Abstract
Bladder muscle specimens from seven patients with neurogenic bladder dysfunction were analyzed to determine whether the muscarinic receptor subtype mediating contraction shifts from M3 to the M2 subtype as found in the denervated, hypertrophied rat bladder. Seven bladder specimens were analyzed from six female and one male patients. Six of the patients had traumatic cervical spinal cord injuries (C4–C7), and the other patient had an L1 congenital myelomeningocele. This was compared with results from bladder specimens obtained from eight organ transplant donors. The affinities of three subtype-selective muscarinic receptor antagonists for inhibition of carbachol-induced contractions were determined. The affinity of the M3 selective antagonists darifenacin or p-fluoro-hexahydrosiladifenadol (p-F-HHSiD) was determined in six of the seven spinal injury patient specimens. The affinity was consistent with M2-mediated contractions in four of these six specimens, intermediate between M2 and M3 in one specimen, and within the M3 range in one specimen. The other specimen, tested only with the M2 selective antagonist methoctramine, showed an M3 affinity. In the organ donors, the affinity of p-F-HHSiD was within the M2 range for six of seven specimens, whereas the affinity of darifenacin was within the M3 range for five of six and intermediate between M2 and M3 for the other specimen tested. The affinity of methoctramine in both organ donor specimens tested was within the M3 range. Whereas normal detrusor contractions are mediated by the M3 receptor subtype, in patients with neurogenic bladder dysfunction as well as certain organ transplant donors, contractions can be mediated by the M2 muscarinic receptor subtype.
Keywords: spinal cord injury
bladder contraction during voiding is mediated by the neurotransmitter ACh acting through muscarinic receptors located on urinary bladder smooth muscle cells. At least three subtypes of muscarinic ACh receptors (M1, M2, and M3) can be distinguished based on the actions of relatively subtype-selective antimuscarinic agents. Five subtypes arising from five separate genes are identifiable by molecular techniques (5). Immunologic, functional, and molecular studies indicate that most tissues, including the human bladder, contain a mixture of subtypes. Immunoprecipitation has determined that both M2 and M3 receptor proteins are present in the human bladder (49); however, affinities of subtype-selective antimuscarinic agents for inhibition of bladder contractions in neurologically intact animal models, including rat, rabbit, and mouse, are consistent with the M3 subtype mediating contraction under normal conditions in vitro (11, 23, 29, 44). In the rat, the potency of subtype-selective antagonists for inhibition of bladder contraction in vivo correlates better with their affinity at M2 than at M3 receptors (29). The affinities of subtype-selective antimuscarinic agents for inhibiting in vitro human bladder contractions have only been published in a few studies using a very small number of specimens (18, 24, 30, 36, 37). These studies also conclude that, although the M2 receptor subtype predominates, the minor population of M3 receptor subtypes mediates contraction of the normal human bladder.
Denervation and spinal cord injury in the rat induce bladder hypertrophy and a change in muscarinic receptor subtype mediating urinary bladder contraction from M3 to M2 (7, 11). Bladder muscle specimens from patients with spinal cord injury were analyzed to determine whether in humans with neurogenic bladder, the muscarinic receptor subtype mediating contraction also shifts from M3 to the M2 subtype.
MATERIALS AND METHODS
Studies were performed with approval of the Temple University School of Medicine Institutional Review Board and informed consent was obtained from all subjects in this study. At the time of open reconstructive surgery, 5 mm × 15 mm full thickness muscle specimens were removed from the bladder dome. A total of seven bladder specimens was analyzed from one male and six female patients. Patient characteristics are listed in Table 1. Six of the patients had traumatic cervical spinal cord injuries (C4–C7) with an average time after injury of 2.6 ± 0.8 yr. The other patient had an L1 congenital myelomeningocele. The average age of the patients was 18.6 ± 0.7 yr (range 17–22 yr). Preoperative bladder capacity was determined during filling cystometry using a fill rate of 30 ml/min. Bladder capacity was defined as the point at which an uninhibited bladder contraction occurred or the intravesical pressure reached 40 cmH2O pressure, whichever came first. Before surgery, all patients were being treated with at least one anticholinergic agent for their neurogenic bladders (Table 1). In addition, bladder tissue was obtained from eight organ donors from the National Disease Research Institute (Table 2). Tissue was extirpated as soon as possible after cessation of circulation, immersed in Viaspan (Belzer UW; Dupont Pharma) cold storage solution [composed of the following (g/l): 50 pentafraction, 35.83 lactobionic acid, 3.4 potassium phosphate monobasic, 1.23 magnesium sulfate heptahydrate, 17.83 raffinose pentahydrate, 1.34 adenosine, 0.136 allopurinol, 0.922 total glutathione, 5.61 potassium hydroxide, pH adjusted to 7.4 with sodium hydroxide or hydrochloric acid], and shipped to the laboratory by courier on wet ice. Six of the eight donors spent time on life support. Experiments were typically carried out the day after procurement in the organ donors and on the same day in the patients with neurogenic bladder.
Table 1.
