Abstract
The search for active toxins for managing weeds or plant diseases is believed to be a promising avenue of investigation. However, the effects of Alternaria toxins on insects have just begun to be investigated. Bioactivities of toxins from four strains of Alternaria alternata on Rosa chinensis and rose aphid Macrosiphum rosivorum were tested in the present study. At a concentration of 50.0 μg/ml, the crude extract (toxin) of strain 7484 was found not to be harmful to rose plants with excised leaf-puncture method (P≥0.079), and rose plants showed enhanced resistance to rose aphids when this Alternaria toxin was sprayed on the plants (P≤0.001). However, this toxin caused no detrimental effects on aphids in insecticidal bioassay at a concentration of 10.0 to 160.0 μg/ml (P≥0.096). Therefore, the Alternaria toxin had significantly induced the resistance of rose plants against rose aphids, demonstrating that the resistance mechanism triggered by the Alternaria toxin in the rose plant may also be used by the plant to defend itself against insects. Further bioassays aimed to discover the olfactory responses of aphids to the toxin-induced volatiles of host plants. The aphids were significantly more attracted to both volatiles emitted and collected from control rose plants than to both volatiles emitted and collected from the toxin-treated rose plants (P≤0.014). This result showed that the toxin-induced resistance related to the volatile changes of host plants.
Keywords: Alternaria alternata, Macrosiphum rosivorum, Rosa chinensis, Toxin, Plant resistance, Olfactory response, Volatile, Headspace collection
1. Introduction
Alternaria alternata (Fr.) Keissl., commonly known as a cosmopolitan saprophyte, produces a variety of secondary metabolites belonging to several classes of phytotoxic chemicals, including either host-specific or nonhost-specific toxins from different strains (Strange, 2003; Chen et al., 2005). Generally, plants sensitive to a specific toxin (genera, species, and even cultivars) fall within the range of the hosts of the pathogen that produces this specific toxin. However, the spectrum of activities of a nonspecific toxin is not limited to phylogenetic specialization of the producer pathogen, and is concentration-dependent (Berestetskiy, 2008).
Amongst toxins produced by fungi, the Alternaria toxins were applied widely. The Alternaria toxins may provide the prospect for biocontrol of weeds (Chen et al., 2005). For instance, Alternaria alternata lycopersici toxin (AAL-toxin) was used to control jimsonweed Datura stramonium and black nightshade Solanum nigrum L. (Abbas et al., 1993). In recent years, it was reported that Alternaria alternata Crofton-weed toxin (AAC-toxin) had a high herbicidal activity on crofton weed, large crabgrass, barnyard grass, redroot pigweed, and eclipta (Qiang et al., 2010), and that alternethanoxins A and B had potential as natural herbicides for Sonchus arvensis biocontrol (Evidente et al., 2009). Similarly, a synthetic analogue of AAL-toxin has significant phytotoxicity to duckweed (Lemna pausicostata), indicating some potentials for development of safe and effective natural herbicides (Abbas et al., 1995). The practical application of maculosin and its synthetic analogues as natural herbicides against spotted knapweed was described in previous works (Strobel et al., 1991; Park et al., 1993; Bobylev et al., 1996). The Alternaria toxins may also have potential as a fungus control agent (Chelkowski and Visconti, 1992). An interesting example was reported by Egusa et al. (2008), wherein pre-inoculation with a nonpathogenic strain of A. alternata or pretreatment with an elicitor prepared from this strain reduced disease symptoms by the pathogen. In addition, the Alternaria toxins were used for screening plant genotypes resistant to disease (Švábová and Lebeda, 2005; Berestetskiy, 2008).
Until now, it is well-known that pathogens or their toxins may induce numerous resistance-related plant responses (Heath and Skalamera, 1997), including (1) changes of primary and secondary metabolites (Hatcher et al., 1995) such as host-plant volatiles, or (2) synthesis of defensive components (Hammerschmidt, 1999), or (3) hypersensitive response (HR) (Kombrink and Schmelzer, 2001), or (4) occurrences of systemic acquired resistance (SAR) and induced systemic resistance (ISR) (Cipollini et al., 2004). Many signalling pathways were involved during these plant defense responses, such as salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), reactive oxygen species (ROS). More importantly, numerous cross-talks between multiple signalling pathways were found (Zhao and Sakai, 2003), for example, between SA pathway (mainly relating to the resistance to fungi) and JA pathway (mainly relating to the resistance to insects). Further, direct cross-effects between plant defenses to pathogens and herbivores may also occur because of the physiological changes of plants. For example, when the Phaseolus lunatus plants were simultaneously exposed to a fungal pathogen (Colletotrichum lindemuthianum) and a herbivore (Epilachna varivestis), extensive cyanogenesis (anti-herbivore defense) inhibited the activity of polyphenol oxidases (PPO, mainly relating to resistance to pathogens) in lima plants, and vice versa (Ballhorn et al., 2010; Ballhorn, 2011). Thus, the ecological cross-effects between herbivores and toxins (or fungi) may be plant-mediated. While SAR and ISR can enhance plant resistance to the original attacker, cross-effects on resistance to other organisms may be an important ecological consequence (Heil and Bostock, 2002), and cross-effects may result from broad biological activity of defenses induced during SAR or ISR that directly alter general pest resistance (Cipollini et al., 2004). For example, negative effects of Alternaria brassicae-infection on leaf beetle Phaedon cochleariae were demonstrated in an agricultural system (Rostás and Hilker, 2002). Thus, we believe that plants may obtain resistance to insects, resulting from the plant’s resistance induced by toxins, especially under controlled conditions.
