Abstract
Acquiring information via observation of others can be an efficient way to respond to changing situations or learn skills, particularly for inexperienced individuals. Many bat species are gregarious, yet few studies have investigated their capacity for learning from conspecifics. We tested whether big brown bats (Eptesicus fuscus) can learn a novel foraging task by interacting with knowledgeable conspecifics. In experimental trials 11 naïve bats (7 juveniles, 4 adults) interacted freely with trained bats that were capturing tethered mealworms, while in control trials 11 naïve bats (7 juveniles, 4 adults) flew with untrained bats. Naïve bats were then assessed for their ability to capture tethered mealworms. While no bat in the control group learned the task, a significant number of experimental bats, including juveniles with little or no experience foraging, showed evidence of learning. Eighty-two per cent of experimental bats and 27% of control bats directed feeding buzzes (echolocation calls associated with prey capture) at the mealworm. Furthermore, seven experimental bats (64%) showed evidence of learning by attacking and/or capturing the mealworm, while no bat in the control group attacked or captured the prey. Analyses of high-speed stereo video recordings revealed increased interaction with demonstrators among bats attacking or capturing the mealworm. At the time they displayed evidence of learning, bats flew closer together during feeding buzzes than during other portions of trials. Our results demonstrate that social interaction with experienced bats, and listening to feeding buzzes in particular, may play an integral role in development of foraging skills in bats.
Keywords: big brown bat, Eptesicus fuscus, feeding buzzes, foraging, information transfer, juvenile development, local enhancement, social learning
Relatively long-lived animals, particularly those whose roosts or food sources change seasonally and over the course of a lifetime, would benefit from the ability to acquire new skills and learn new information throughout their lives. Flexibility, innovation, and learning ability should be especially important for these types of organisms. Acquiring skills that are not innate and responding to changing situations require animals to use individual learning, social information, such as watching, following, imitating, or listening, or some combination of the two to behave appropriately (Cavalli-Sforza & Feldman 1983; Boyd & Richerson 1985). Group-living animals especially may benefit from gaining information based on the behaviour of conspecifics. This might include obtaining social information about roosting, nesting, or foraging sites, learning which foods are safe for consumption based on cues from others, or learning a new way of accessing food through interactions with knowledgeable conspecifics (e.g., Galef & Laland 2005; Bonnie & Earley 2007; Seppanen et al. 2007). Obtaining information in these ways might benefit the observer by allowing it to conserve energy that would be required otherwise to find a resource alone, preventing it from harm caused by ingesting unpalatable items, or increasing its foraging efficiency, respectively.
Young animals, especially, may benefit from social information when they are first learning to forage and locate roosts as parental care comes to an end. Various young mammals have been shown to learn foraging techniques from their mothers (e.g., golden hamsters, Mesocricetus auratus—Previde & Poli 1994; black rats, Rattus rattus—Terkel 1996). However, young animals can also learn foraging-related skills from individuals other than their mothers. For example, Thornton (2008) found that meerkat (Suricata suricatta) pups learn about novel foods from helpers that are feeding them, young-of-the-year perch (Perca fluviatilis) learn to eat a new food item from experienced demonstrator fish (Magnhagen & Staffan 2003), and juvenile ringdoves (Streptopelia risoria) learn food choice and foraging techniques from both kin and non-kin (Hatch & Lefebvre 1997).
Many of the more than 1,100 described species of bats (Order Chiroptera), including big brown bats (Eptesicus fuscus), are gregarious, spending much time roosting, foraging, seeking hibernacula and caring for young in the company of conspecifics (e.g., Guthrie 1933; Davis & Hitchcock 1965). Despite the opportunities for social learning and information transfer bats could experience (Wilkinson & Boughman 1999), few studies have experimentally tested these phenomena in bats. When tested, bats have shown the capacity to socially learn methods of obtaining food (E. fuscus, Myotis lucifugus, and Antrozous pallidus— Gaudet & Fenton 1984; Trachops cirrhosus—Page & Ryan 2006), food location (Phyllostomus discolor—Wilkinson 1987), and flavor preference (Carollia perspicillata—Ratcliffe & ter Hofstede 2005). In addition, there is evidence that Nycticeius humeralis (Wilkinson 1992) and Myotis bechsteinii (Kerth & Reckardt 2003) exchange information about roosting (both species) and foraging (N. humeralis) sites.
While these studies demonstrate that bats can learn socially in some instances, few species of bats have been tested, and none of these studies focused on learning in juveniles. Furthermore, previous social learning studies in general often do not quantify the mechanism(s) by which social learning has occurred. While it is not well established that young E. fuscus typically forage with their mothers (Brigham & Brighman 1989 report one such instance), this species frequently forages in the vicinity of other bats. This foraging situation may allow young individuals to gain foraging skills via interaction with more experienced individuals. In addition, food availability may change seasonally or from year to year, making it beneficial for adults to acquire foraging information from one another as well. If bats are learning from conspecifics, then flying near, interacting with, and listening to knowledgeable individuals may maximize the amount of information they receive. With these factors in mind, the following questions motivated our research. 1) Does learning from conspecifics play a role in the development of foraging skills in E. fuscus? 2) If juveniles learn socially, is this ability limited to young bats, or can adults also learn a new foraging task from other bats? 3) Is the extent of interaction with experienced bats associated with likelihood of social learning? To address these questions, we tested whether young E. fuscus with little or no previous experience flying or foraging could learn a novel foraging task by observing, listening to, and interacting with experienced conspecifics. We also tested the ability of adult bats, which had experience capturing free-flying prey in the wild, to learn the same novel foraging task through exposure to trained conspecifics. Finally, we analysed synchronized audio and high-speed video recordings from these interactions to look for behavioural patterns potentially related to social learning and quantify any association between the amount of inter-bat interaction (smaller inter-bat distances, following or chasing behaviour), auditory food-related cues, and likelihood of learning.
