Abstract
Although the rate of the sudden infant death syndrome (SIDS) has decreased over the last two decades, medical examiners and coroners are increasingly unwilling to use the SIDS diagnosis, particularly when there is an unsafe sleeping environment that might pose a risk for asphyxia. In order to reliably classify the infant deaths studied in a research setting in the mixed ancestory population in Cape Town, South Africa, we tested a classification system devised by us that incorporates the uncertainty of asphyxial risks at an infant death scene. We classified sudden infant deaths as: A) SIDS (where only a trivial potential for an overt asphyxial event existed); B) Unclassified—Possibly Asphyxial-Related (when any potential for an asphyxial death existed); C) Unclassified—Non-Asphyxial-Related (e.g., hyperthermia); D) Unclassified—No autopsy and/or death scene investigation; and E) Known Cause of Death. Ten infant deaths were classified according to the proposed schema as: SIDS, n = 2; Unclassified—Possibly Asphyxial-Related, n = 4; and Known Cause, n = 4. A conventional schema categorized the deaths as 6 cases, SIDS, and 4 cases, Known Cause, indicating that 4/6 (67%) of deaths previously classified as SIDS are considered related importantly to asphyxia and warrant their own subgroup. This new classification schema applies a simpler, more qualitative approach to asphyxial risk in infant deaths. It also allows us to test hypotheses about the role of asphyxia in sudden infant deaths, such as in brainstem defects in a range of asphyxial challenges.
Keywords: Bed sharing, Death scene investigation, Overlaying, Sudden infant death syndrome, Sudden unexpected death in infancy, Bedcovers, Sleep environment, Autopsy
Introduction
Sudden infant death syndrome has been defined by an expert panel of pediatric and forensic pathologists as the sudden, unexpected death of an infant less than 1 year of age with onset of the fatal episode apparently occurring during sleep and that remains unexplained after a thorough investigation, including performance of a complete autopsy and review of the circumstances of death and clinical history [1]. After the initiation of the national infant safe sleep campaign in the United States in the 1980s and 1990s, there has been over a 50% decline in the overall rate of deaths attributed to SIDS [2–4]. Yet, during this same time-frame, the diagnosis of SIDS has come under increasing scrutiny by death investigators (coroners and medical examiners) [5, 6]. Greater use of infant death scene investigation and doll re-enactment has raised the concern that deaths that in the past might have been classified as SIDS may actually represent asphyxial deaths. Indeed, a recent study of 206 sudden infant deaths in Detroit, Michigan, reported circumstances consistent with asphyxia in approximately 85% of such deaths [7]. These circumstances included prone or face-down sleep position, soft bedding, bed sharing, overlaying, sofa deaths, and face-covering. As the declining rate of SIDS deaths has plateaued over the last few years there has been a corresponding increase in the infants whose cause and manner of death has been listed as unknown or unclassified [2, 5, 6]. The relatively stable infant death mortality over the last several years suggests that a diagnostic shift may be occurring away from the diagnosis of SIDS towards unknown/unclassified [2, 5, 6]. In addition, we believe that there may be an increasing willingness to classify some deaths as true “positional asphyxia” or “suffocation” [5]. Although infant death scene investigations have shown that potential asphyxial risks are the most common reason for abandoning a SIDS diagnosis in favor of an unknown/unclassified cause of death, other risk factors such as hyperthermia and/or other autonomic dys-regulation may also be a factor [8–10]. Nevertheless, we feel that the potential for an external asphyxial stressor (such as suffocation, overlaying, or re-breathing) needs to be included in any classification schema of sudden and unexpected infant death.