With an increasing plantation area of the cut rose Rosa chinensis Jacq. cv. Movie Star, the infestation of the rose aphid Macrosiphum rosivorum Zhang has often been found all the year round in greenhouses, and is responsible for serious economic losses to both the potted and cut rose production industries. However, as mentioned above, it remains unclear whether insect pests were detrimentally or beneficially affected by Alternaria toxins. Although a few studies on the effects of fungi of the genus Alternaria on insects were reported, to the best of our knowledge, the mechanisms of resistance to insects induced by Alternaria toxins were not clear. So in the present study, we examined toxicities of toxins produced by four strains of A. alternata to rose leaves and aphids. The resistance against aphids induced by the Alternaria toxins and the olfactory responses of aphids to volatiles of rose plants were also studied.
2. Materials and methods
2.1. Plants and insects
Plants were sampled from the susceptible rose cultivar Movie Star (about two years old) in the greenhouses for China rose cut flower production in three counties (Kunming, Kunyang, and Yiliang) in Yunnan province, southwest of China, and grown in a fungus-free greenhouse compartment of our laboratory at about 24 °C and 80% relative humidity (RH) with a 16 h:8 h light:dark photoperiod regime provided by fluorescent lamps. Rose plants were used for the bioassays when they had developed 6–10 expanded leaves after a cultivation period of four weeks.
Rose aphids were collected from naturally occurring rose aphid colonies in the different greenhouses for rose production in three counties where the rose plants were collected. Aphids from different colonies separately and continuously reared on seedlings of the rose cultivar Movie Star were grown inside other greenhouse compartments as described above. Then, aphids randomly chosen from different compartments were used in the following bioassays.
2.2. Toxin purification
Samples (stems and leaves) were collected from both wild and cultivated rose plants (Rosa rugosa Thunb.). For isolation of Alternaria fungi, the procedure followed by Kaul et al. (2008) was adopted with slight modifications. In the present study, from a total of ten fungus strains of A. alternata, four strains (7484, 0845, 0393, and 0203) were used for the production of toxins.
Each of the Alternaria toxins was obtained from the culture of A. alternata strains by first transferring conidia to potato dextrose agar (PDA) medium and thereafter transferring mycelial culture to 200 ml potato dextrose broth in five 500-ml conical flasks at 25 °C for 10 d. A total of 6 L of culture filtrate was extracted with ethyl acetate and then the extract was passed through a column of macroporous resin D101, the column being eluted with alcohol. The alcohol-diluted extraction was concentrated by a rotary evaporator at 50 °C (pressure 0.075 MPa) until partially purified crude solid extracts were obtained.
2.3. Leaf-puncture assay
The toxicities of toxins of four strains of A. alternata were assayed by leaf-puncture bioassay on rose leaves of R. chinensis (Abbas et al., 1993). The fully expanded leaves from greenhouse-grown plants were washed for 10 min in running tap water, sterilized in 1% (mass fraction) sodium hypochlorite for about 1 min, then aseptically rinsed thoroughly with sterile distilled water. Finally the leaves were placed on moistened filter paper and punctured by a sterile needle on the lower surface.
Toxins were dissolved with sterile deionized water (from 20.0 to 140.0 μg/ml with a step of 30.0 μg/ml). Droplets (10 μl) of the test solution were applied on the wounded leaves and then incubated in transparent plastic boxes at 24 °C under 16-h photoperiod, with the lower surface upwards. After 48 h of incubation, the diameters (mm) of the necrotic lesions were measured with a microscope caliper (Olympus, Tokyo, Japan). At the same time, rose leaves with droplets of sterile water served as a control. Ten replicates were undertaken for each concentration as well as for the control.
2.4. Resistance bioassay
To test the Alternaria toxin-induced resistance against aphids, 5-ml solution of toxins at a concentration of 50.0 μg/ml was evenly sprayed on rose plants (leaves and stems) with a sprayer similar to a perfume container. After one day, each potted rose was infested with 10 adult wingless aphids by means of a wetted fine brush and a stereomicroscope, and the whole plant was covered with a cage of fine-mesh gauze to avoid aphids moving to neighbouring plants. At the same time, rose plants infected by 10 aphids and sprayed with water served as a control. Six replicates were undertaken for treated and control rose plants. All rose plants were kept in the glasshouse under the conditions described in Section 2.1. The number of aphids in each of cages was respectively recorded each day until the 10th day after infestation.