METHODS
Study Subjects
We selected fourteen naïve young (estimated ages: 21–51 days (X±SD = 34±10 days) and eight adult (≥ 1 year old) big brown bats (Eptesicus fuscus) to be “observer” bats. “Observer” refers to the naive individual whose ability to learn a novel foraging task, after exposure to others, was assessed. Except for one set of twins born in captivity, all bats were wild-caught in Maryland. Juvenile ages were estimated from epiphyseal gap measurements and forearm length (Kunz 1974; Burnett & Kunz 1982), by physical appearance (e.g., naked versus with fur), and by comparison to known-age individuals born in the lab. Five bats were estimated to be between 21 and 26 days old, four between 32 and 40 days of age, and four between 41 and 51 days old when they began their time in the experiment (one bat’s age was not recorded). Age and experimental start date of bats in control vs. experimental groups was balanced (control X±SD age: 35±12 days; experimental X±SD age: 35±9 days), and we assigned individuals from the two sets of juvenile twins used to the opposite condition (control versus experimental) from their siblings.
We used twelve adult and one young E. fuscus as “demonstrators” for the experimental or control group. “Demonstrator” refers to bats that were either a) naive, but had experience with the flight room (control demonstrators), or b) were trained to capture a tethered prey item (experimental demonstrators), and were flown with observers during experiments. We trained six adult bats (two males, four females) to catch a tethered mealworm (Tenebrio molitor) hanging from the ceiling of a 7 × 6 × 2.5 m anechoic flight chamber (Fig. 1) to serve as demonstrators for the experimental group. Bats were trained by feeding them mealworms from a tether and repeatedly drawing their attention to tethered mealworms while restricting their food intake outside of training sessions. We also used one adult female who learned to take a tethered mealworm as an observer in 2006 and then served as a demonstrator the following year. In addition, we used one young male (~5.5 weeks old) as a demonstrator after he learned to catch mealworms as an observer.
Figure 1.
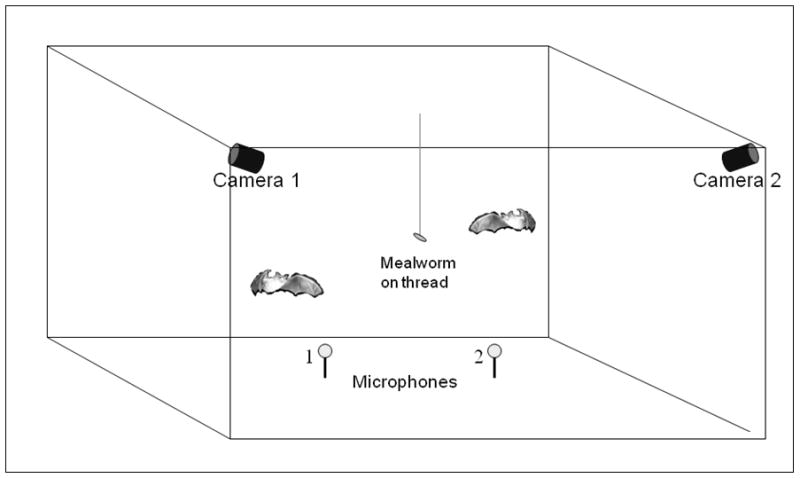
Schematic of flight room set-up showing positioning of high-speed cameras, ultrasound-sensitive microphones, and tethered mealworm. Drawing not to scale.
To ensure that bats actively searched for the mealworm, rather than rely primarily on spatial memory to find the prey, the location of the mealworm was varied from day to day during training and trials. The mealworm was generally within 1–2 m of the center of the flight room. Once a bat took the tethered mealworm, there was no food item available in the room until the researcher presented a new mealworm on the tether. We used the remaining five adults (two males, three females), who had experience flying in the flight room but did not know how to catch tethered mealworms, as “demonstrators” for the control group. We never observed control demonstrators emitting buzzes towards or attempting to capture the mealworm.
Bats were maintained on a reverse 12-h day-night cycle (lights off from 8:30 until 20:30) and, when not flying in experiments, were housed in cages containing three or four bats each. This research was conducted with approval from the Institutional Animal Care and Use Committee at the University of Maryland (protocol R-05-15) under a state collecting permit. As a condition of the permit, bats were not released at the conclusion of the study. Some individuals were, however, subsequently used for other experiments.
Experimental Procedure
Young E. fuscus learn to fly between 18 and 35 days of age (Kurta & Baker 1990), and we tested juveniles ~1–3 weeks following collection from the wild (bats that could already fly when captured) or from becoming volant (bats born captivity or collected when pre-volant). Adults began testing approximately 2 months after capture. Prior to the start of the experiment, we released pre-volant juveniles in the flight room on several days until they learned to fly, as measured by successfully flying in several continuous loops around the room and landing on the wall rather than the floor. We tested a total of 22 observer bats. Sixteen bats (10 juveniles and six adults) were tested during July-September 2006, and the remaining six (four juveniles and two adults) were tested during July-August 2007. Half of the observers were assigned to a control group, and the remaining 11 to an experimental group. Each group consisted of seven juveniles and four adults. Juveniles were tested for up to 18 days, while adults were tested for up to 10 days. All bats that did not eat during testing were fed in their cages later in the day after, but not immediately following, testing (typically at least 2 h later). Bats always had access to fresh water. On days juvenile observers were tested, we fed them the same amount of mealworms they were fed during the non-experimental period to avoid food deprivation. We fed adult observers approximately 1/3 of the usual amount of mealworms. This level and duration of food deprivation was not harmful to the animals, as evidenced by their continued active behaviour, lack of excessive weight loss (< 20% loss from pre-study weight), and return to pre-testing weight with no apparent ill effects following the study.
