| 17 | 0 | 0 | 0 | 15.070 | 16.421 |
| 18 | 0 | 0 | 0 | 17.425 | 16.421 |
| 19 | 0 | 0 | 0 | 18.541 | 16.421 |
| 20 | 0 | 0 | 0 | 17.518 | 16.421 |
2.6. Validation of the model
The second-order polynomial regression equation obtained from the experimental data can be used to predict the response at any levels of the variables within the range of the experimental design. In order to determine the accuracy of the model, the concentrations of three factors (glucose, yeast extract, and magnesium carbonate), which had a major influence on succinic acid production, were randomly selected within the design space. The remaining components of the medium in this experiment were at fixed levels. Four sets of experiments were carried out.
3. Results and discussion
3.1. Medium components screened for succinic acid production with one-at-a-time method
In order to select the key parameters for succinic acid production with A. succinogenes strain BE-1, the one-at-a-time method was conducted as described above. It showed that the key parameters were glucose (22.50 g/L), yeast extract (7.50 g/L), magnesium carbonate (22.50 g/L), inoculum size (5%), and fermentation time (12 h). The initial fermentation pH was at 7.00. This resulted in the production of 14.80 g/L of succinic acid in 12 h at 37 °C (data not shown). The by-products were also determined by HPLC, and the yields of formic acid, acetic acid, and lactic acid were 2.8, 3.2, and 2.3 g/L, respectively. Thus, the medium obtained by the one-at-a-time method was: glucose 22.50 g/L, yeast extract 7.50 g/L, K2HPO4 1.37 g/L, KH2PO4 1.53 g/L, NaCl 1.50 g/L, MnCl2 0.07 g/L, CaCl2 0.38 g/L, and MgCO3 22.50 g/L (pH 7.00).
3.2. CCD
A three-variable-five-level matrix of CCD was employed to determine the optimized conditions and the interactive effects. Glucose, yeast extract, and magnesium carbonate were selected as the factors for CCD. The succinic acid concentrations for each individual run along with the predicted responses are summarized in Table 2. The highest succinic acid concentration of 18.86 g/L was attained at 12 h when the concentrations of glucose, yeast extract, and magnesium carbonate were 30.00, 10.00, and 30.00 g/L, respectively, with 5% inoculum volume (Run 8). Also, the lowest succinic acid concentration was 0.104 g/L, which was gained when glucose, yeast extract, and magnesium carbonate concentrations were 22.50, 3.30, and 22.50 g/L, respectively (Table 2). Based on the analysis of the software, the optimized concentrations of glucose, yeast extract, and magnesium carbonate were 27.43, 9.56, and 23.32 g/L and the predicted concentration of succinic acid was 19.08 g/L. This was a 28.9% improvement over that attained with the one-at-a-time method.
The response data were analyzed in the Design-Expert software. The application of RSM yielded the following regression equation, which is an empirical relationship between succinic acid and the test variables in coded units:
| Y=16.41+2.47A+4.07B+0.39C+0.53AB+0.41AC+1.68BC−1.50A2−3.18B2−0.89C2, | (3) |
where Y is the succinic acid produced as a function of glucose (A), yeast extract (B), and magnesium carbonate (C). The statistical significance of the above equation was checked by the F test, and the ANOVA for the response surface quadratic model is shown in Table 3. The model F value of 14.95 and values of probability (P)>F (0.0001) indicated that the model terms were significant. The regression equation showed that the R 2 was 0.9308 (Table 3), which indicated aptness of the model (Isar et al., 2006). This was similar to R 2 of 0.936 reported by Singh and Chhatpar (2010). This result indicates that approximately 93% of the variability in the dependent variable (response) can be explained by this model. The R 2 value is always between 0 and 1. The closer the R 2 is to 1.0, the stronger the model and the better it predicts the response (Aghaie et al., 2009). The adjusted R 2, which corrects the R 2 value for the sample size and for the number of terms, was 0.8686. The lack of fit F value was 1.81 (the lack of fit value was not significant in relation to the pure error). Non-significant lack of fit indicated that the model was a good fit (Song et al., 2007). The adequate precision value, which measured the signal to noise ratio, was 14.432. The ratio greater than 4 is desirable. Thus, this model could be used to navigate the design space (Ghosh and Hallenbeck, 2010).
Table 3.