To test the effect of change of concentrations on the reproduction of aphids, another bioassay was conducted as described above except for the toxin concentration. Five toxin solutions (from 20.0 to 140.0 μg/ml with a step of 30.0 μg/ml) were used for each toxin and water served as the control. Six replicates were conducted for each concentration. The numbers of aphids were recorded 10 d after infestation to calculate the inhibitory index of rose aphid reproduction. The inhibitory index (II) was calculated using the formula as follows: II (%)=(n c−n t)/n c×100%, where n c is the control number, the mean number of aphids on control plants, and n t is the treated number, the mean number of aphids on toxin-treated plants.
2.5. Toxicity to aphids
To determine whether aphids were directly affected by the Alternaria toxin of strain 7484, its toxicity to aphids was tested under laboratory conditions using the slide-dipping method (commonly used for the determination of insecticidal activity) as described by Stribley et al. (1983). With a stereomicroscope and a wetted fine brush, 20 adults were affixed to double face scotch tape stuck tightly to side on the dorsal part and the slides were then dipped into the toxin solution for 10 s and the excess was blotted off with filter paper. Aphids were maintained at 22 °C and 70% RH.
The toxicity was tested at five different concentrations of 10.0 to 160.0 μg/ml and six replicates were undertaken for each concentration. The same method was used for the control treatment using water. Mean mortality percentage was recorded 24 h after treatment (all insects that responded to touching with the fine brush were considered to be alive) (Kazem and El-Shereif, 2010).
2.6. Collection of headspace volatiles
Volatiles of rose plants were collected from growing, potted rose plants (including control plants and rose plants treated with a solution of toxin of strain 7484 in the same way as described in Section 2.4). Growing conditions were the same as those used in Section 2.1. Sampling methods were as follows (Fraser et al., 2003):
Three potted plants were placed in a 100 cm×60 cm×60 cm glass tank with a removable glass top fitted with openings for air exchange. Charcoal-filtered air was pumped into the tank at 3 L/min, and air with volatiles in the headspace was withdrawn by vacuum at 1 L/min. Excess air escaped through a vent in the glass top. The vacuum line was connected to a glass collection cartridge containing 200 mg of Super Q polymer adsorbent (80/100 mesh, Alltech, Deerfield, IL, USA). Volatiles were collected for 24 h (16 h:8 h light:dark photoperiod regime) and extracted from the trap using 10 ml of dichloromethane per cartridge. Extracted samples were stored in glass vials at −80 °C until use. Four to six collections were made to obtain enough volatiles to compare olfactory responses of aphids to different host plants (treated and control plants).
2.7. Olfactory bioassays
The experiments were carried out using a Y-shaped glass tube olfactometer covered by a black cloth to test whether aphid adults were differentially attracted to the control rose leaves or the leaves from the rose plants treated with a toxin of strain 7484, and to the two volatiles collected from these two rose materials. The glass tube contained a stem that was 2 cm in diameter and 20 cm long with two arms that were 2 cm in diameter and 20 cm long (Rayamajhi et al., 2006).
In a first series of choice experiments two rose leaves were used. The two leaves were separately placed into 1-L glass bottles. Each arm of the Y-tube led to a 1-L glass bottle. In all cases, comparisons were made between treated rose leaves and control rose leaves, or clean air. Airflow was charcoal-filtered to remove organic contaminants and then passed through a band of needle valves attached to flow meters that regulated the volume of airflow to 300 ml/min. The airflow passed through the source odour bottles and then into either side of the Y-tube, converging at the base of the tube. The Y-tube was horizontally positioned. Aphid adults were individually released at the base of the Y-tube, and walked toward the source odours. Ten Y-tubes were used at the same time. In each test, 10 insects were placed separately into 10 Y-tubes. Ten replicates were undertaken (totally 100 insects were used to test their olfactory responses to each odour source). After 1 h, a positive response was recorded if an aphid went forward ca. 2 cm beyond either of the arm entrances. After an experiment was performed, the Y-tube and source odours were rotated 180° and a second bioassay was conducted. After two assays, the Y-tube was cleaned with 90% ethanol and placed into drying oven (100 °C) to dry for 15 min before the next assay.
In the following experiments, similar olfactory bioassays were conducted with the exception of odour sources: the two volatiles were collected from treated rose plants and control plants instead of leaves themselves (compared between treated and control volatiles or clean air). The two volatiles (0.1% diluted by deionized water) were separately placed into the 50-ml glass vials and then led into the Y-tube.
2.8. Statistical analysis
Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The toxicity of toxin on the rose leaves was analyzed using general linear models, in particular, analysis of variance (ANOVA). The toxicity to aphids was analyzed by Student’s t-test of independent samples. Olfactory responses of aphids to odour sources were compared using Pearson’s χ 2 test according to Fisher’s exact test (two-sided). Statistical differences were determined by Student-Newman-Keuls post-hoc tests.