Spinal cord-injured patient information
| PT No. | Sex | Age | SCI Level | DOI | Duration, yr | Preop Capacity, ml | Preoperative Bladder Meds | Med Duration, mo |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 20 | C5 | 1990/08/05 | 6.67 | 133 | Oxy, 5 mg BID | 60 |
| 2 | F | 17 | C5R, C7L | 1994/12/31 | 2.58 | 95 | Oxy, 5 mg TID | 8 |
| 3 | F | 22 | L1 spina bifida | NA | 22.00 | 63 | Oxy, 5 mg TID; Hyos, 0.375 mg BID; Dox, 8 mg QID | 6 |
| 4 | F | 18 | C4–5 | 1996/12/26 | 1.50 | 233 | Oxy, 5 mg QID; Imip, 10 mg TID | 13 |
| 5 | F | 18 | C6 | 1996/07/12 | 2.33 | 350 | Tol, 2 mg BID; Hyos, 0.375 mg BID | >1 |
| 6 | F | 18 | C7 | 1997/07/02 | 2.00 | 180 | Oxy-XL, 20 mg QD; Prob, 15 mg TID; Prob, 30 mg QHS | 7 |
| 7 | M | 17 | C5 | 1999/04/05 | 1.50 | 360 | Oxy-XL, 15 mg QD | 12 |
PT, patient; SCI, spinal cord injury; DOI, date of injury; NA, not available; BID, twice per day; QD, once per day; TID, three times per day; QID, taken four times per day; QHS, taken at bedtime; Oxy, oxybutynin; Oxy-XL, oxybutynin extended release (M3-selective antimuscarinic); Tol, tolterodine (nonselective antimuscarinic); Hyos, hyoscyamine (nonselective antimuscarinic); Imip, imipramine (tricyclic antidepressant that inhibits reuptake of serotonin and norepinephrine and that has some antimuscarinic effects); Prob, propantheline (non-subtype-selective antimuscarinic); Dox, doxazosin (a nonspecific α-1 antagonist); M, male; F, female; Preop, preoperative; Med, medication.
Table 2.
Donor information
| PT No. | Assay Date | Sex | Age, yr | Hours Post | Admit Date | Harvest Date (Life Support Duration) | History |
|---|---|---|---|---|---|---|---|
| 1 | 1996/12/03 | NA | NA | NA | NA | NA | NA |
| 2 | 1997/07/14 | M | 23 | NA | NA | 1997/07/12 (21:00) | Head trauma |
| 3 | 2000/04/14 | M | 21 | 11.5 | 2000/04/11 | 2000/04/14 (1:17) | Head trauma |
| 4 | 2000/04/20 | M | 47 | 23.5 | 2000/04/15 | 2000/04/19 (6:52) | CVA |
| 5 | 2000/05/19 | M | 35 | 17.5 | 2000/05/17 | 2000/05/17 (19:32) | Gun shot wound |
| 6 | 2000/07/08 | M | 22 | 13.5 | 2000/07/6 | 2000/07/07 (5:37) | Gun shot wound |
| 7 | 2001/01/16 | F | 69 | 16 | 2001/01/14 | 2001/01/15 (15:45) | Mitral valve prolapse |
| 8 | 2001/01/22 | F | 43 | 16 | 2001/01/19 | 2001/01/21 (21:08) | Hypertension; Crohn's; chronic sinusitis |
Hours Post, time the tissue was on ice in transport media between the time of organ harvest and the in vitro assay. Life support duration indicates hours:minutes that the donor was on life support between declaration of brain death and organ harvest. CVA, cerebral vascular accident. NA, not available.
Muscle strips
Multiple bladder smooth muscle strips approximately 2 × 8 mm (mucosa free) were dissected from the specimen under fourfold magnification such that the direction of the smooth muscle bundles was primarily aligned along the length of the strip. Strips were stretched slowly to 1.0 g of isometric tension in tissue baths containing 15 ml of modified Tyrode solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3, and 5.6 mM glucose) and equilibrated with 95% O2-5% CO2 at 37°C. The strips were tested for their ability to contract in response to electric field stimulation using bipolar platinum electrodes oriented 1 cm apart along the length of the strip and a stimulus intensity of 8 V, 30 Hz, 1-ms duration. Strips that did not contract in response to this electric field stimulation were not used in the analysis.
Carbachol dose response
After equilibration to the bath solution for 30 min, separate groups of bladder strips (n = 3–8) were incubated for 30 min in the presence or absence of selected concentrations of subtype-selective muscarinic receptor antagonists: methoctramine (0.1, 1.0, and 10 μM), p-fluoro-hexahydrosiladifenadol (p-F-HHSiD, 0.1, 1.0, and 10 μM), and darifenacin (0.03 μM). Each muscle strip was used as either an antagonist-free control or exposed to one concentration of antagonist, and thus each strip only underwent one cumulative dose-response curve. Dose-response curves were derived from the peak tension developed after cumulative addition of carbachol in one-half log increments (10 nM to 1 mM final bath concentration). An EC50 value for each strip was determined from a nonlinear least squares sigmoidal curve fit of the data (Origin, OriginLab, Northampton, MA). Dose ratios were determined based on the average of the EC50 values of antagonist-free strips (n = 3–8). The EC50 values determined in the presence of antagonist were used to generate Schild plots to calculate antagonist pA2 values for each individual patient specimen (3). If the slope of the Schild plot was not significantly different from unity, the slope of the Schild plot was constrained to unity to calculate the pKb value. Not all of the antagonists could be tested in all of the specimens due to insufficient specimen size.