Experimental trials
On each day of testing, we allowed each observer in the experimental group to interact freely with a trained bat (demonstrator) while the demonstrator captured and consumed 15 mealworms from a tether approximately one meter in length. This constituted one test session. With the exception of one day on which two sessions were conducted for the same bats (one in the morning and one in the afternoon), observer bats were flown in one session per day. If a bat hid out of view in the room, we retrieved and released it or placed it on the wall. After each mealworm was taken, we immediately suspended another tethered mealworm from the ceiling. Bats were free to fly or land on the wall between capture events. We alternated the demonstrator bat with which each observer flew daily such that observers were generally not paired with the same demonstrator two days in a row and flew with each available trained demonstrator roughly an equal number of times.
Control trials
We treated bats in the control group in the same manner as those in the experimental group, except that they were flown with untrained (naïve) bats. Control sessions lasted 7 min each, during which the “demonstrator” and the observer could interact freely. Trained bats frequently captured all 15 mealworms in less than 7 min, and attempts to allow untrained bats to fly for longer resulted in the bats landing on the walls or ceiling, rather than continuing to fly. To give control bats the same cues as experimental bats, we climbed a step ladder and appeared to present tethered mealworms at least seven but no more than 15 times during each control trial. We alternated the demonstrator with which each observer flew daily such that observers were generally not paired with the same demonstrator two days in a row and flew with each available control demonstrator equally often. Two juveniles in the control group were inadvertently flown with a trained demonstrator for one session each early in the experiment. These errors represent less than 1% of all test sessions and did not affect the outcome of the study.
Except for the first two to three days of the study in 2006, we gave each observer bat (in experimental and control groups) the opportunity to fly alone in the presence of a mealworm for three minutes immediately following interaction with the demonstrator. The purpose of this was to assess behaviours potentially related to learning to capture the mealworm. Because it was often not possible to distinguish observer from demonstrator bats during test sessions, learning behaviour of observers might have gone unnoticed if bats were only observed in pairs. If, during its time alone, a bat roosted on the wall for extended periods (e.g., > 1 minute) or hid out of view, an experimenter approached the bat to encourage it to fly again. If the bat directed a feeding buzz (detected using either a Pettersson D100 or D240x heterodyne bat detector (Pettersson Electronik, AB, Uppsala, Sweden) set to 35kHz) towards the mealworm, or otherwise appeared to show interest in the tethered mealworm (e.g., repeatedly flying near the mealworm), we extended the 3 min period until the bat stopped flying (2006) or until an additional 3 min passed (2007). If the bat directed a buzz towards the mealworm during the additional 3 min, we extended the time by another 3 min. If a bat learned to capture the mealworm, we tested it alone by offering 10 to 20 tethered mealworms on two additional consecutive days to ensure that it retained this behaviour.
Data Collection
Set-up and equipment
We tested bats in a large, carpeted flight room (Fig. 1), with walls and ceiling covered with acoustic foam. The room was equipped with low intensity and long wavelength overhead lighting (> 650 nm, red filters, Reed Plastics, Rockville, MD) to minimize availability of visual cues (Hope & Bhatnagar 1979). The experimenters also used red light emitting diode (LED) headlamps to observe behaviour and keep track of bats during experiments. We made synchronized stereo video and audio recordings using two high-speed (240 frames/sec in 2006; 250 frames/sec in 2007) infrared-sensitive video cameras (in 2006: Kodak MotionCorder Analyzers, Model 1000, Eastman Kodak Company, San Diego, California, USA; in 2007: Photron PCI-R2, Photron USA, Inc., San Diego, California, USA) and two ultrasound-sensitive microphones (UltraSound Advice, London, UK) amplified (UltraSound Advice, London, UK) and recorded at 250 kHz/channel (Wavebook, IOTech, Cleveland, Ohio, USA; Fig. 1). We recorded eight-second segments of synchronized high-speed video and audio from experimental and control sessions. We also viewed trials in real-time using an infrared-sensitive Sony NightShot camcorder (Sony Electronics, San Diego, California, USA).
Social learning
We scored the response of each observer bat into one of four categories with regard to how it interacted with the mealworm (henceforth referred to as category): 1: no buzz, 2: buzz only, 3a: attack without capture, or 3b: attack with capture. We used a combination of visual and auditory information (see Table 1) to categorize responses and based our assessment on times when bats were flying alone or we could otherwise clearly identify which bat was the observer. For example, if it appeared that an observer might have buzzed at the mealworm while flying with a demonstrator but we were uncertain which bat emitted the buzz, we did not attribute buzzing behaviour to the observer bat at this time. For subsequent analyses, we combined bats that attacked with or without capture (categories 3a and 3b) into one group (category 3). We made this decision because juvenile bats that made repeated attacks on the mealworm (emitting feeding buzzes while flying towards the mealworm and knocking it from the string) appeared to identify the mealworm as a prey item and attempt to capture it, but lacked the coordination to successfully take the prey from the tether. When being trained, adult bats learning to take tethered mealworms frequently produced buzz sequences directed at the prey and knocked the prey to the ground prior to mastering the capture task. Because juveniles were fed each day, some individuals may have lacked sufficient motivation to continue attempting to capture the mealworm as their motor coordination increased. Because our goal was to assess whether bats were learning from conspecifics to recognize an item as prey and how to approach it (skills which might be learned socially), rather than to assess the flight skills and coordination of individuals (which could likely only be acquired via individual learning/practice), we considered bats to have displayed evidence of learning socially about acquiring the prey if they produced feeding buzzes and attacked the prey item (knocked it to the ground), regardless of whether they successfully took prey from the tether during the experimental period. We used a Fisher’s exact test to compare evidence of learning from individuals in control versus experimental groups.