Analysis of variance (ANOVA) for the quadratic modela
| Source | Sum of squares | Degree of freedom | Mean square | F value | Prob>F | Significance |
| Model | 506.70 | 9 | 56.30 | 14.95 | 0.0001 | Significant |
| Residual (error) | 37.66 | 10 | 3.77 | |||
| Lack of fit | 24.26 | 5 | 4.85 | 1.81 | 0.2652 | Not significant |
| Pure error | 13.40 | 5 | 2.68 | |||
| Total | 544.35 | 19 |
Coefficient of determination (R 2)=0.9308; Adjusted R 2=0.8686; Coefficient of variation (CV)=15.39%; Adeq precision=14.432
The F test and the corresponding P values along with the parameters were estimated (Table 4). The smaller the P value, the larger the significance of the corresponding coefficient (Qi et al., 2009). The parameters estimated and the corresponding P values suggest that, among the independent variables, glucose (A) and yeast extract (B) have significant effects on succinic acid production. The quadratic terms of glucose (A), yeast extract (B), and interaction between yeast extract (B) and magnesium carbonate (C) also have significant effects on succinic acid yield.
Table 4.
Significance of the coefficients of regression
| Model term | Parameter estimated | Standard error | F value | P value |
| β0 | 16.41 | 0.79 | ||
| β1 | 2.47 | 0.53 | 22.11 | 0.0008** |
| β2 | 4.07 | 0.53 | 60.03 | <0.0001** |
| β3 | 0.39 | 0.53 | 0.54 | 0.4796 |
| β12 | 0.53 | 0.69 | 0.59 | 0.4602 |
| β13 | 0.41 | 0.69 | 0.36 | 0.5645 |
| β23 | 1.68 | 0.69 | 6.00 | 0.0342* |
| β11 | −1.50 | 0.51 | 8.63 | 0.0149* |
| β22 | −3.18 | 0.51 | 38.65 | <0.0001** |
| β33 | −0.89 | 0.51 | 3.02 | 0.1131 |
P<0.01 indicates that model terms are highly significant at the 1% level
P<0.05 indicates that model terms are significant at the 5% level
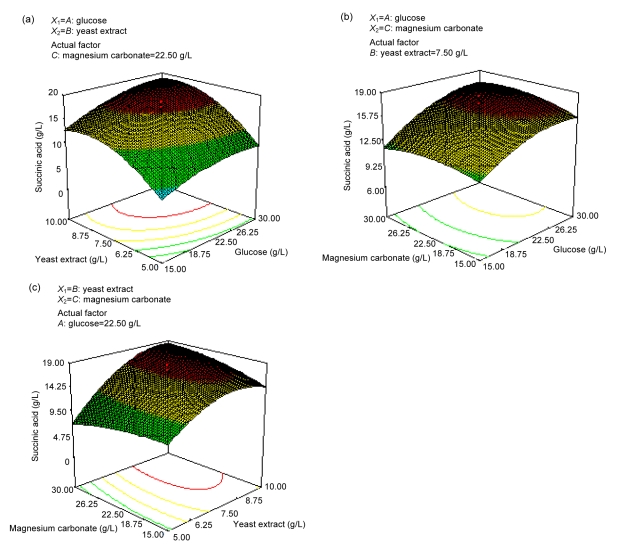
The response surface curves were then plotted to study the interaction among different factors, and to determine the optimal concentration of each factor for maximum succinic acid production. As shown in Fig. 1a, the effects of glucose and yeast extract on the production of succinic acid were determined when the other factor was at its center point. When yeast extract was at a low level, the yield of succinic acid was low. Significant improvement in succinic acid production can be obtained by increasing the amount of yeast extract to some extent.
Fig. 1.
Response surface plots and contour plots
(a) Combined effects of glucose and yeast extract with constant magnesium carbonate (22.50 g/L); (b) Combined effects of glucose and magnesium carbonate with constant yeast extract (7.50 g/L); (c) Combined effects of yeast extract and magnesium carbonate with constant glucose (22.50 g/L)
The effects of glucose and magnesium carbonate are shown in Fig. 1b. Magnesium carbonate is considered as one of the main factors to control the pH of the fermentation broth, and provides magnesium ions for phosphoenolpyruvate carboxykinase, which is a key enzyme in succinic acid production (Lee et al., 1999). When the glucose was at a low level, an increase in magnesium carbonate did not improve the succinic acid concentration. However, if the glucose and magnesium carbonate were at high levels, more succinic acid could be attained.
The interaction of yeast extract and magnesium carbonate on the concentration of succinic acid, when glucose concentration was at its center point, was statistically significant, as shown in Fig. 1c. When the concentration of magnesium carbonate was 15.00 g/L, the succinic acid concentration increased at some extent with the amount of yeast extract added less than 7.50 g/L; while with yeast extract at a higher concentration (>10.00 g/L), adding enough magnesium carbonate can lead to a higher concentration of succinic acid.