Taken together, the current epidemiologic, forensic, and research information prompted us to devise a classification schema for sudden unexpected infant deaths (SUID) that takes into account the potential lethal role of asphyxia in the pathogenesis of these deaths, and which translates to the everyday practice of forensic death investigators caught in the dilemma of differentiating lethal asphyxia from sub-lethal asphyxia in vulnerable infants. The proposed classification schema is based upon the review of the clinical history, autopsy, and death scene investigation but places a special emphasis upon the possible role of asphyxia. We define asphyxia based upon subjective observations at the scene that include physical evidence or strong suspicion of occlusion of the airway (e.g., suffocation in soft bedding), mechanical impedance of respiration (e.g., overlaying), and/or potential rebreathing of exhaled gases in the face-down position. Asphyxia is severe hypoxia and hypercapnia combined, with hypoxia defined as oxygen levels in the lung, blood, or other tissues below the normal level of sea level, and hypercapnia as arterial carbon dioxide levels above 40 mmHg. Of note, postmortem measurements of blood oxygen and carbon dioxide levels are unreliable, and the concentration of exhaled gases at the scene investigation is not necessarily a meaningful reflection of the microenvironment of the infant's face at the time of death; moreover, there is no established biochemical and/or tissue marker of asphyxia for diagnostic use in infants at autopsy (see below). Thus, the estimation of the role of asphyxia is typically based upon the judgment and experience of the forensic pathologist/investigator. In the following study, we developed and tested a classification schema in a high risk SIDS (as defined in the reference) population, i.e., the mixed ancestry population of Cape Town, South Africa in whom the SIDS rate is among the highest in the world, i.e., 3.8/1000 [11]. The Division of Forensic Medicine and Pathology at the University of Stellenbosch, as part of the National Forensic Pathology Service (South Africa), investigated 10 SUID deaths [12] as a feasibility sample that is the basis of the classification schema devised by us.
Materials and methods
Development of classification schema
We propose classifying SUID deaths into one of five categories: Group A) SIDS (where only a trivial potential for an overt asphyxial event existed); Group B) Unclassified—Possibly Asphyxial-Related (when any potential for an asphyxial death existed); Group C) Unclassified—Non-Asphyxial-Related (e.g., hyperthermia); Group D) Unclassified—No autopsy and/or death scene investigation; and Group E) Known Cause of Death (Table 1). Cases in Group A meet the criteria of the standard definition of SIDS of the sudden and unexpected, sleep-related death of an infant <12 months of age that is unexplained by a complete autopsy, death scene investigation and medical record review [1]. In SIDS, we propose that the circumstances of mild asphyxia (e.g., prone sleep position on a firm surface) may or may not be present (Fig. 1). Group B is used whenever a death is potentially due to significantly soft bedding (e.g., quilts, multiple blankets, and soft pillows), there is an adjacent adult bed-sharer, or a prone sleeping infant's face is found face-down in a potential re-breathing environment creating a possibility of asphyxia (Fig. 2). Despite the strong circumstances of asphyxia, their contribution to death cannot be proven today by current methods. In Group C, the cause of death is uncertain but there are no circumstances of asphyxia. Such causes of death include hyper- or hypothermia, or an underlying natural disease of unclear significance relative to the pathogenesis of death, e.g., mild infection. Cases in which there is no autopsy and/or death scene investigation are classified as Group D, in line with current thinking that the proper classification of a case a requires complete autopsy and/or death scene investigation. Group E includes all known (established) causes of death, including severe infection, lethal congenital malformations, and inborn errors of metabolism. Unequivocal or intentional trauma is also included, notably cases of definitive overlaying (Fig. 3) or intentional suffocation admitted by the parent or caretaker.
Table 1.
Proposed classification schema for sudden and unexpected infant death
| A. SIDS: Sudden and unexpected death of an infant <12 months of age that is unexplained by a compete autopsy, death scene investigation and medical record reviewa |
| B. Unclassified—Possibly Asphyxial-Related: implies a possible asphyxiating environmentb |
| C. Unclassified—Non-asphyxial-Relatedc |
| D. Unclassified—No autopsy and/or death scene investigation |
| E. Known cause of death |
Circumstances of no or at most mild asphyxia, e.g. prone sleeping on a firm surface
Strong circumstances of asphyxia such as adult bed-sharing, soft bedding, or potential re-breathing
Cause of death uncertain, but no circumstances of asphyxia (e.g., hyper- or hypothermia, underlying natural disease of unclear significance)
Fig. 1.