Table 1.
Learning categories.
| Category | Observed Behaviour | Evidence | Interpretation |
|---|---|---|---|
| 1. No Buzz | No feeding buzz produced or attempts to capture mealworm | No buzz audible on bat detector; no visual evidence of bat approaching mealworm | No learning: Bat does not notice mealworm and/or does not recognize it as a prey item. |
| 2. Buzz only | Emitted feeding buzz(es) while approaching mealworm | Detected by bat detector, combined with visual observation of the bat’s location and direction through NightShot camera | Investigation: Bat investigates mealworm but never makes an attack or capture; bat may or may not recognize mealworm as a prey item. |
| 3a. Attack without capture | Contact with the mealworm in association with feeding buzzes directed towards the mealworm—i.e., hitting it with the nose and knocking it from the string while producing a feeding buzz | Detected via bat detector and visual observation through camera | Learning (but lacked motor skills for successful capture): Bat recognizes mealworm as prey item and attacks it in capture attempt(s) but does not demonstrate the foraging skills necessary to capture it. |
| 3b. Attack with capture | Successful, repeated capture and consumption of the mealworm from the tether | Detected via bat detector, observation through camera, and subsequent chewing sounds/visual observation of bat chewing combined with absence of mealworm from string | Learning (with motor skills for successful capture): Bat recognizes the mealworm as a prey item and demonstrates the foraging skills necessary to capture and consume it. |
Flight behaviour
We tested whether increased inter-bat interaction was associated with increased likelihood of learning the foraging task. To quantify level of inter-bat interaction during trials, we analysed high-speed videos to assess both chasing/following behaviour and in-flight inter-bat distances. We predicted that there would be an association between observer bats flying close to demonstrator bats and learning the foraging task. If naïve bats were attending to the feeding behaviours of knowledgeable bats, or if knowledgeable bats were behaving competitively towards naïve bats as the latter learned the foraging task, shorter inter-bat distances and higher prevalence of following or chasing behaviour are expected for trials in which observer bats that eventually learned the task were flying. If this is the case, we expect bats attacking or capturing the prey item (category 3) to fly closer to demonstrator bats and engage in chasing/following behaviour more frequently than category 1 (no evidence of learning) bats throughout the experiment. Furthermore, we wanted to test whether observer-demonstrator flight distance decreased over time only for category 3 bats, which could indicate that as observers fly increasingly close to demonstrators or follow them more frequently, they are more likely to acquire information from demonstrators and learn the task. This result, if found, might also indicate an increased level of competition between observers beginning to learn the task and demonstrators.
We conducted video analysis on 145 8-s recordings from 22 observer bats. Some category 2 and 3 bats that never successfully consumed a tethered mealworm eventually stopped displaying buzzing and attacking behaviour. All such individuals were juveniles and may have given up and waited to be fed later in the day after repeated unsuccessful capture attempts. Trials occurring from this point forward are not included in the 145 analysed recordings. Aside from these trials, we used all available recordings for category 3 bats (n = 67 recordings from 7 bats) and a minimum of 6 recordings per bat including first, middle, and last days (defined below) as available for category 1 (n = 52 recordings from 10 bats) and 2 (n = 26 recordings from 5 bats) bats. We only included portions of recordings in which both bats were flying and visible in both camera views within the calibrated space and did not include recordings with fewer than 200 frames (~800 ms) meeting these criteria. In cases with more than one useable recording from the same bat on the same day, we combined data from these recordings for analyses of inter-bat interactions. In total, these 145 recordings came from 99 distinct test sessions (29 from category 3 bats, 22 from category 2 bats, and 48 from category 1 bats). The total number of frames used in the analyses was 113 710, and the mean number of frames used per session was 1149 (±SD 944), with number of frames ranging from 226 to 5531.
To account for any behavioural changes over time, sessions were divided into three ordered periods: 1) the first day a bat flew in the experiment, or the first day with a useable recording, as long as it was not after the third day in the experiment; 2) any days between the days described in period 1 and period 3; and 3) the last day flying (category 1) or buzzing/attacking/catching days (categories 2 and 3)—this was the last day flying for bats in category 1 (or the last available day, up to three days back), or days on which category 2 and 3 bats buzzed at, attacked, and/or captured the mealworm. Days after a category 2 or 3 bat had first emitted buzzes at the mealworm but did not do so on that day were not included in the analyses. For one control bat in category 2 (a juvenile that died after 5 days of testing), only one analyzable day was available, and this was both the first day and the first day the bat buzzed at the mealworm; this session was counted as time period 3.
Using a custom Matlab programme that allowed us to mark and plot the 3-dimensional flight trajectories of each bat (see Chiu et al. 2008), we calculated the mean in-flight inter-bat distance (IBD) between observer and demonstrator bats for each video file analysed. We used a generalized linear mixed model (GLMM) to compare mean distances in different learning categories and time periods. This analysis accounted for the repeated measures nature of most of the data (we had more than one data point for 20 of the 22 bats tested) by considering bat ID in the model.