3.3. Validation of model
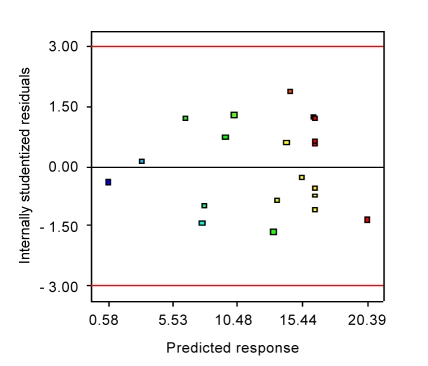
The model was validated for the three variables within the design space. The conditions and results of four experiments are listed in Table 5. The result shows that under the following conditions: glucose 25.00 g/L, yeast extract 10.00 g/L, and magnesium carbonate 25.00 g/L, the concentration of succinic acid (18.23 g/L) nearly reached the optimized concentration, viz. 19.08 g/L. The predicted values and actual experimental values were compared, and then the residual and percentage error were calculated in Table 5. It was observed that the percentage errors between the actual and predicted values for succinic acid production varied from 3.02% to 6.38%. This range of the percentage error accepted was similar in the confirmation experiments of optimization of enzymatic hydrolysis conditions (Qi et al., 2009). Therefore, the empirical models were reasonably accurate, and the RSM analysis is indeed a useful technique to predict and optimize the fermentation media. Usually, it is necessary to check the adequacy of the model to ensure that it provides maximum approximation on relationship between response and factors. The residuals from the least squares are an important tool for judging the model adequacy. Fig. 2 shows the plot of residuals vs. the predicted response. The residual plots of the model are randomly distributed without any trends. This result indicates good predictions of maximum response along with constant variance and adequacy of the quadratic models.
Table 5.
Confirmation experiments
| No. | Glucose (g/L) | Yeast extract (g/L) | MgCO3 (g/L) | Succinic acid concentration |
|||
| Actual (g/L) | Predicted (g/L) | Residual (g/L) | Error (%) | ||||
| 1 | 20.00 | 5.00 | 30.00 | 6.32 | 6.040 | 0.280 | 4.43 |
| 2 | 20.00 | 10.00 | 35.00 | 15.89 | 16.905 | −1.015 | 6.38 |
| 3 | 20.00 | 5.00 | 20.00 | 9.22 | 8.736 | 0.484 | 5.25 |
| 4 | 25.00 | 10.00 | 25.00 | 18.23 | 18.780 | −0.550 | 3.02 |
Fig. 2.
Plot of internally studentized residuals vs. predicted response
4. Conclusions
RSM was successfully applied to the optimization of succinic acid fermentation medium components. The predicted model of succinic acid production was developed in terms of fermentation factors by RSM, and an ANOVA test was performed. The glucose, yeast extract, and interactive effect of yeast extract and magnesium carbonate were the most significant factors in succinic acid production. The optimum values for glucose, yeast extract, and magnesium carbonate concentrations were found to be 27.43, 9.56, and 23.32 g/L, respectively. This resulted in a predicted value 19.08 g/L, which was increased by 28.9% compared with 14.80 g/L obtained from the one-at-a-time method. The validation of the model was also conducted, and the percentage errors between the actual and predicted values for succinic acid production varied from 3.02% to 6.38%. Therefore, the RSM approach can be quite efficient and useful for the optimization of succinic acid fermentation conditions, and the optimal conditions provide important parameters for scaled-up industry succinic acid fermentation with A. succinogenes strain BE-1.