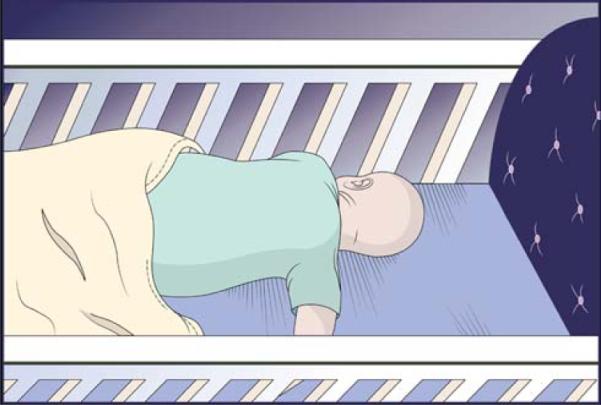
The cause of death in this infant is classified as Group A, SIDS. The circumstances of mild asphyxia may or may not be present. Here the infant's head is turned to the side. He is on a firm mattress in a crib with no pillow
Fig. 2.
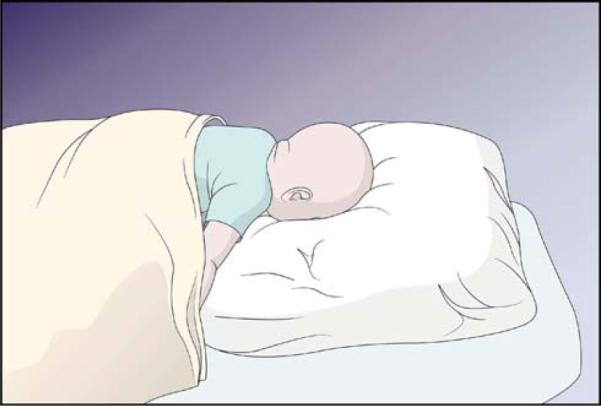
The cause of death in this infant is classified as Group B, Unclassified Possibly Asphyxial-Related when any potential for an asphyxial death exists. The infant is sleeping in an adult bed with soft bedding and her head is found-face down in that bedding
Fig. 3.

The cause of death is classified as Group E, Known cause of death, due to accidental asphyxiation. The large adult, compromised by alcohol, is completely overlaying the body of the infant
Analysis of the proposed classification schema
In accordance with standardized protocols, a review of the clinical history and complete autopsy, including bacterial and viral studies (all cases), HIV testing (all cases), and metabolic studies as indicated by clinical history and/or hepatic steatosis, was performed in each case. In addition, a death scene investigation was done by trained death scene investigators from the Division of Forensic Medicine and Pathology at the University of Stellenbosch following the format set forth in the Centers for Disease Control (CDC) SUID reporting form [13]. The death scene investigation with photography was performed at the first visit. The body and documentation were presented to the forensic pathologist in charge as autopsy is statutorily mandated in all cases of sudden unexpected deaths in South Africa. This study received Institutional Review Board approval; informed consent from the mother and/or father for research was obtained in each case.
Comparison of proposed classification schema to standard schema
We compared our classification schema to the San Diego schema in which the deaths are separately classified as unclassified sudden infant death [1]. In the latter category, alternative diagnoses of natural or unnatural conditions are equivocal, including cases for which autopsies are not performed.