To test our hypothesis regarding chasing/following behaviour, we considered a combination of the angle between bats’ flight paths (inter-bat angle—IBA) and IBD to determine how often bats in each learning category flew in close, following/chasing configurations with demonstrators. Using position data from video analysis (described above), we calculated the proportion of each analysed test session bats flew in a following formation (as opposed to converging or diverging flight) with an IBA of < 30° and an IBD of < 1m simultaneously. This flight configuration represents one bat tightly following or chasing the other. Because the data were not normally distributed and contained many zeroes, we compared the percentage of sessions with following occurring at least 10% of the time (i.e., in ≥ 10% of useable frames) versus < 10% of the time between bats in each of the three learning categories and across time. We chose a 10% criterion because the overall mean percentage of frames representing the following configuration (including all learning categories and times) was approximately 10%.
For each 8-s recording used, we evaluated whether we could identify which was the observer and which was the demonstrator in the video and audio recordings to help determine which bat was following which. We identified bats based upon written notes of individual behaviour during trials, and based upon matching which bat was known to capture the mealworm with which bat emitted a feeding buzz using position data of each bat relative to the two microphones. For a variety of reasons, positive identification of both bats was possible for only about one quarter of recordings (34 of 145).
Feeding buzz analysis
A feeding buzz (FB) refers to calls made as a bat initiates an attack on a prey item, and calls in FBs are characterized by increasingly shorter duration and pulse interval (PI-- time from start of one pulse to start of the next pulse), as well as a decrease in call frequency (e.g., Griffin et al. 1960; Surlykke & Moss 2000). For the following analysis, we identified FBs via visual and auditory examination of recordings, then confirmed by measuring PI from oscillograms and spectrograms to ensure that PI was < 13ms and dropped to < 8ms (Surlykke & Moss 2000). In data collected from bats in categories 2 and 3 flying with demonstrators from the first study day through the last day each bat emitted buzzes towards or first took the mealworm, we recorded 28 8-s recordings in which at least one feeding buzz was identified, both audio and video recordings were available for analysis, and both bats were flying and visible in the calibrated space during the entire FB(s). We calculated the mean IBD during each FB (when PIs were < 13 ms) and during another portion of the same trial. We then used the detailed information available from these 28 pairs of values recorded with 8 different observer bats on 19 test days (sessions) to examine mean IBD at the time of the buzzes compared with other times during the same recordings.
If naïve bats attend to the feeding behaviours of knowledgeable bats, or if knowledgeable bats behave competitively towards naïve bats as the latter learn the task, we expected the IBD during feeding buzzes to be smaller than at other times during the recording. To test this prediction, for each feeding buzz we calculated the mean IBD during the buzz and compared this value to the mean IBD during another 260 ms (mean buzz duration = 257 ms) period in the same 8-s recording. Depending upon availability of 260 consecutive ms with both bats flying in the calibrated space, this period began (in order of preference): approximately 1 s prior to the buzz, more than 1 s prior to the buzz, approximately 1 s after the buzz, or more than 1 s after the buzz. We conducted separate analyses for recordings on days before versus after the observers present began emitting buzzes towards or attacking the mealworm. We compared the IBD during feeding buzzes versus the other portion of each recording in a generalized linear mixed model (GLMM) accounting the repeated measures nature of the data (i.e., more than one recording per bat). For a recording containing two feeding buzzes, we used the mean IBD during the buzzes and the mean IBD during two other portions of the recording.
RESULTS
Social Learning
Observer bats in the experimental group, including juveniles with little or no prior experience foraging, were significantly more likely to direct feeding buzzes towards and attack the mealworm than bats in the control group. Because we predicted that bats exposed to knowledgeable demonstrators were more likely to learn the task, we used a one-tailed test to assess the significance of our findings. A significantly greater number of experimental bats (82%-- six young and three adult) than control bats (27%-- three young) directed feeding buzzes towards the mealworm (Fisher’s exact test, one-tailed: P = 0.015). Furthermore, seven bats in the experimental group (64%-- five young and two adult) and no bat in the control group showed evidence of learning the task by attacking the mealworm and knocking it from the tether (Fisher’s exact test, one-tailed: P = 0.002). Four of the seven bats (two young and two adult) successfully captured the mealworm after directing feeding buzzes towards it. Bats began to display attacking/catching behaviour after an average of 6.1±2.5 (X±SD) sessions (range of 2–9 sessions) of exposure to knowledgeable demonstrators. A greater number of juveniles exposed to knowledgeable bats attacked the mealworm, compared to juveniles in the control group (Fisher’s exact test, one-tailed: P = 0.010). No bat of any age in the control group ever attacked the mealworm. We found no consistent pattern between age and likelihood of learning the task. The ages in days (X±SD) of young bats displaying no evidence of learning (category 1), only emitting buzzes towards the mealworm (category 2), and attacking/catching the mealworm (category 3), were 35±12, 38±15, and 34±6, respectively.
Flight Behaviour and Inter-bat Interactions
Bats that eventually attacked or captured the mealworm (category 3) flew significantly closer to demonstrators than did other bats (Fig. 2). In addition, we found a higher prevalence of following/chasing behaviour in sessions from category 3 bats than in sessions with bats that never buzzed at the mealworm (category 1; Fig. 3). We found no significant trend with regard to inter-bat distance (IBD) or following/chasing behaviour based on number of days bats had flown in the experiment (GLMM: time period P > 0.05 in both cases). However, we did find that bats flew closer together during feeding buzzes than at another time within the same 8-s recording after, but not before, the observer bat present began to display buzzing/attacking behaviour (Fig. 4).
Figure 2.