3. Results
3.1. Toxicity to rose plants and resistance against aphids of Alternaria toxins
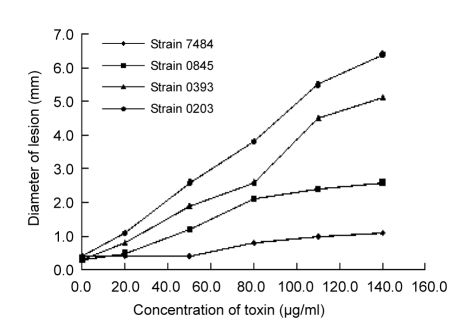
Alternaria toxins of four strains were tested by leaf-puncture assay at 20.0 to 140.0 μg/ml on the excised leaves of roses. All toxins caused phytotoxicity at all concentrations with the exception of 20.0 and 50.0 μg/ml of the toxin of strain 7484 when compared with the control (using only water, i.e., at 0.0 μg/ml in Fig. 1). Further, the toxicity increased with the increasing concentrations of toxins. At the highest concentration of 140.0 μg/ml of toxins from 0203, 0393, 0845, and 7484 strains, lesions reached 6.4, 5.1, 2.6, and 1.1 mm in diameters, respectively. The diameters of lesions produced by the toxin from strain 7484 were significantly lower than those produced by three other toxins at the same concentrations (P≤0.041) except 20.0 μg/ml (P≥0.088), whilst the diameters of lesions on leaves treated with the toxin from strain 7484 did not significantly differ between the first three concentrations (20.0, 50.0, and 80.0 μg/ml) and the control (P≥0.079) (P≤0.047 at 110.0 and 140.0 μg/ml compared to control).
Fig. 1.
Effect of concentrations of toxins from four strains (7484, 0845, 0393, and 0203) of Alternaria alternata on size of necrotic lesion on excised rose leaves
The concentration of 0.0 μg/ml served as control. Ten replicates were conducted for each concentration
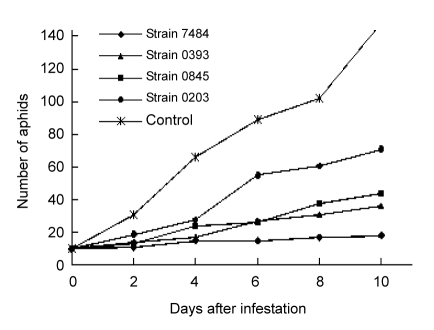
The effects of four toxins against aphid M. rosivorum were tested by spraying the toxin solutions on the potted rose plants (Fig. 2). The toxin of strain 7484 was used on the intact rose leaves (not punctured) at 50.0 μg/ml in this bioassay, and rose plants did not show any necrotic lesions or other symptoms, demonstrating that it was not harmful to rose plants under the condition tested. However, among these four toxins, 7484 possessed the strongest inhibitory effect on aphids. Ten days after inoculation, the mean number of total aphids was 18.2, having totally increased only 1.82-fold, while 3.63-, 4.41-, 7.13-, and 11.11-fold increases were recorded by calculating the mean aphid numbers in bioassays for toxins of strains 0393, 0845, 0203, and control (water), respectively.
Fig. 2.
Resistance to rose aphids induced by toxins from four strains (7484, 0845, 0393, and 0203) of Alternaria alternata
The solutions of four toxins (50.0 μg/ml) were evenly sprayed on rose plants (water served as control). Six replicates were conducted for each toxin
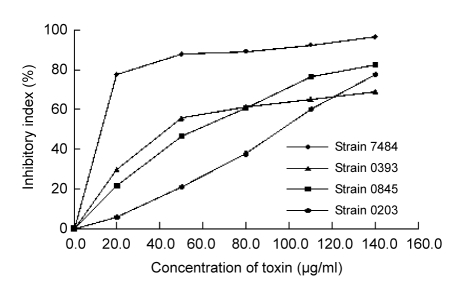
With increasing concentrations of toxins of four strains, the inhibitory effects of toxins on aphids showed a general increase. For the toxin of strain 7484, the inhibitory index had already reached 87.7% at 50.0 μg/ml that was not harmful to rose plants (P≤0.001, compared to control). The toxins of three other strains also showed significant inhibitory effects on aphids at higher concentrations (≥50.0 μg/ml) as shown in Fig. 3. However, at these concentrations the rose plants showed a large number of necrotic lesions.
Fig. 3.
Effect of concentrations of toxins on resistance to rose aphids induced by toxins from four strains (7484, 0845, 0393, and 0203) of Alternaria alternata
Four toxin solutions (from 20.0 to 140.0 μg/ml) were separately sprayed on rose plants (0.0 μg/ml, i.e., water, served as control). Six replicates were conducted for each concentration
3.2. Toxicity to aphids
The measurement of mean percentage mortality of aphids resulting from treatments with different concentrations of the toxin from strain 7484 using slide-dipping method is presented in Table 1. Although at higher concentrations, the mortalities were very low (≤2.19%), and did not increase with increasing concentrations when compared with the control treatment (water).
Table 1.