Results
Analysis of the proposed classification schema
The authors of this study are part of an ongoing international study of infant and stillbirth deaths, including in Cape Town, South Africa. For the purpose of an initial test of the classification schema presented in this article, we analyzed the first 10 cases of SUID to present to the study from the mixed ancestory population in Cape Town (Table 2). All cases had a complete autopsy and death scene investigation. The median postnatal age of all cases was 2 postnatal months, with a range from 0.5–7 months. Seventy-one percent of the cases (5/7) were born prematurely (<37 gestational weeks at birth), and 50% (5/10) were male. All cases were found in the early morning after a sleep period and the deaths were associated with sleep. All infants were bed-sharing at the time of death (Table 2). Of these cases, 67% (6/9) were found prone. We classified the cases as: Group A, SIDS, n = 2; Group B, Unclassified—Possibly Asphyxia-Related, n = 4; Group C, Unclassified—Non-asphyxial-Related, n = 0; Group D, No autopsy and/or death scene investigation, n = 0; Group E, Known Cause of death, n = 4 (Table 2). The 4 known causes of death represented one case each of: 1) disseminated tuberculosis with severe hilar adenopathy and airway obstruction; 2) disseminated cytomegalovirus with myocarditis and pneumonitis; 3) E. coli. pneumonia; and 4) hepatic hemangioendothelioma with right heart failure. In none of these 4 cases was the diagnosis suspected clinically. The clinical presentation of all of these established causes mimicked SIDS with mild or no apparent clinical prodrome, as well as sleep-related death in bed sharing environments. In all of these cases, death was discovered after the night sleep period. The difference from SIDS in these four cases was the determination at autopsy of unequivocal and severe lethal disease.
Table 2.
Clinicopathologic features of 10 SUID cases classified according to the proposed schema
| Feature | Group A | Group B | Group C | Group D | Group E |
|---|---|---|---|---|---|
| SIDS n = 2 | Possible asphyxia n = 4 | Not possible asphyxia n = 0 | No autopsy or DSI n = 0 | Known cause n = 4 | |
| Gestational age | 36 weeks | 33 weeks | No cases | No cases | 37 weeks |
| Median, range | (35–37 weeks) | (32–34 weeks) | (34–40 weeks) | ||
| Postnatal age | 2 months | 2 months | No cases | No cases | 2 months |
| Median, range | (1–3 months) | (0.5–7 months) | (1–4 months) | ||
| Preterm | 50% (1/2) | 100% (3/3) | No cases | No cases | 50% (1/2) |
| Male | 50% (0/2) | 50% (2/4) | No cases | No cases | 75% (3/4) |
| Found after sleep period in morning | 100% (2/2) | 100% (4/4) | No cases | No cases | 100% (4/4) |
| Bed sharing | 2/2 (100%) | 100% (4/4) | No cases | No cases | 100% (4/4) |
| Number (range) of bed sharers | 1 (1) | 1.5 (1–3) | No cases | 1.5 (1–4) | |
| Soft bedding | 0% (0/2) | 50% (2/4) | No cases | No cases | 25% (1/4) |
| Found face-down | 0% (0/2) | 0% (0/4) | No cases | No cases | 0% (0/3) |
| Found prone | 100% (2/2) | 50% (2/4)a | No cases | No cases | 67% (2/3) |
| Autopsy findings | Non-significant | Non-significant | No cases | No cases | Diagnostic |
Legend: There were no cases classified as Group C or Group E. Information is not available for each variable in every case DSI death scene investigation
Two infants were found prone, the other two found supine
The basis of the classification of 4 of 6 unexplained deaths as Group B was based upon bed sharing with adults, very soft bedding, and the lack of a known cause of death (Table 2). Of note, all of the 6 infants in the category were born prematurely. Although bed sharing in large adult beds was seen in all deaths in this study, including the four with known causes (Table 2), the difference between the classification of Group A versus Group B was based upon the subjective interpretation of the forensic pathologist concerning the potential for a causal role of asphyxia. Two of the six unexplained deaths were classified as SIDS because bed-sharing was not thought to play a significant role in the pathogenesis of death. In one of these cases, the bed sharer was another small child; the second child was sharing the bed with an adult in a significantly removed area of a large bed as to not have posed a risk to the infant. In all of the four cases classified as Group B, the infant was bed sharing with one or more adults and/or one or more children and with compounding circumstances that set the circumstances apart from those of the two SIDS infants. In one of these four cases, the infant was born prematurely at 33 gestational weeks and died two weeks later in a bed sharing environment (with mother and two brothers); these circumstances were considered to cause potential asphyxia because of the possibility of overlaying of the very small infant (2,400 grams at autopsy). In two additional cases, over-wrapping in particularly was considered a compounding factor. Using the standard classification schema of Krous et al. [1], the South African death investigators in this study (SW, JD) classified the ten cases reported here as SIDS, n = 6; Unclassified—none, and Known Cause of Death, 4. The main difference between the classification schema is that 2/6 (33%) unexplained cases were considered as SIDS in the proposed schema, compared to 6/6 (100%) in the standard schema, and 4/6 (67%) of the unexplained cases were considered possibly related to asphyxia, whereas this option is not stipulated in reference current schema [1].