Footnotes
Project supported by the National Major Special Project on New Varieties Cultivation for Transgenic Organisms (No. 2009ZX08009-130B), the National Natural Science Foundation of China (No. 50874112), and the National High-Tech R&D Program (863) of China (No. 2009AA06Z320)
References
- 1.Açıkel Ü, Erşan M, Açıkel YS. Optimization of critical medium components using response surface methodology for lipase production by Rhizopus delemar . Food Bioprod Process. 2010;88(1):31–39. doi: 10.1016/j.fbp.2009.08.003. [DOI] [Google Scholar]
- 2.Aghaie E, Pazouki M, Hosseini MR, Ranjbar M, Ghavipanjeh F. Response surface methodology (RSM) analysis of organic acid production for Kaolin beneficiation by Aspergillus niger . Chem Eng J. 2009;147(2-3):245–251. doi: 10.1016/j.cej.2008.07.008. [DOI] [Google Scholar]
- 3.Aktaş N, Boyacı IH, Mutlu M, Tanyolaç A. Optimization of lactose utilization in deproteinated whey by Kluyveromyces marxianus using response surface methodology (RSM) Bioresour Technol. 2006;97(18):2252–2259. doi: 10.1016/j.biortech.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A. Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol. 2008;31(5):647–654. doi: 10.1002/ceat.200800063. [DOI] [Google Scholar]
- 5.Ghosh D, Hallenbeck PC. Response surface methodology for process parameter optimization of hydrogen yield by the metabolically engineered strain Escherichia coli DJT135. Bioresour Technol. 2010;101(6):1820–1825. doi: 10.1016/j.biortech.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Isar J, Agarwal L, Saran S, Saxena RK. A statistical method for enhancing the production of succinic acid from Escherichia coli under anaerobic conditions. Bioresour Technol. 2006;97(13):1443–1448. doi: 10.1016/j.biortech.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Isar J, Agarwal L, Saran S, Kaushik R, Saxena RK. A statistical approach to study the interactive effects of process parameters on succinic acid production from Bacteroides fragilis . Anaerobe. 2007;13(2):50–56. doi: 10.1016/j.anaerobe.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Kılıç M, Bayraktar E, Ateş S, Mehmetoglu Ü. Investigation of extractive citric acid fermentation using response-surface methodology. Process Biochem. 2002;37(7):759–767. doi: 10.1016/S0032-9592(01)00277-1. [DOI] [Google Scholar]
- 9.Lee PC, Lee WG, Lee SY, Chang HN. Effects of medium components on the growth of Anaerobiospirillum succiniciproducens and succinic acid production. Process Biochem. 1999;35(1-2):49–55. doi: 10.1016/S0032-9592(99)00031-X. [DOI] [Google Scholar]
- 10.Lee PC, Lee SY, Hong SH, Chang HN. Isolation and characterization of a new succinic acid-producing bacterium, Mannheimia succiniciproducens MBE L55E, from bovine rumen. Appl Microbiol Biotechnol. 2002;58(5):663–668. doi: 10.1007/s00253-002-0935-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee PC, Lee SY, Hong SH, Chang HN, Park SC. Biological conversion of wood hydrolysate to succinic acid by Anaerobiospirillum succiniciproducens . Biotechnol Lett. 2003;25(2):111–114. doi: 10.1023/A:1021907116361. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Yang M, Wang D, Li W, Wu Y, Zhang Y, Xing J, Su Z. Efficient conversion of crop stalk wastes into succinic acid production by Actinobacillus succinogenes . Bioresour Technol. 2010;101(9):3292–3294. doi: 10.1016/j.biortech.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 13.Mohana S, Shrivastava S, Divecha J, Madamwar D. Response surface methodology for optimization of medium for decolorization of textile dye Direct Black 22 by a novel bacterial consortium. Bioresour Technol. 2008;99(3):562–569. doi: 10.1016/j.biortech.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Qi BK, Chen XR, Shen F, Su Y, Wan YH. Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind Eng Chem Res. 2009;48(15):7346–7353. doi: 10.1021/ie8016863. [DOI] [Google Scholar]
- 15.Sharma S, Malik A, Satya S. Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J Hazard Mater. 2009;164(2-3):1198–1204. doi: 10.1016/j.jhazmat.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Chhatpar HS. Optimization of protease production by Streptomyces sp. A6 using statistical approach for reclamation of shellfish waste. World J Microbiol Biotechnol. 2010;26(9):1631–1639. doi: 10.1007/s11274-010-0339-1. [DOI] [Google Scholar]
- 17.Song XJ, Zhang XC, Kuang CH, Zhu LY, Guo N. Optimization of fermentation parameters for the biomass and DHA production of Schizochytrium limacinum OUC88 using response surface methodology. Process Biochem. 2007;42(10):1391–1397. doi: 10.1016/j.procbio.2007.07.014. [DOI] [Google Scholar]
- 18.Wu H, Li ZM, Zhou L, Xie JL, Ye Q. Enhanced anaerobic succinic acid production by Escherichia coli NZN111 aerobically grown on gluconeogenic carbon sources. Enzy Microb Technol. 2009;44(3):165–169. doi: 10.1016/j.enzmictec.2008.10.016. [DOI] [Google Scholar]
- 19.Yuan LL, Li YQ, Wang Y, Zhang XH, Xu YQ. Optimization of critical medium components using response surface methodology for phenazine-1-carboxylic acid production by Pseudomonas sp. M-18Q. J Biosci Bioeng. 2008;105(3):232–237. doi: 10.1263/jbb.105.232. [DOI] [PubMed] [Google Scholar]
- 20.Zheng P, Dong JJ, Sun ZH, Ni Y, Fang L. Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes . Bioresour Technol. 2009;100(8):2425–2429. doi: 10.1016/j.biortech.2008.11.043. [DOI] [PubMed] [Google Scholar]