Statistical and data analysis
Results are reported as means ± SE or 95% confidence intervals for affinity values. Statistically significant differences in the affinity values and departure from unity in the slopes derived from the Schild plots were determined using the 95% confidence intervals.
RESULTS
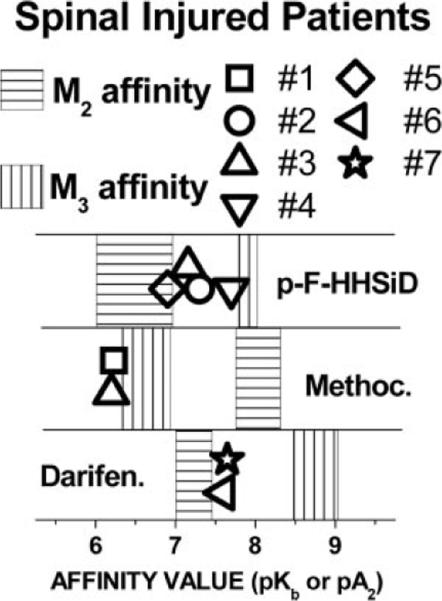
Preoperative bladder capacity in the neurogenic bladder patients averaged 202 ± 41 ml (range 63–360). Of the seven patients with neurogenic bladder, six had uninhibited detrusor contractions during filling. The average uninhibited bladder contraction was 77 ± 21 cmH20 (range 30–140). The other patient reached 40 cmH20 pressure before an uninhibited contraction occurred; thus filling was stopped and that infused volume was defined as bladder capacity. At the concentrations used, the inhibition of in vitro contraction by all of the antagonists was surmountable by carbachol, and thus all behaved as competitive antagonists. The affinity of the M3 selective antagonist darifenacin or p-F-HHSiD was determined in six of the seven specimens (Fig. 1, patients 2–7). The affinities were consistent with M2-mediated contractions in four of these six specimens (patients 3, 5, 6, and 7), intermediate between M2 and M3 in one specimen (patient 2), and consistent with M3-mediated contractions in one specimen (patient 4). One of these six neurogenic bladder specimens (patient 3) and the other neurogenic specimen (patient 1) were tested with the M2-selective antagonist methoctramine, and low affinities were found for both, consistent with M3-mediated contractions. There was no striking relationship between antagonist affinity and preoperative bladder capacity, level of spinal injury, time after injury, or preoperative medications.
Fig. 1.
Affinity of subtype-selective antimuscarinics for inhibiting carbachol-induced contraction of urinary bladder muscle strips in vitro from spinal injured patients. The shaded areas represent the affinity ranges of antimuscarinics [p-fluoro-hexahydrosiladifenadol (p-F-HHSiD), methoctramine (Methoc), and darifenacin (Darifen)] for the individual receptor subtypes reported in the literature (15, 29). Reported affinity ranges for the M2 subtype is shaded with horizontal lines, and reported affinity for the M3 subtype is shaded with vertical lines. Symbols refer to the affinities that were determined by Schild analysis of the bladder tissue obtained from the individual patient numbers indicated in Table 1.
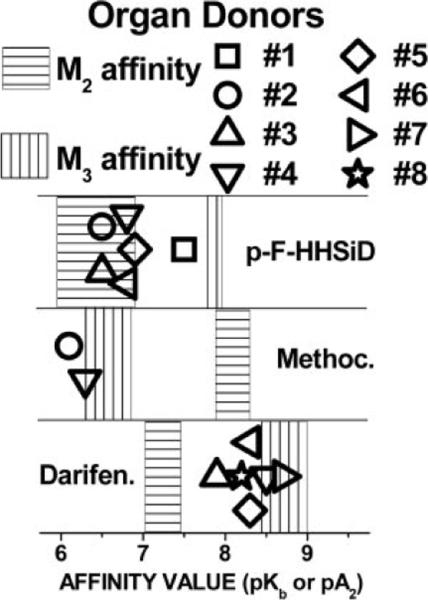
In five bladder specimens from the organ donors (Fig. 2, donors 3–6 and 8), the affinity of both p-F-HHSiD and darifenacin was determined. In four of the five (donors 4–6 and 8), the affinity of p-F-HHSiD was within the reported range for M2-mediated contractions, while the affinity of darifenacin was not different from the range of affinities reported for the M3 subtype. In the other specimen (donor 3), the affinity of p-F-HHSiD was within the reported M2 range and was intermediate between M2 and M3 range for darifenacin. In one tissue, only p-F-HHSiD was used (donor 1) and had an affinity consistent with M3 receptors mediating contraction. In one tissue (donor 7), only darifenacin was used, and an affinity not different from the M3 range was found. In one specimen (donor 2), p-F-HHSiD and methoctramine were used; the p-F-HHSiD affinity was consistent with M2 receptors, while the methoctramine affinity was consistent with M3 receptors mediating contraction. Strikingly, the methoctramine affinity was low (consistent with M3-mediated contractions) in all specimens tested (patients 1 and 3 and donors 2 and 4). In every study we have previously performed, with the single exception of rat bladder after selective alkylation of M3 receptors in the presence of isoproterenol (12), we have always found a low methoctramine affinity consistent with M3 receptors mediating contraction. After confirming this in two human specimens from each group, we did not continue to determine the affinity of methoctramine using this limited patient and donor tissue.