Discussion
The new classification schema proposed here does not eliminate ambiguity in diagnosing SUID deaths, but rather, makes that ambiguity a part of the classification process. From our collective experience in death investigation, we believe that differentiating between a true asphyxial death (such as overlaying or suffocation by a plastic bag) and undetermined/unclassified death is rarely problematic in as much as an asphyxial or suffocation diagnosis commonly requires a considerable amount of certainty. As it has in the past, ambiguity with this new schema exists primarily between the diagnosis of Group A. SIDS and Group B. Unclassified—Possibly Asphyxial-Related. To minimize that ambiguity in the proposed schema, Group B is used whenever the possibility that significantly soft bedding (e.g., quilts and multiple blankets), an adult bed-sharer (especially in small, preterm infants), or a prone sleeping infant's found face down in the bedding in a potential re-breathing environment could have caused the death. Using a standard schema [1], the South African death investigators in this study classified the 10 SUDI cases reported here as 6 SIDS and 4 Known Causes of Death. The differences between the two classification systems reflects the differences between accepting any possibility of asphyxia in the new classification system versus requiring a higher (albeit unquantified) asphyxial risk to classify a death as unclassified/undetermined in the standard classification schema. The new classification system relieves the death investigator from having to at least semi-quantify the asphyxial risk and simply state that a potentially lethal asphyxial risk is present. The utilization of this new classification schema appears to reduce the number of cases designated as SIDS compared to other more commonly used classifications methods, as seen in our pilot study in which there were 2/10 (20%) instead of 6/10 (60%) SIDS cases. Nevertheless, we believe this proposed system is helpful because it reflects the reality of the uncertainty of day-to-day practice and helps focus basic research towards specific underlying mechanisms.
A major factor in classifying cases as Group B was the subjective consideration of the role of asphyxia in bed sharing. The risk of bed sharing is considered to be related to asphyxia from overlaying from a body part of an adult or older sibling and/or adult soft bedding, e.g., pillows [14]. The incidence of bed sharing in the mixed ancestory population in the Cape Flat is currently unknown, but this very small study raises the question that it may be over a majority of cases. In industrialized countries across socioeconomic groups bed sharing persists, in part because it is perceived as beneficial to child care by increasing parental bonding and facilitating breast feeding through the night [15]. Its high incidence among lower socio-economic groups, however, reflects at least in part lack of funds and space (crowding) for cribs [16].