Measurement of toxicity of Alternaria alternata toxin extracted from strain 7484 to Macrosiphum rosivorum aphids with slide-dipping method
| Toxin concentration (μg/ml) | Mortality of aphids (%)a |
Pc | |
| Toxin | Controlb | ||
| 10.00 | 1.31±1.29 | 0.86±0.34 | 0.431 |
| 20.00 | 0.97±0.59 | 0.95±0.63 | 0.967 |
| 40.00 | 1.04±0.92 | 0.78±0.30 | 0.598 |
| 80.00 | 1.94±1.25 | 0.81±0.65 | 0.096 |
| 160.00 | 1.56±1.05 | 0.91±0.78 | 0.248 |
Six replicates were conducted for each concentration as well as for control, and data are expressed as mean±SD
Water served as control
Student’s t-test of the independent samples was used to compare means of mortality of aphids
3.3. Olfactory responses of aphids to volatiles
The adult aphids of M. rosivorum were significantly more attracted to the control rose leaves (90.5% vs. 9.5%) in olfactory bioassays (Table 2), while significantly less to the toxin-treated leaves (18.4% vs. 81.6%), as compared to clean air. When directly compared in the olfactory assays, the adults significantly preferred control leaves (95.1%) over toxin-treated leaves.
Table 2.
Comparison of olfactory responses of adult Macrosiphum rosivorum aphidsa
| Bioassay group | Odour source | Aphids with response |
Aphids without response (%) | |||
| Numberb | Percentage (%)c | Pearson’s χ2 value | Pd | |||
| Rose leavese | ||||||
| 1 | Untreated | 6.7±1.4 | 90.5 | 7.066 | 0.008 | 26.0 |
| Clean air | 0.7±0.2 | 9.5 | ||||
| 2 | Treated | 0.9±0.5 | 18.4 | 6.039 | 0.014 | 51.0 |
| Clean air | 4.0±0.7 | 81.6 | ||||
| 3 | Untreated | 7.7±2.1 | 95.1 | 7.529 | 0.006 | 19.0 |
| Treated | 0.4±0.1 | 4.9 | ||||
| Collected volatilesf | ||||||
| 1 | Untreated | 7.3±1.6 | 89.9 | 11.491 | 0.001 | 19.0 |
| Clean air | 0.8±0.4 | 10.1 | ||||
| 2 | Treated | 1.4±0.3 | 24.6 | 7.827 | 0.005 | 43.0 |
| Clean air | 4.3±1.2 | 75.4 | ||||
| 3 | Untreated | 8.4±1.7 | 93.8 | 15.274 | 0.000 | 10.0 |
| Treated | 0.6±0.2 | 6.2 | ||||
| Toxin and solvent | ||||||
| 1 | Toxin | 3.5±1.1 | 52.2 | 0.709 | 0.400 | 33.0 |
| Clean air | 3.2±0.8 | 47.8 | ||||
| 2 | Solvent | 2.9±0.9 | 45.1 | 0.406 | 0.524 | 36.0 |
| Clean air | 3.5±1.3 | 54.9 | ||||
There are ten randomly selected moths in a treatment (ten replicates)
The number of aphids is expressed as mean±SD
The aphids that did not respond to the treatment within testing time (1 h) were excluded when calculating the percentages of attracted or repelled aphids
Based on Fisher’s exact test (exact sig. (two-sided)) of Pearson’s χ 2 test when comparing the aphid number
Responses of aphids to volatiles emitted from leaves of the rose plants not treated with toxin solution (only water used) and from leaves of those treated with toxin solution, or clear air
Responses of aphids to volatiles collected from leaves of the rose plants not treated with toxin solution and from leaves of those treated with toxin solution, or clear air
Similarly, adults were significantly more attracted to the volatiles collected from the control rose plant materials using the headspace method (89.9%), but significantly less to the volatiles collected from the host plants treated with toxin of strain 7484 (24.6%), than to the empty vial. When comparing olfactory responses to these two volatiles, the adults were significantly more attracted to the control odour (93.8%).
No significant differences were observed when olfactory bioassays were compared between toxin itself and clean air, or between solvent (dichloromethane) and clean air (Table 2).
Among all bioassays, the mean number of insects that showed no response was the lowest in the bioassay directly comparing the two volatiles collected from the control roses and the roses treated by the toxin of strain 7484 (Table 2).
4. Discussion
It is well-known that the Alternaria toxins can be harmful to the plants (Berestetskiy, 2008). Our results showed that all crude toxins extracted from the culture filtrates of four strains (7484, 0845, 0393, and 0203) of A. alternata had phytotoxicity on the rose leaves at a higher concentration (>50.0 μg/ml). This is commonly due to plant responses induced by toxins (Kombrink and Schmelzer, 2001). As mentioned above, the virulence of toxins is related to host plant species and concentration of toxins applied (Berestetskiy, 2008). In addition, the toxicity of toxins of strain 7484 is the lowest among these four toxins. Especially at a lower concentration (≤50.0 μg/ml), the toxins of strain 7484 showed no bioactivity on excised rose leaves (Fig. 1).
Meanwhile, the toxin of strain 7484 possessed the strongest inhibitory effect on the reproduction of rose aphid M. rosivorum (Figs. 2 and 3) among these four toxins when they were sprayed on leaves of potted rose plants, wherein its inhibitory index had reached 87.7% at 50.0 μg/ml (Fig. 3). Furthermore, any infectious symptoms were not seen on leaves under the same conditions (Fig. 3). For toxins of strain 7484, the reduced toxicity to rose plants and increased resistance to aphids might be obtained after further isolation and purification.