Fig. 2.
Affinity of subtype-selective antimuscarinics for inhibiting carbachol-induced contraction of urinary bladder muscle strips in vitro from organ donors. Notation is the same as indicated for Fig. 1 except characteristics of donor numbers are indicated in Table 2.
Table 3 provides a summary of the antagonist affinity data.
Table 3.
Summary of experimental data
| Spinal Injured Patients |
Organ Donors |
||||||
|---|---|---|---|---|---|---|---|
| Pt. No. | Meth | p-F-HHSiD | Dar | Pt. No. | Meth | p-F-HHSiD | Dar |
| 1 | 6.2±0.5 (M3) | 1 | 7.5±0.4 (M3) | ||||
| 2 | 7.3±0.3 (INT) | 2 | 6.1±0.2 (M3) | 6.5±0.2 (M2) | |||
| 3 | 6.2±0.2 (M3) | 7.2±0.4 (M2) | 3 | 6.5±0.2 (M2) | 7.9±0.6 (INT) | ||
| 4 | 7.7±0.5 (M3) | 4 | 6.8±0.2 (M3) | 6.3±0.1 (M2) | 8.7±0.3 (M3) | ||
| 5 | 6.9±0.3 (M2) | 5 | 6.9±0.1 (M2) | 8.3±0.2 (M3) | |||
| 6 | 7.6±0.5 (M2) | 6 | 6.8±0.2 (M2) | 8.3±0.6 (M3) | |||
| 7 | 7.7±0.3 (M2) | 7 | 8.9±0.2 (M3) | ||||
| 8 | 6.6±0.2 (M2) | 8.2±0.2 (M3) | |||||
Values are means ± 95% confidence intervals expressed as –Log (pKb or pA2). For each specimen, the affinity of the muscarinic receptor antagonists was determined as described in materials and methods for inhibiting carbachol-induced bladder contraction. The determined value was compared with the published affinity of the M2 and M3 receptors for the antagonists. If the determined affinity (95% confidence range) overlaps the range of the reported values for either subtype, the subtype within the range is shown in parenthesis after the affinity value. If the determined value (95% confidence interval) is between the reported range for the two subtypes or within the range of both subtypes, this affinity value is considered intermediate (INT) between the two subtypes. Meth, methoctramine; p-F-HHSiD, p-fluoro-hexahydrosiladifenadol; Dar, darifenacin.
DISCUSSION
Human detrusor contractions are thought to be mediated by the M3 receptor subtype. This assumption is based in part on data from animal studies and very limited data in human tissue (18, 24, 30, 36, 37). Our study is the first to demonstrate that in individuals with a neurogenic bladder dysfunction from spinal cord injury or myelodysplasia, detrusor contractions can also be mediated by the M2 muscarinic receptor subtype. This was also seen in certain bladders from organ donors.
Bladder contraction occurs from ACh-induced excitation of postjunctional muscarinic cholinergic receptors on bladder smooth muscle. Subtype-selective antimuscarinic agents are available that are at least 10-fold selective for each of the M1–M3 subtypes (15, 16). The MT3 toxin is at least 30-fold selective for the M4 subtype (15, 16, 31). No M5-selective antagonists are currently available. M1 receptors have a high affinity for pirenzepine (PZP), a low affinity for methoctra-mine, and an intermediate affinity for p-F-HHSiD. M2 receptors have a high affinity for methoctramine and a low affinity for PZP and p-F-HHSiD. M3 receptors have a high affinity for 4-diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP), darifenacin, and p-F-HHSiD, an intermediate affinity for PZP, and a low affinity for methoctramine (15). Affinity values derived from Schild plot analysis of the inhibition of carbachol-induced contractions of the rat urinary bladder are consistent with M3 receptors mediating contraction (10, 49). This is also consistent with the response seen in other animal models demonstrating that, although M2 receptors are more abundant (27, 49), it is the M3 receptor subtype that is important in the control of bladder contractions across a range of species.
Muscarinic receptor subtypes have been analyzed in human bladder using several different approaches. Binding studies in human bladder as well as cultured human bladder smooth muscle cells have demonstrated greater numbers of M2 than M3 receptors (27, 28, 32, 33, 39). The mRNA encoding M2 and M3 has been reported to be present in equal amounts (50). With the use of antibodies to the muscarinic subtypes, ~80% of precipitable receptors are M2, with the rest mainly M3 (49). There are limited functional data from human bladder. Muscarinic receptor stimulation of human bladder tissue induces phosphatidyl inositol (PI) hydrolysis (2, 39, 48), which is consistent with involvement of the M3 subtype because M1, M3, and M5 subtypes preferentially couple to PI turnover. This is further supported by a study similar to ours using “normal” human bladders. In these bladder strips, darifenacin had a high pA2 value similar to that for guinea pig bladder, with low affinities of methoctramine and pirenzepine. The authors concluded that contraction in human bladder is mediated through M3 receptors, although the exact source of this human tissue and patient characteristics were not specified in this abstract (37). Similarly, in a series of bladder specimens taken from 10 males and 11 females undergoing cystectomy for bladder cancer, the affinities of six different muscarinic antagonists (PZP, methoctramine, 4-DAMP, tropicamide, oxybutynin, and tolterodine) were consistent with M3-mediated contractions (18).