The classification schema here is heavily based upon the premise that asphyxia plays a major, albeit not exclusive, role in the pathogenesis of sudden infant death. This premise is based largely upon forensic observations at the scene in which potential asphyxiating situations are commonly found, e.g., upper airway compression in the face-down position, soft bedding, and bed sharing [17, 18]. There is also experimental evidence that the prone (face-down) position leads to rebreathing exhaled gases, particularly in soft bedding, which is followed in turn by asphyxia [19]. This asphyxia is postulated to lead to death if effective life-saving mechanisms (e.g., autoresuscitation and/or arousal) do not intervene [20]. Yet, other terminal pathways to SIDS may occur that involve thermoregulatory and cardiovascular mechanisms as opposed to respiratory mechanisms [8–10]. Abnormal cardiovascular control is suggested by subclinical evidence of abnormal heart rate and heart rate variability in infants who subsequently die of SIDS and by tracings at the time of death in SIDS infants that indicated episodic tachycardia and/or bradycardia in the terminal phase [21–23]. Yet, respiratory and autonomic terminal pathways are not mutually exclusive of one another as they are sub-served by the same brainstem and other neuronal populations and are heavily integrated [24]. The possibility that SIDS infants experience repeated asphyxial episodes prior to death is supported by reports in SIDS cases of “hypoxic tissue markers”, i.e., hyperplasia of pulmonary neuroendocrine cells [25], reduced hypoxanthine oxidase in the vitreous humor [26], and elevated vascular endothelial growth factor in the cerebrospinal fluid [27], and brainstem gliosis [28, 29]. The possibility that these biochemical and tissue markers of hypoxia be used in routine forensic practice to “measure” asphyxia at or around the time of death needs to be determined through further research.
The significance of the possible diagnostic shift in SIDS is important not only for the epidemiologists and nosologists, but also for those researchers studying the etiology and basic pathogenesis of SUID, of which SIDS is a part. One leading hypothesis is that an important subset of SIDS is due to a developmental disorder of the brainstem which leads to impaired protective responses to life-threatening stressors during infant sleep such as asphyxia, hypercarbia, and hypoxia [24]. The focus upon the brainstem is because it plays a critical protective role in the response to asphyxia and other stressors; brainstem-mediated defense systems involve chemosensitivity to carbon dioxide and chemosensitivity to oxygen, autoresuscitation, and arousal with head lifting and turning to escape asphyxiating microenvironments by gaining fresh air [24]. Evidence is mounting that suggests the majority of sudden infant deaths are associated with intrinsic brainstem defects in markers of the neurotransmitter serotonin (5-HT) and in regions of the medulla oblongata that are critical to protective responses to sleep-related asphyxia [24, 30]. In the proposed classification schema, a diagnosis of SIDS implies a vulnerable infant, perhaps due to a brainstem defect, in whom even a seemingly trivial asphyxial stressor triggers death due to an inability to mount protective responses to asphyxia (Fig. 4). At the other end of the spectrum is the normal infant without a brainstem or other underlying vulnerability in whom the degree of asphyxia is so severe that it is lethal under all circumstances; of course, at this end of the spectrum, infants with a brainstem defect will also die (Fig. 4).
Fig. 4.

SIDS is conceptionalized as a disorder of protective asphyxial challenge responses in which the vulnerable infant, due to brainstem pathology in neuronal networks that mediate responses to asphyxia, is compromised and even a mild asphyxial challenge causes death. At the other end of the spectrum, the asphyxial challenge Is so severe, that all infants, with or without underlying brainstem pathology, die
In conclusion, this pilot study demonstrates the feasibility of the proposed classification schema in sudden and unexplained infant death. Although the schema is feasible, a larger sample size (study in progress) may well contain more problematic cases of greater difficulty to classify. The small sample size in this study likewise precludes conclusions regarding the true incidence of either unexplained or explained deaths in the larger population of infant deaths among the mixed ancestory in Cape Town. At best, we can only suggest that implementation of this schema would result in a reduction in the number of deaths classified as SIDS. Given the caveat of the sample size, however, the observed results give an important first glimpse into what may be causing infant deaths among the Cape Town mixed ancestry population. It appears that the trained death scene investigators can, albeit subjectively, easily recognize potential asphyxial challenges at a death scene that `could' have caused an infant death. We do not believe that it is either necessary or advisable to quantify these subjective observations between different parties using the classification schema. In addition to the ease and simplicity of application, the value of our proposed classification system is that it allows for testing of the hypothesis that brainstem and other vulnerabilities cause SIDS in infants in whom minor or no significant external asphyxial element is present. Further, this classification schema allows for study of the possibility that brainstem and other abnormalities may contribute to the Group B. “Unclassified-Possibly Asphyxia-Related” deaths by postulating that these abnormalities may prevent infants from being able to extricate themselves from a mild-to-moderate asphyxiating environment. Finally, this schema is simple and classifies deaths in a manner directly transferable from a research format to a working diagnosis used by many of the medical examiners/coroners throughout the world.