Fugal toxins can induce plant’s changes of resistance-related metabolites, including different oligosaccharides, lipids, peptides, proteins, benzoquinones, and terpenoids. However, such compounds are commonly believed to enable plants to resist phytopathogen attack (Montesano et al., 2003). Some nonphytopathogenic Alternaria toxins have also been developed to control other phytopathogens (Chen et al., 2005). In addition, some Alternaria toxins were used for biocontrol of certain weeds (Wan et al., 2001). Furthermore, our results (Figs. 2 and 3) showed that Alternaria toxin had detrimental effects on the reproduction of aphids.
Not only may Alternaria fungus affect the behaviour and performance of insects on their shared host plant (Rostás and Hilker, 2002), but also its toxin often produces the same effects on insects and its host plants (Berestetskiy, 2008). In the present study, the results showed that Alternaria toxin could impact the aphid’s behaviour and performance (Figs. 2 and 3; Table 2). Thus, ecological cross-effects between this fungus and rose aphid on their shared host plant could also be deduced, having negative cross-talk between these two pest organisms.
Although the application of Alternaria toxin of strain 7484 on host plants had detrimental effects on aphids, we could show that this toxin itself was non-harmful to rose aphids, not having acute or contact toxicity, or insecticidal activity (Table 1). Therefore, the plant’s changes induced by the Alternaria toxins may be responsible for the strongest inhibitory effect on aphids (Figs. 2 and 3). Conversely, although previous researches on plant-mediated effects of fungal infection of host plant on insects were largely conducted, it is difficult to ascertain whether such effects were induced or not. Maybe some mycelia or the toxins produced by fungi still remained in the plants used in assays. It is also difficult to determine the toxicity of toxins in the plants on insects. Therefore, Alternaria toxin that was nontoxic to insects was used to test these induced effects on aphids in this paper. According to the obtained results (Figs. 2 and 3; Table 1), such plant-mediated inhibitory effect was induced by toxin. The rose plant was able to indirectly defend itself against attacks by herbivores, demonstrating that the resistance mechanism triggered by the Alternaria toxin in rose plant may also be used by plants to defend themselves against insects, which may be helpful in understanding the interactions between insect and fungus in the tripartite system consisting of fungus, plant, and insect. In such a tripartite system, toxins or its producers may have beneficial or detrimental effects, or no effects, on insects for different species of pathogens and insects. Such effects may be direct, plant-mediated or both. Further, the plant-mediated effects on insects may result from: (1) toxins themselves produced by fungi; (2) some plant’s chemicals induced by toxins. In the present study, the Alternaria toxin produced a detrimental influence on aphids by inducing the resistance-related changes of the rose plants.
In the meantime, the results from olfactory bioassays showed that the volatiles of the control and toxin-treated rose plants clearly affected the behaviour of the rose aphid M. rosivorum (Table 2). The adult aphids were significantly more attracted by volatiles emitted from the control potted rose plants, and significantly repelled by those from the toxin-treated plants, when comparisons were undertaken between these two rose leaves or clear air (P≤0.014). Likewise, similar results were obtained from the bioassays using volatiles collected from these two rose plant materials with the headspace method (P≤0.005), while no differences between clear air, toxin itself, and solvent had been shown in olfactory bioassays (P≥0.400). The adults similarly responded to the toxin, solvent, and clear air. However, they responded significantly differently to both volatiles. Therefore, based on these results (Figs. 2 and 3; Tables 1 and 2), the volatiles played an important role in selecting host plants. As abovementioned, fungi may induce the changes of plant volatiles so that insects are affected (Hammack, 2003). For instance, healthy Melaleuca quinquenervia leaves attracted more female Oxyops vitiosa weevils in olfactory bioassays when compared to the leaves infected with rust Puccinia psidii (Rayamajhi et al., 2006).
Insects can recognize their optimal host plants from an array of the fungus-infected plants according to the cues of volatiles and leave the infected host plants to search other plants as a result of some fungus-induced changes of plant volatiles (Rostás et al., 2003). So the plants may avoid being attacked by insects. In a no-choice situation, insects may reject feeding on the fungus-infected plants because of the changes of plant volatiles. So the development and reproduction of insects may be detrimentally affected by infection. In the present study, this was responsible for the lower increase of aphids on the toxin-treated rose plants when compared with that on the control plants (Figs. 2 and 3).