Studies in normal bladders from all species studied to date indicate that the M3 subtype mediates bladder contraction. These determinations are based on the assumption that only one of the receptor subtypes mediates the response. In some of our pathological human bladder specimens from patients with neurogenic bladder dysfunction or organ donors, it appears that both M2 and M3 receptors may be mediating the contractile response depending on which subtype-selective antagonist was used. We interpret the apparent contradictory data (i.e., a low p-F-HHSiD affinity indicating M2-mediated contractions together with a low methoctramine affinity indicating M3-mediated contractions) to be consistent with a scenario in which either the M2 or the M3 receptor can mediate contraction. When the M2 receptor is inhibited by methoctramine, the M3 receptor mediates contraction. When the concentration of methoctramine is high enough to block both the M2 and the M3 receptor, contraction is inhibited. This would yield an affinity consistent with blockade of the least sensitive receptor to methoctramine, in this case the M3. The converse is also true, i.e., p-F-HHSiD would not affect contraction until both M2 and M3 receptors are inhibited and thus a low affinity would be obtained. In five of the organ donor specimens, the affinity of two different M3-selective antagonists (darifenacin and p-FHHSiD) was determined. In four of these five, an affinity consistent with M2 receptors was found using the less selective antagonist (p-F-HHSiD), whereas the affinity of the antagonist with a greater selectivity for M3 receptors (darifenacin) was high, consistent with M3 receptors mediating contractions. The relative selectivity of these two antagonists may offer some explanation of these findings.
There are multiple reports of an M2 receptor contribution to bladder contraction under certain experimental conditions or in different pathological states. Bilateral removal of the major pelvic ganglia in rats renders the bladder denervated because unlike other mammalian species, the adult rat bladder does not contain intramural ganglia (25, 46). Because these bladders cannot empty on their own, they become hypertrophic within 24 h after denervation even though they are emptied with manual external abdominal pressure twice a day. In denervated bladders, the affinity of subtype-selective antimuscarinic receptor drugs to inhibit carbachol-induced contractions is consistent with M2 or a combination of M2- and M3-mediated bladder contractions, while nondenervated sham-operated control bladders display affinities consistent with M3-mediated contractions. After denervation, there is a 60% increase in M2 receptor density with no change in M3 receptor density compared with sham-operated controls (11). M2 receptors also participate in contraction of the bladder from rats with a T9 level spinal cord injury who do not spontaneously regain the ability to urinate (7). Rats with the T9 level injury who were able to spontaneously urinate and control animals demonstrate bladder contractions mediated by the M3 receptor subtype. Other conditions affecting smooth muscle in which there is M2-mediated contraction include experimental esophagitis in cat (41), a model of acute cholecystitis in guinea pig (8), and arterial hypertension (47). In addition, alteration of experimental conditions in smooth muscle studies can shift the subtypes mediating contraction from M3 to M2. This includes inhibition of the sarcoplasmic reticulum ATPase (13) and after inactivation of M3 receptors using the irreversible inhibitor 4-DAMP mustard in an environment of increased intracellular cAMP (12, 29).
There are several mechanisms through which M2 receptors may produce bladder contraction. Data obtained after selective alkylation of M3 receptors with 4-DAMP mustard suggest that M2 receptors, through activation of a GTP binding protein associated with inhibition of adenylyl cyclase (Gi), may be involved in inhibition of β-adrenergic receptor-induced relaxation (12, 29). Based on cloned receptors expressed in CHO cells, a particular receptor subtype does seem to preferentially couple with one type of G protein. However, there is substantial evidence of significant promiscuity in this coupling mechanism such that, depending on the type of cell and the biochemical state of the cell, a given receptor may be able to couple with several different types of G proteins so that a single receptor subtype may mediate several different cellular signals (45, 49). Whereas M2 receptors are traditionally thought to couple to the Gi class of GTP binding proteins, we have demonstrated, using a coimmunoprecipitation assay, that M2 receptors can also couple to Gq in human bladder (49). Thus differential coupling of M2 to different G proteins under different conditions may alter its effect on bladder tissue. There are several different mechanisms of M2 action, demonstrated in smooth muscle other than the bladder, that may also be active in the detrusor. These include opening of nonselective cation channels, resulting in depolarization and influx of calcium, which is seen in ileum (4), and inhibition of conductance through potassium channels as seen in canine colon (21). M2 receptors could also activate protein kinase C (PKC) to induce contraction (21, 48).
Selective alkylation experiments in mice demonstrates that in most strains, no indirect contractile role for M2 receptors exists (19, 20) as can be shown in rat bladder (12, 29) and guinea pig ileum (43). This is confirmed by muscarinic receptor subtype knockout experiments in mice. In M3 knockout mice, very little residual M2-mediated contractions are found (35), and in M2 knockout mice, maximal contractions to carbachol in bladder are not reduced and carbachol potency is only slightly decreased (42). The regulation of the interplay between M2- and M3-mediated contractions is not clear; however, receptor density may play a role. After denervation in the rat, there is an increase in the number of M2 receptors but not M3 (11). There is also evidence that the M2/M3 interaction is regulated by intracellular calcium. Low levels of muscarinic stimulation and hence low levels of calcium induce a translocation in PKC to the membrane, while at greater levels of receptor stimulation PKC activation is inhibited (40). We have shown, using combinations of M2- and M3-selective antagonists, that there is no synergy between these subtypes in normal rat bladder contraction. However, in the denervated, hypertrophied bladder there is a synergistic interaction between the two subtypes in that the contractile response from the combination of M2 and M3 subtypes is greater than what would be predicted from the sum of each subtype acting alone (13).