Key points
There is evidence that death investigators are increasingly unwilling to diagnose SIDS when there is a possible asphyxial component in an infant's death.
A classification scheme of Sudden Unexpected Infant Deaths (SUID) is presented which incorporates the role of asphyxia (bed sharing, soft bedding, rebreathing) that is often found at infant death scenes using the designator of `Unknown—possibly asphyxia related.'
Using the designation of `Unknown—possibly asphyxia related' in the classification of SUID appears to reduce the incidence of SIDS in a given population.
Allowing asphyxia to be a part of classifying SUID may correlate with seritonergic brainstem defects previously described in SIDS.
Acknowledgments
We appreciate the help of Mr. Richard A. Belliveau in manuscript preparation. Drs. Eugene E. Nattie and Henry F. Krous provided critical reviews.
References
- 1.Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114(1):234–8. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Hauck FR, Tanabe KO. International trends in sudden infant death syndrome: stablization of rates requires further action. Pediatrics. 2008;122:660–6. doi: 10.1542/peds.2007-0135. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell EA, Brunt JM, Everard C. Reduction in mortality from sudden infant death syndrome in New Zealand: 1986-92. Arch Dis Child. 1994;70(4):291–4. doi: 10.1136/adc.70.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal SM. Sudden infant death syndrome in South Australia 1968-97. Part I: changes over time. J Pediatr Child Health. 2000;36(6):540–7. doi: 10.1046/j.1440-1754.2000.00563.x. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163(8):762–9. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 6.The American Academy of Pediatrics The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116(5):1245–55. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- 7.Pasquale-Styles MA, Tackitt PL, Schmidt CJ. Infant death scene investigation and the assessment of potential risk factors for asphyxia: a review of 209 sudden unexpected infant deaths. J Forensic Sci. 2007;52(4):924–9. doi: 10.1111/j.1556-4029.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams SM, Mitchell EA, Stewart AW, Taylor BJ. Temperature and the sudden infant death syndrome. Pediatr Perinat Epidemiol. 1996;10(2):136–49. doi: 10.1111/j.1365-3016.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 9.Fleming PJ, Levine MR, Azaz Y, Wigfield R, Stewart AJ. Interactions between thermoregulation and the control of respiration in infants: possible relationship to sudden infant death. Acta Pediatr Suppl. 1993;82(Suppl 389):57–9. doi: 10.1111/j.1651-2227.1993.tb12878.x. [DOI] [PubMed] [Google Scholar]
- 10.Harper RM. Autonomic control during sleep and risk for sudden death in infancy. Arch Ital Biol. 2001;139:185–94. [PubMed] [Google Scholar]
- 11.Molteno CD, Ress E, Kibel MA. Early childhood mortality in Cape Town. S Afr Med J. 1989;75:570–4. [PubMed] [Google Scholar]
- 12.Dempers JJ, Folkerth RD, Kinney HC, Odendaal H, Sens MA, Randall BB, Wadee SA. The institution of a standardized SIDS investigation protocol in South Africa: feasibility study of 11 cases in the Western Cape. International SIDS Meeting; Portsmouth, England. Jun, 2008. Getting citation. [Google Scholar]
- 13.CDC for Disease Control and Prevention: Guidelines for death scene investigation of sudden, unexplained infant deaths: Recommendation of the interagency panel on sudden infant death scene investigation. MMWR. 1996;45(RR-10):1–6. [Google Scholar]