Moreover, the toxins used on the rose plants, instead of infection by fungi, may make plants to obtain the fungus-induced resistance to insects especially under controlled conditions, as shown in this study. We argue that the observed phenomenon represents SAR and/or ISR, because the toxin itself is not harmful to aphids (Table 1). So a proactive defense barrier (Zhang, 2003) in plants for controlling insect pests can be built to a certain extent, instead of desinsection after infestation. Such a biologically based strategy may be a new idea for insect control. However, this needs to remain a hypothesis without its toxicity test to animals and humans. Although a considerable number of toxins from pathogens are harmful to animals or humans, it is still possible to obtain some active and safe chemicals from pathogen-produced toxins. For example, some Alternaria toxins (or its analogues) not harmful to mammalian were reported by Abbas et al. (1995). Most microbial phytotoxins are biodegradable, water-soluble, and non-halogenated compounds. They are also more benign toxicologically and environmentally, compared to synthetic herbicides (Evidente et al., 2009). In most cases, these toxins were used as herbicides. However in this study, the results showed that the Alternaria toxin was non-harmful to rose plants and aphids, and possessed significant plant-mediated inhibitory effects on aphids in the same time. In addition, our results suggested that some active chemicals for controlling insects might be found from the plant’s volatiles induced by toxin. Further studies are needed to elucidate the active chemicals in Alternaria toxins or toxin-induced volatiles of plants and the physiological changes in rose plants when treated with Alternaria toxins.
Acknowledgments
The authors would like to thank Prof. Yu-hui CHEN, College of Life Science, Southwest Forestry University, China, who helped in the isolation and identification of Alternaria fungi.
Footnotes
Project supported by the Scientific and Technological Department (No. 2008CD140) and the Education Department (No. 08z0027) of Yunnan Province in China
References
- 1.Abbas HK, Vesonder RF, Boyette CD, Peterson SW. Phytotoxicity of AAL-toxin and other compounds produced by Alternaria alternata to jimsonweed (Datura stramonium) Can J Bot. 1993;71(1):155–160. doi: 10.1139/b93-017. [DOI] [Google Scholar]
- 2.Abbas HK, Tanaka T, Shier WT. Biological activities of synthetic analogues of Alternaria alternata toxin (AAL-toxin) and fumonisin in plant and mammalian cell cultures. Phytochemistry. 1995;40(6):1681–1689. doi: 10.1016/0031-9422(95)00470-R. [DOI] [PubMed] [Google Scholar]
- 3.Ballhorn DJ. Constraints of simultaneous resistance to a fungal pathogen and an insect herbivore in lima bean (Phaseolus lunatus L.) J Chem Ecol. 2011;37(2):141–144. doi: 10.1007/s10886-010-9905-0. [DOI] [PubMed] [Google Scholar]
- 4.Ballhorn DJ, Pietrowski A, Lieberei R. Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean (Phaseolus lunatus L.) J Ecol. 2010;98(1):226–236. doi: 10.1111/j.1365-2745.2009.01591.x. [DOI] [Google Scholar]
- 5.Berestetskiy AO. A review of fungal phytotoxins: from basic studies to practical use. Appl Biochem Microbiol. 2008;44(5):453–465. doi: 10.1134/S0003683808050013. [DOI] [PubMed] [Google Scholar]
- 6.Bobylev MM, Bobyleva LI, Strobel GA. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa) J Agric Food Chem. 1996;44(12):3960–3964. doi: 10.1021/jf960091c. [DOI] [Google Scholar]
- 7.Chelkowski J, Visconti A. Alternaria: Biology, Plant Diseases and Metabolites. Amsterdam, the Netherlands: Elsevier; 1992. pp. 449–541. [Google Scholar]
- 8.Chen S, Dai X, Qiang S, Tang Y. Effect of a nonhost-selective toxin from Alternaria alternata on chloroplast-electron transfer activity in Eupatorium adenophorum . Plant Pathol. 2005;54(5):671–677. doi: 10.1111/j.1365-3059.2005.01249.x. [DOI] [Google Scholar]
- 9.Cipollini D, Enright S, Traw MB, et al. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol Ecol. 2004;13(6):1643–1653. doi: 10.1111/j.1365-294X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- 10.Egusa M, Akamatsu H, Tsuge T, Otani H, Kodama M. Induced resistance in tomato plants to the toxin-dependent necrotrophic pathogen Alternaria alternata . Physiol Mol Plant Pathol. 2008;73(4-5):67–77. doi: 10.1016/j.pmpp.2009.02.001. [DOI] [Google Scholar]
- 11.Evidente A, Punzo B, Andolfi A, Berestetskiy A, Motta A. Alternethanoxins A and B, polycyclic ethanones produced by Alternaria sonchi, potential mycoherbicides for Sonchus arvensis biocontrol. J Agric Food Chem. 2009;57(15):6656–6660. doi: 10.1021/jf9014944. [DOI] [PubMed] [Google Scholar]