As a result of using specimens from patients with neurogenic bladder dysfunction, our results differ from previous published results in human bladder that concluded that the M3 subtype mediates bladder contraction (18, 37). Some of our donor tissue also shows M2-mediated contractions. While it is not likely that bladder hypertrophy or neurogenic lesions are responsible for this finding, one explanation is that the physiology of this tissue is different from normal because of the transport solution and ischemia or anoxia. Another possible reason is due to bladder inactivity while the donor was on life support or due to long transport times. An additional explanation may be that we determined antagonist affinities in each individual patient specimen as opposed to averaging results from all individuals. Many studies in the past have correlated the affinities of antagonists in inhibition of bladder contraction with their affinities for each of the five muscarinic receptor subtypes expressed in clonal cell lines, assuming that the subtype that gives the highest correlation coefficient mediates bladder contraction (1, 18, 22, 29). This method gives the same weight to drugs with no subtype selectivity as those that are highly selective. Often this type of analysis yields similar significant correlations for more than one subtype. An underlying assumption is that a single subtype mediates contraction. Our data and others indicate that both M2 and M3 muscarinic receptor subtypes are involved in mediating contraction of bladder smooth muscle (9, 12, 26, 29). Another issue in determining the muscarinic receptors that mediates normal human bladder contraction is that tissue is taken at the time of surgery performed for some pathological process, so whether this tissue truly represents normal bladder must be questioned. This also includes organ transplant donor controls, some of which we found in this investigation to have M2-mediated bladder contractions. A major difference in our study from those published previously is that we are looking at neurogenic bladder, which is different from that used in other studies. Irrespective of the physiological changes from the neurological injury, another factor to consider in these patients may be the concomitant use of antimuscarinic and other medications.
Prior studies described above have used inactivation of the M3 receptor with 4-DAMP mustard to produce M2-mediated contractions (12, 24, 29). At least six of the seven patients were on long-term antimuscarinics to treat their neurogenic bladders before surgery (see Table 1 for specifics). Although these medications do not covalently inactivate the muscarinic receptor like 4-DAMP mustard does, it is possible that the long-term use of muscarinic antagonists may serve to modulate the M3 or M2 contractile response. Chronic atropine treatment may increase muscarinic receptor density; however, this is dependent on the method of drug administration. When atropine was administered for 14 days through an osmotic pump at 5 mg·kg−1·day−1 to rats, we found that muscarinic receptor density increased by ~43% (34). In another study in which atropine was given for 14 days by daily intraperitoneal injection (20 mg/kg), we found that no increase in muscarinic receptor density occurred (17). The effect of the antimuscarinics on the density of muscarinic receptors in our patients is unknown. Nonetheless, the only issue that this would cloud is whether contraction in the untreated neurogenic bladder is predominantly mediated by the M3 receptor. Our data show that bladder contractions in these patients are mediated by the M2 receptor, whether it is due to the physiological changes of the injury or due to any contribution of their concomitant medications. Another factor in our neurogenic bladder patients may be the effect of functional bladder outlet obstruction from vesicosphincter dyssynergia. Bladder outlet obstruction for 3 days in the rat yields a hypertrophied bladder, some degree of functional denervation, and an M2-mediated component of contraction (9).
Our data may have clinical implications. In patients with a suprasacral spinal cord injury, some of the bladder contraction can be M2 mediated. Thus in these patients, addition of a relatively selective M2 antagonist or a drug that has both M2 and M3 blocking activity might be expected to be more effective in increasing bladder capacity than one that is predominantly M3 selective. However, intravenous administration of the highly M3-selective antagonist darifenacin has been shown to effectively suppress unstable bladder contractions provoked by rapid fluid infusion (50–100 ml infused at 20 ml/s) in a group of eight male patients with suprasacral spinal cord injury (14). An advantage of an M2-selective agent would be less dry mouth because salivation is primarily mediated by M3 receptors (38). On the other hand, an M2-selective antagonist might be expected to produce increased cardiac side effects because the M2 receptor mediates the effects of the vagus nerve on the heart. A larger unanswered question is whether these results are applicable to the large number of patients who are clinically neurologically intact and use antimuscarinic medications for overactive bladder. One theory of overactive bladder in otherwise neurologically intact individuals is that of partial detrusor denervation (6). In our rat model of bladder denervation, there is a shift in the muscarinic receptor subtypes responsible for bladder contraction from M3 to M2. In these patients who have decentralization of their bladders, there was also a shift in the muscarinic receptor subtype mediating contraction from M3 to M2. These data indicate that if there is some element of denervation in patients with overactive bladder, then there might also be a shift from M3- to M2-mediated contraction. This deserves further investigation.