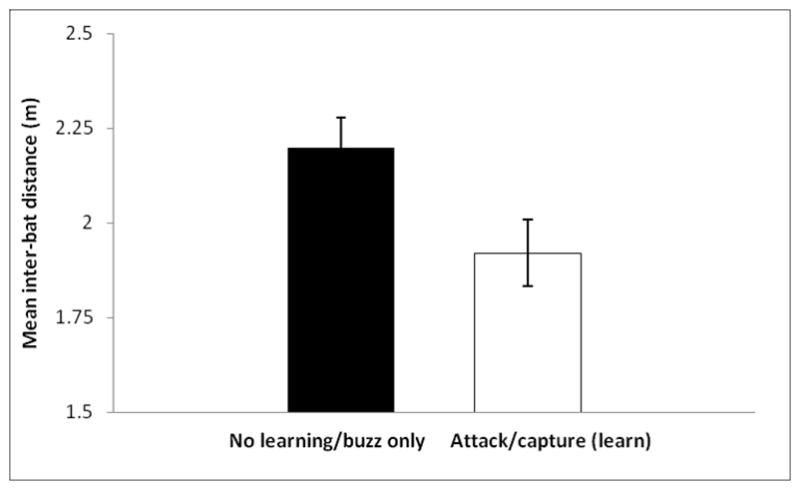
Mean inter-bat flight path distances for test sessions in which observer bats that did not buzz at or buzzed at but did not attack the mealworm flew (n = 70 sessions, 15 bats) versus those in which observer bats eventually attacking and/or capturing the mealworm flew (n = 29 sessions, 7 bats). Error bars represent one standard error.
Figure 3.
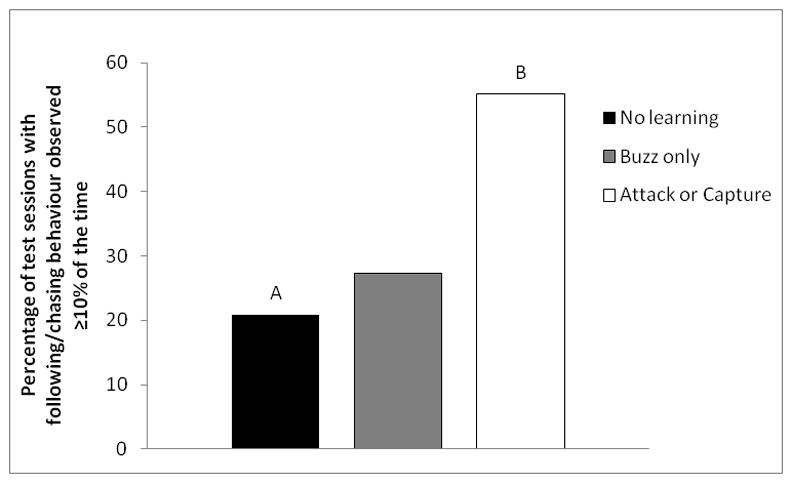
The percentage of test sessions in each learning category in which following or chasing behaviour, defined as one bat trailing the other by < 1m with a flight path < 30° from the leading bats’ flight path, was present ≥10% of the time. Different letters indicate significant differences in means. No buzz: n = 48 sessions, 10 bats; buzz only: n = 22 sessions, 5 bats; attack or catch: n = 29 sessions, 7 bats.
Figure 4.
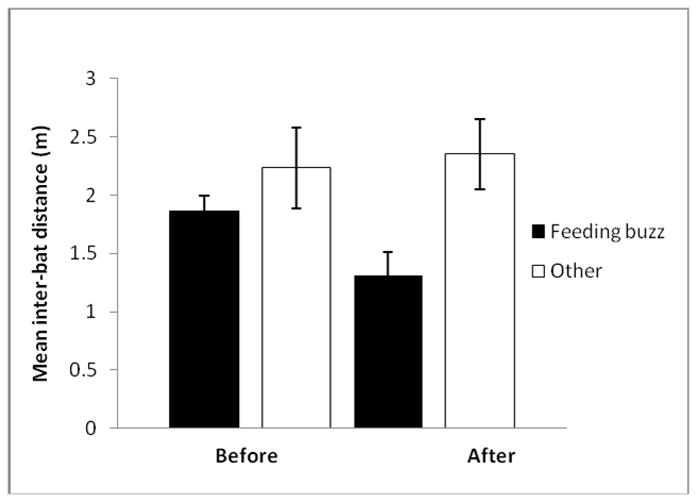
In recordings containing a feeding buzz(es), the mean IBD in meters during the feeding buzz and at another segment of approximately equal length (260 ms) for each recording. “Before” (n = 15 recordings from 6 bats) and “after” (n = 13 recordings from 5 bats) refer to whether the observer bat present had yet begun to emit buzzes towards or attack the mealworm when the recording was recorded. Error bars represent one standard error.
Analysis of video data revealed significant differences in mean inter-bat distances (IBDs) between bats in different learning categories. Specifically, smaller IBD was positively related to observers attacking or capturing the prey item. Category 1 and 2 bats maintained a significantly larger mean distance from demonstrator bats than did bats in category 3 (GLMM: F1,20 = 4.84, P = 0.0398), with category 3 bats flying an average of 0.278 m closer to demonstrators (Fig. 2).