- 12.Fraser AM, Mechaber WL, Hildebrand JG. Electroantennographic and behavioural responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J Chem Ecol. 2003;29(8):1813–1833. doi: 10.1023/A:1024898127549. [DOI] [PubMed] [Google Scholar]
- 13.Hammack L. Volatile semiochemical impact on trapping and distribution in maize of northern and western corn rootworm beetles (Coleoptera: Chrysomelidae) Agric Forest Entomol. 2003;5(2):113–122. doi: 10.1046/j.1461-9563.2003.00171.x. [DOI] [Google Scholar]
- 14.Hammerschmidt R. Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol. 1999;55(2):77–85. doi: 10.1006/pmpp.1999.0215. [DOI] [Google Scholar]
- 15.Hatcher PE, Paul ND, Ayres PG, Whittaker JB. Interactions between Rumex spp., herbivore and a rust fungus: the effect of Uromyces rumicis infection on leaf nutritional quality. Funct Ecol. 1995;9(1):97–105. doi: 10.2307/2390095. [DOI] [Google Scholar]
- 16.Heath MC, Skalamera D. Cellular interactions between plants and biotrophic fungal parasites. Adv Bot Res. 1997;24:195–225. doi: 10.1016/S0065-2296(08)60074-9. [DOI] [Google Scholar]
- 17.Heil M, Bostock R. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann Bot. 2002;89(5):503–512. doi: 10.1093/aob/mcf076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaul S, Wani M, Dhar KL, Dhar MK. Production and GC-MS trace analysis of methyl eugenol from endophytic isolate of Alternaria from rose. Ann Microbiol. 2008;58(3):443–445. doi: 10.1007/BF03175541. [DOI] [Google Scholar]
- 19.Kazem MGT, El-Shereif SAEHN. Toxic effect of capsicum and garlic xylene extracts in toxicity of boiled linseed oil formulations against some piercing sucking cotton pests. American-Eurasian J Agric Environ Sci. 2010;8(4):390–396. [Google Scholar]
- 20.Kombrink E, Schmelzer E. The hypersensitive response and its role in local and systemic disease resistance. Eur J Plant Pathol. 2001;107(1):69–78. doi: 10.1023/A:1008736629717. [DOI] [Google Scholar]
- 21.Montesano M, Brader G, Palva ET. Pathogen derived elicitors: searching for receptors in plants. Mol Plant Pathol. 2003;4(1):73–79. doi: 10.1046/j.1364-3703.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Stierle A, Strobel GA. Metabolism of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa) Phytochemistry. 1993;35(1):101–106. doi: 10.1016/S0031-9422(00)90516-8. [DOI] [Google Scholar]
- 23.Qiang S, Wang L, Wei R, Zhou B, Chen S, Zhu Y, Dong Y, An C. Bioassay of the herbicidal activity of AAC-toxin produced by Alternaria alternata isolated from Ageratina adenophora . Weed Technol. 2010;24(2):197–201. doi: 10.1614/WT-D-09-00016.1. [DOI] [Google Scholar]
- 24.Rayamajhi MB, Van TK, Pratt PD, Center TD. Interactive association between Puccinia psidii and Oxyops vitiosa, two introduced natural enemies of Melaleuca quinquenervia in Florida. Biol Control. 2006;37(1):56–67. doi: 10.1016/j.biocontrol.2005.10.013. [DOI] [Google Scholar]
- 25.Rostás M, Hilker M. Asymmetric plant-mediated cross-effects between a herbivorous insect and a phytopathogenic fungus. Agric Forest Entomol. 2002;4(3):223–231. doi: 10.1046/j.1461-9563.2002.00147.x. [DOI] [Google Scholar]
- 26.Rostás M, Simon M, Hilker M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic Appl Ecol. 2003;4(1):43–62. doi: 10.1078/1439-1791-00132. [DOI] [Google Scholar]
- 27.Strange RN. Introduction to Plant Pathology. Chichester, UK: John Wiley & Sons; 2003. [Google Scholar]
- 28.Stribley MF, Moores GD, Devonshire AL, Sawick RM. Application of the FAO-recommended method for detecting insecticide resistance in Aphis jabae Scopoli, Sitobion avenae (F.), Metopolophium dirhodum (Walker) and Rhopalosiphum padi (L.) (Hemiptera: Aphididae) Bull Entomol Res. 1983;73:107–115. doi: 10.1017/S0007485300013845. [DOI] [Google Scholar]
- 29.Strobel G, Kenfield D, Bunkers G, Sugawara F, Clardy J. Phytotoxins as potential herbicides. Cell Mol Life Sci. 1991;47(8):819–826. doi: 10.1007/BF01922462. [DOI] [Google Scholar]
- 30.Švábová L, Lebeda A. In vitro selection for improved plant resistance to toxin-producing pathogens. J Phytopathol. 2005;153(1):52–64. doi: 10.1111/j.1439-0434.2004.00928.x. [DOI] [Google Scholar]
- 31.Wan ZX, Qiang S, Xu SC, Shen ZG, Dong YF. Culture conditions for production of phytotoxin by Alternaria alternata and plant range of toxicity. Chin J Biol Control. 2001;17(3):10–15. [Google Scholar]
- 32.Zhang LH. Quorum quenching and proactive host defense. Trends Plant Sci. 2003;8(5):238–244. doi: 10.1016/S1360-1385(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Sakai K. Multiple signalling pathways mediate fungal elicitor-induced β-thujaplicin biosynthesis in Cupressus lusitanica cell cultures. J Exp Bot. 2003;54(383):647–656. doi: 10.1093/jxb/erg062. [DOI] [PubMed] [Google Scholar]