Whereas normal detrusor contractions are thought to be mediated by the M3 receptor subtype, in patients with neurogenic bladder dysfunction and certain organ donors, contractions can be mediated by the M2 muscarinic receptor subtype. These findings challenge the assumption on which medical therapy is based for treatment of patients with voiding dysfunction. There may also be implications for drug development. If the change in function and number of receptor subtypes in denervated bladder is a common phenomenon in other pathological states, then the therapies developed for the treatment of these conditions based on the effect in normal tissue may be clinically ineffective. Results from clinical trials with the M3-selective receptor antagonist darifenacin may help to clarify the relative importance of M2 and M3 receptors in overactive bladder. To our knowledge, there are currently no M2 subtype-selective antagonists in development for clinical use. Our data also point out the variability between individual patient response to selective antimuscarinic therapy. Thus the present data indicate that different individuals or groups of patients might benefit from different antimuscarinic profiles, i.e., M3-selective, M2-selective, or nonselective drugs. Caution should be used therefore in pooling data from different patients and assuming the average results represent all patients. Currently, clinical trials require this pooling of patient data because there is currently no way of stratifying patients a priori into separate groups based on their potential clinical response to antimuscarinic drugs with different selectivity profiles.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-43333.
REFERENCES
- 1.Alberts P. Classification of the presynaptic muscarinic receptor subtype that regulates 3H-acetylcholine secretion in the guinea pig urinary bladder in vitro. J Pharmacol Exp Ther. 1995;274:458–468. [PubMed] [Google Scholar]
- 2.Andersson KE, Holmquist F, Fovaeus M, Hedlund H, Sundler R. Muscarinic receptor stimulation of phosphoinositide hydrolysis in the human isolated urinary bladder. J Urol. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- 3.Arunlakshan O, Schild HO. Some qualitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton TB, Zholos AV. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 5.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 6.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. discussion 68–73. [DOI] [PubMed] [Google Scholar]
- 7.Braverman A, Legos J, Young W, Luthin G, Ruggieri M. M2 receptors in genito-urinary smooth muscle pathology. Life Sci. 1999;64:429–436. doi: 10.1016/s0024-3205(98)00582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braverman AS, Bartula LL, Myers SI, Parkman HP, Ruggieri MR. Inflammation changes the muscarinic receptor subtype and signal transduction pathway that mediates gallbladder contraction (Abstract) Gastroenterology. 2000;118:A197. [Google Scholar]
- 9.Braverman AS, Karlovsky M, Pontari MA, Ruggieri MR. Aging and hypertrophy change the muscarinic receptor subtype mediating bladder contraction from M3 towards M2. J Urol. 2002;167:170. doi: 10.1152/ajpregu.00009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braverman AS, Kohn IJ, Luthin GR, Ruggieri MR. Prejunctional M-1 facilitory and M-2 inhibitory muscarinic receptors mediate rat bladder contractility. Am J Physiol Regul Integr Comp Physiol. 1998;274:R517–R523. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1654–R1660. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braverman AS, Ruggieri MR. Selective alkylation of rat urinary bladder muscarinic receptors with 4-DAMP mustard reveals a contractile function for the M2 muscarinic receptor. J Recept Signal Transduct Res. 1999;19:819–833. doi: 10.3109/10799899909042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braverman AS, Tallarida RJ, Ruggieri MR., Sr. Interaction between muscarinic receptor subtype signal transduction pathways mediating bladder contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R663–R668. doi: 10.1152/ajpregu.00116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bycroft J, Leaker B, Wood S, Knight S, Shah J, Craggs M. The effect of darifenacin on neurogenic detrusor overactivity in patients with spinal cord injury. Proc 33rd Int Continence Soc Annu Mtg Florence; Italy. 2003. pp. 74–76. [Google Scholar]
- 15.Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 16.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 17.Cawley TA, Jr, Shickley TJ, Ruggieri MR, Luthin GR. Effect of chronic neuroleptic treatment on central and peripheral muscarinic receptors. J Pharmacol Exp Ther. 1993;267:134–139. [PMC free article] [PubMed] [Google Scholar]
- 18.Chess-Williams R, Chapple CR, Yamanishi T, Yasuda K, Sellers DJ. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol. 2001;21:243–248. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 19.Choppin A. Muscarinic receptors in isolated urinary bladder smooth muscle from different mouse strains. Br J Pharmacol. 2002;137:522–528. doi: 10.1038/sj.bjp.0704897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choppin A, Eglen RM. Pharmacological characterization of muscarinic receptors in mouse isolated urinary bladder smooth muscle. Br J Pharmacol. 2001;133:1035–1040. doi: 10.1038/sj.bjp.0704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole WC, Carl A, Sanders KM. Muscarinic suppression of Ca2+−dependent K current in colonic smooth muscle. Am J Physiol Cell Physiol. 1989;257:C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino G, Barbieri A, Chiossa E, Tonini M. M4 muscarinic autoreceptor-mediated inhibition of 3H-acetylcholine release in the rat isolated urinary bladder. J Pharmacol Exp Ther. 1997;283:750–756. [PubMed] [Google Scholar]