- 14.Thach BT. Tragic and sudden death. Potential and proven mechanisms causing sudden infant death syndrome. EMBO Rep. 2008;9(2):114–8. doi: 10.1038/sj.embor.7401163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna JJ, Ball HL, Gettier LT. Mother-infant cosleeping, breastfeeding and sudden infant death syndrome: what biological anthropology has discovered about normal infant sleep and pediatric sleep medicine. Am J Phys Anthropol. 2007;(Suppl 45):133–61. doi: 10.1002/ajpa.20736. [DOI] [PubMed] [Google Scholar]
- 16.Brenner RA, Simons-Morton BG, Bhaskar B, Ravenus M, Das A, Clemens JD. Infant parent bed sharing in an inner-city population. Arch Pediatr Adolesc Med. 2003;157:33–9. doi: 10.1001/archpedi.157.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Kemp JS, Kowalski RM, Burch PM, Graham MA, Thach BT. Unintentional suffocation by rebreathing: a death scene and physiologic investigation of a possible cause of sudden infant death. J Pediatr. 1993;122(6):874–80. doi: 10.1016/s0022-3476(09)90010-5. [DOI] [PubMed] [Google Scholar]
- 18.Patel AL, Harris K, Thach BT. Inspired CO(2) and O(2) in sleeping infants rebreathing from bedding: relevance for sudden infant death syndrome. J Appl Physiol. 2001;91(6):2537–45. doi: 10.1152/jappl.2001.91.6.2537. incidence of SIDS with bed sharing. [DOI] [PubMed] [Google Scholar]
- 19.Kemp JS, Unger B, Wilkins D, et al. Unsafe sleep practices and an analysis of bedsharing among infants dying suddenly and unexpectedly: results of a four-year, population-based, death-scene investigation study of sudden infant death syndrome and related deaths. Pediatrics. 2000;106(3):E41. doi: 10.1542/peds.106.3.e41. bed sharing and hypercapnia. [DOI] [PubMed] [Google Scholar]
- 20.Thach BT, Lijowska A. Arousals in infants. [Review] [11 refs] Sleep. 1996;19(10 Suppl):S271–3. doi: 10.1093/sleep/19.suppl_10.s271. [DOI] [PubMed] [Google Scholar]
- 21.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36(2):113–22. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 22.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93(1):44. [PubMed] [Google Scholar]
- 23.Poets CF. Apparent life-threatening events and sudden infant death on a monitor. Paediatr Respir Rev. 2004;5(Suppl A):S383–6. doi: 10.1016/s1526-0542(04)90068-1. [DOI] [PubMed] [Google Scholar]
- 24.Kinney HC, Richerson GB, Dymecki SB, Darnall RA, Nattie EE. The brainstem, serotonin, and sudden infant death syndrome: a review. Annu Rev Pathol Mech Dis. 2009;4:517–49. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol. 2007;10:106–16. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- 26.Vege A, Chen Y, Opdal SH, Saugstad OD, Rognum TO. Vitreous humor hypoxanthine levels in SIDS and infectious death. Acta Paediatr. 1994;83(6):634–9. doi: 10.1111/j.1651-2227.1994.tb13096.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones KL, Krous HF, Nadeau J, Blackbourne B, Zielke HR, Gozal D. Vascular endothelial growth factor in the cerebrospinal fluid of infants who died of sudden infant death syndrome: evidence for antecedent hypoxia. Pediatrics. 2003;111:358–63. doi: 10.1542/peds.111.2.358. [DOI] [PubMed] [Google Scholar]
- 28.Kinney HC, Burger PC, Harrell FE, Jr, Hudson RP., Jr. `Reactive gliosis' in the medulla oblongata of victims of the sudden infant death syndrome. Pediatrics. 1983;72(2):181–7. [PubMed] [Google Scholar]
- 29.Takashima S, Armstrong D, Becker L, Bryan C. Cerebral hypoperfusion in the sudden infant death syndrome? Brainstem gliosis and vasculature. Ann Neurol. 1978;4(3):257–62. doi: 10.1002/ana.410040312. [DOI] [PubMed] [Google Scholar]
- 30.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296(17):2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]