We defined following/chasing behaviour as bats flying in a following formation (not converging or diverging) with an IBD of < 1m while simultaneously flying with trajectories < 30° apart. We found a significant difference in prevalence of chasing/following behaviour in test sessions with bats from different learning categories (GLMM: F2,19 = 3.99, P = 0.036), but not across time (same GLMM: F2,32 = 0.33, P = 0.72). Because we predicted that sessions in which bats displaying learning behaviour flew were more likely to contain chasing or following behaviour, we used one-tailed tests for pairwise comparisons. The percentage of sessions in which bats displayed following/chasing behaviour ≥10% of the time was more than two times greater for category 3 bats (55%; n = 29 test sessions from 7 bats) than for category 1 bats (21%; n = 48 test sessions from 10 bats; pairwise comparison from GLMM above, one-tailed test with Bonferroni correction: F1,19 = 7.00, P = 0.024; Fig. 3, supplemental videos S1–S3).
Of the 145 8-s video/audio recordings used in the analysis, we could confidently identify which bat was which in 34 recordings. Of these recordings, 11 (from 10 sessions, 7 observers, and 4 demonstrators) contained following/chasing behaviour as described above, and eight of these contained category 3 bats. Of these eight trials, the demonstrator led at least some of the time in 87.5% of trials, compared with 62.5% of trials showing the observer leading. In considering total frame numbers containing following, 60% of these frames represent the demonstrator following the observer, and 40% represent the observer following the demonstrator.
Behaviour during feeding buzzes
For recordings on days before observer bats buzzed at or attacked the mealworm (before), we found no significant difference in IBD during feeding buzzes vs. at another time in a recording (GLMM: F1,5 = 1.06, P = 0.35; n = 15 recordings). However, for recordings occurring once observer bats had begun directing buzzes towards or attacking the mealworm (after), we found that bats flew, on average, more than 1m closer to one another during feeding buzzes than at other points in the same recording (GLMM: F1,4 = 8.25, P = 0.045; n = 13 recordings; Fig. 4). For 55% of the 29 feeding buzzes analysed, IBD decreased from the beginning of a feeding buzz (Fig. 5, supplemental videos S2 and S3).
Figure 5.
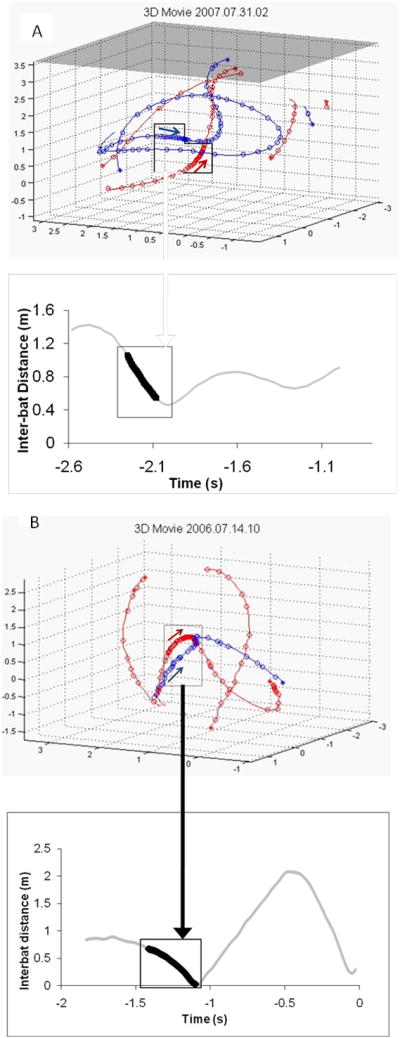
Sample plots showing bat flight paths and IBD over time during recordings from the days two observer bats first attacked (A) or captured (B) the mealworm. Red and blue represent each bat’s flight path, and each circle represents a vocalization emitted by that bat. The box corresponds to the emission of a feeding buzz by one of the bats, and the arrows indicate the flight direction of each bat at the time of the buzz. In the time by distance plots, the darkened line and box show the timing of the buzz. In A, the red bat first emits a feeding buzz towards the mealworm, then the blue bat emits a buzz in the direction of the mealworm. In B, the red bat emits the feeding buzz and flies ahead of the blue bat (also see supplemental video S2). Because both observer and demonstrator were potentially emitting buzzes towards the mealworm, it is not clear which bat emitted the feeding buzz in these recordings, but IBD dropped steeply during the duration of the feeding buzz in both A and B.
DISCUSSION
Our results demonstrate that Eptesicus fuscus can learn a novel foraging task via exposure to knowledgeable conspecifics and that higher levels of interaction between demonstrators and naïve bats, including smaller inter-bat distance and increased following/chasing behaviour, are positively related to this learning. Furthermore, we found evidence that naïve bats learning the task (category 3) show increased levels of interaction with demonstrators during feeding buzzes over time as they learn. Our data indicate that the presence of knowledgeable, foraging bats may be important for newly volant E. fuscus first learning to catch insects. Observation of and interaction with other foraging bats, while developing increasing flight skills and agility, appear to facilitate rapid acquisition of foraging skills in young E. fuscus. While the number of adults we were able to test limited the power of some analyses, our observations indicate that learning from others is not limited to a particular developmental stage in this species. These results are consistent with those of Gaudet & Fenton (1984), who found that adult E. fuscus could learn a different foraging task from others.
We found that increased inter-bat interaction is positively associated with increased likelihood of learning. The results do not allow us to infer whether interaction increased learning, vice versa, or both. It is possible that some observers were more likely to interact closely with (and perhaps attend more closely to the behaviour of) demonstrators, and therefore had greater opportunities for learning the foraging task. This inference is supported by the finding that bats eventually attacking or capturing the mealworm flew, on average, closer to demonstrator bats and displayed more following/chasing behaviour throughout the experiment than did bats failing to buzz towards the mealworm. It is also possible that as observer bats began to learn the foraging task, interaction increased as the naïve bat began to attend more closely to the demonstrator’s feeding behaviour, or even as a result of competition for the prey item. These latter scenarios are supported by the fact that bats eventually attacking or capturing the mealworm flew closer to demonstrators during feeding buzzes (compared with other points in the same recordings) only after they began to show evidence of learning. A combination of these scenarios is supported by the fact that we found following and leading by both observer and demonstrator bats.