- 23.Durant PA, Shankley NP, Welsh NJ, Black JW. Pharmacological analysis of agonist-antagonist interactions at acetylcholine muscarinic receptors in a new urinary bladder assay. Br J Pharmacol. 1991;104:145–150. doi: 10.1111/j.1476-5381.1991.tb12399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fetscher C, Fleichman M, Schmidt M, Krege S, Michel MC. M(3) muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol. 2002;136:641–643. doi: 10.1038/sj.bjp.0704781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabella G, Uvelius B. Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res. 1990;262:67–79. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- 26.Gillberg PG, Sundquist S, Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol. 1998;349:285–292. doi: 10.1016/s0014-2999(98)00214-3. [DOI] [PubMed] [Google Scholar]
- 27.Goepel M, Gronewald A, Krege S, Michel MC. Muscarinic receptor subtypes in porcine detrusor: comparison with humans and regulation by bladder augmentation. Urol Res. 1998;26:149–154. doi: 10.1007/s002400050038. [DOI] [PubMed] [Google Scholar]
- 28.Harriss DR, Marsh KA, Birmingham AT, Hill SJ. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J Urol. 1995;154:1241–1245. [PubMed] [Google Scholar]
- 29.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Kobayashi S, Suzuki M, Miyata K, Takeuchi M, Yamada T, Honda K. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:97–103. doi: 10.1007/s00210-002-0554-x. [DOI] [PubMed] [Google Scholar]
- 31.Jerusalinsky D, Kornisiuk E, Alfaro P, Quillfeldt J, Ferreira A, Rial VE, Duran R, Cervenansky C. Muscarinic toxins: novel pharmacological tools for the muscarinic cholinergic system. Toxicon. 2000;38:747–761. doi: 10.1016/s0041-0101(99)00196-8. [DOI] [PubMed] [Google Scholar]
- 32.Kiwamoto H, Ma FH, Higashira H, Park YC, Kurita T. Identification of muscarinic receptor subtypes of cultured smooth muscle cells and tissue of human bladder body. Int J Urol. 2001;8:557–563. doi: 10.1046/j.1442-2042.2001.00370.x. [DOI] [PubMed] [Google Scholar]
- 33.Kondo S, Morita T, Tashima Y. Muscarinic cholinergic receptor subtypes in human detrusor muscle studies by labeled and non labeled pirenzepine, AFDX-116 and 4-DAMP. Urol Int. 1995;54:150–153. doi: 10.1159/000282710. [DOI] [PubMed] [Google Scholar]
- 34.Levin RM, Ruggieri MR, Lee W, Wein AJ. Effect of chronic atropine administration on the rat urinary bladder. J Urol. 1988;139:1347–1349. doi: 10.1016/s0022-5347(17)42916-8. [DOI] [PubMed] [Google Scholar]
- 35.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamae K, Yoshida M, Murakami S, Iwashita H, Ohtani M, Masunaga K, Ueda S. Pharmacological effects of darifenacin on human isolated urinary bladder. Pharmacology. 2003;69:205–211. doi: 10.1159/000073665. [DOI] [PubMed] [Google Scholar]
- 37.Newgreen DT, Taylor AM. Characterisation of functional muscarinic receptors in human bladder (Abstract) Br J Pharmacol. 1996;119:45P. [Google Scholar]
- 38.Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B. Tolterodine—a new bladder-selective antimuscarinic agent. Eur J Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- 39.Ruggieri MR, Bode DC, Levin RM, Wein AJ. Muscarinic receptor subtypes in human and rabbit bladder. Neurourol Urodyn. 1987;6:119–128. [Google Scholar]
- 40.Sohn UD, Chiu TT, Bitar KN, Hillemeier C, Behar J, Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol Gastrointest Liver Physiol. 1994;266:G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- 41.Sohn UD, Harnett KM, Cao W, Rich H, Kim N, Behar J, Biancani P. Acute experimental esophagitis activates a second signal transduction pathway in cat smooth muscle from the lower esophageal sphincter. J Pharmacol Exp Ther. 1997;283:1293–1304. [PubMed] [Google Scholar]
- 42.Stengel PW, Yamada M, Wess J, Cohen ML. M(3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- 43.Thomas EA, Baker SA, Ehlert FJ. Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileum. Mol Pharmacol. 1993;44:102–110. [PubMed] [Google Scholar]
- 44.Tobin G, Sjogren C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]acetylcholine in the rabbit urinary bladder. Eur J Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- 45.Tucek S, Michal P, Vlachova V. Modelling the consequences of receptor-G-protein promiscuity. Trends Pharmacol Sci. 2002;23:171–176. doi: 10.1016/s0165-6147(00)01996-9. [DOI] [PubMed] [Google Scholar]
- 46.Uvelius B, Gabella G. Intramural neurones appear in the urinary bladder wall following excision of the pelvic ganglion in the rat. Neuro-report. 1995;6:2213–2216. doi: 10.1097/00001756-199511000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Van Zwieten PA, Doods HN. Muscarinic receptors and drugs in cardiovascular medicine. Cardiovasc Drugs Ther. 1995;9:159–167. doi: 10.1007/BF00877757. [DOI] [PubMed] [Google Scholar]
- 48.Walsh MP, Horowitz A, Clement-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem Cell Biol. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- 49.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi O, Shishido K, Tamura K, Ogawa T, Fujimura T, Ohtsuka M. Evaluation of mRNAs encoding muscarinic receptor sub-types in human detrusor muscle. J Urol. 1996;156:1208–1213. [PubMed] [Google Scholar]