We found that in the majority of test sessions (55%) recorded from bats eventually attacking or catching the mealworm, bats displayed close chasing/following behaviour ≥ 10% of the time, while this was only true in 21% of sessions examined from bats that did not emit buzzes towards the mealworm. Our criteria of < 30° degree IBA and < 1m IBD represent close following behaviour, considering the flight speed of this species. Assuming a mean flight speed of 3.5 m/s in an enclosed room (Craft et al. 1958, Chiu et al. 2008), a trailing bat flying in this configuration would be, at most, about 285 ms behind the leading bat. The angle constraint indicates that bats are traveling in the same direction, and this close IBD may relate to the amount and quality of information available to observer bats. Considering the darkened conditions, bats were likely attending to auditory cues from demonstrators. Spherical spreading loss and attenuation of high-frequency sounds result in lower levels of acoustic energy further from a sound source (Lawrence & Simmons 1982). Chiu et al. (2008) found that bats flying in a set-up similar to the one used in this experiment exhibited increased levels of silent behaviour (presumably to avoid echolocation interference) the closer together they flew, particularly when flying within 1m of one another. If observer bats were obtaining acoustic information from demonstrator bats, flying closer to the knowledgeable bats may have increased the amount and quality of information they could obtain by listening to cues from the knowledgeable bat. A field study of foraging red bats (Lasiurus borealis) revealed chasing behaviour that likely facilitated eavesdropping on the feeding-related cues of conspecifics (Hickey & Fenton 1990).
The chasing/following behaviour we observed could also represent a demonstrator chasing an observer during competition for the prey item, as has been previously observed in some bat species. For example, Rydell (1986) reported that female northern bats (Eptesicus nilssoni) defend foraging areas via aggressive chasing and vocalizations. In addition, aerial “dogfights” wherein foraging E. fuscus chase one another have been reported in the field (Simmons et al. 2001). In a set-up similar to the one in this study, Chiu et al. (2010) observed (sometimes aggressive) chasing behaviour frequently when two trained adult E. fuscus competed for a single mealworm. We could only confidently identify which bat was in the lead in eight trials containing following/chasing behaviour and with category 3 bats flying. In the majority of these trials (87.5%), demonstrators were leading at least some of the time; however, 60% of total following time was representative of observers flying in the lead. The small number of trials available for this level of detailed analysis does not allow for broad conclusions regarding the following/chasing behaviour observed, but these trials do confirm that both observer and demonstrator bats showed following or chasing behaviour. Hickey and Fenton (1990) found that four of five tagged red bats foraging in the wild chased and were chased equally often. Given the set-up of our study, we usually did not know which bat captured the mealworm when a pair of bats was flying unless it was the demonstrator, so we do not have information about prey capture success relative to chasing behaviour. However, Chiu et al. (2010) found that the bat spending more time following/chasing generally had more success capturing the prey item and sometimes appeared to chase the leading bat away from the prey item, indicating that the trailing bat was behaving in a territorial manner. Our findings support the idea that chasing/following could be indicative of both observers following demonstrators to gain information and demonstrators chasing observers in a competitive manner.
Observer bats in this study presumably had the opportunity to eavesdrop on search and approach phase echolocation calls, feeding buzzes, and chewing sounds to learn about the location and nature of the tethered mealworm. Barclay (1982) found that little brown bats (Myotis lucifugus), and most likely E. fuscus, were attracted to the echolocation calls of other individuals in feeding situations, and that subadults were particularly responsive to these calls. Similarly, Gillam (2007) demonstrated that feeding buzzes attract Brazilian free-tailed bats (Tadarida brasiliensis), and Ruczynski et al. (2007) found that hearing conspecific echolocation calls helps noctule bats (Nyctalus noctula) locate roosts. In addition, Dechmann et al. (2009) found that echolocation calls mediate group foraging and passive information transfer about feeding activities in the insectivorous lesser bulldog bat (Noctilio albiventris). The nature of our study allowed us to make detailed observations of behaviour surrounding feeding buzzes, and our findings show that once bats began to buzz at or attack the mealworm, they flew, on average, closer to the demonstrators (or vice versa) than at other points during the same 8-s recordings. This finding strongly suggests an increase in attention to the feeding behaviour of the demonstrator over time by observers.
In addition to echolocation-related cues, other researchers (e.g., Fenton et al. 1983—Nycteris grandis; Page & Ryan 2006—Trachops cirrhosus) have noted that bats respond to the chewing sounds of conspecifics. We also made this observation during our study. Because our findings are consistent with bats using auditory cues to locate prey, it is possible that the behaviour we observed can be explained by local enhancement. If this was the case, naïve bats may have learned about the general location and nature of the prey item by listening to experienced bats forage. Once the attention of naïve bats was drawn to the correct area and prey item, they may have learned on their own how to capture the prey. This may also help explain why some bats attacked the mealworm without successful capture—perhaps they were able to make use of socially mediated information (location/type of prey) but failed to learn to capture the prey.
We made several noteworthy observations about the behaviour of young bats during the experiment. We tested both juveniles that had never foraged outside our lab (captured when pre-volant, or born in captivity) and those that had probably foraged briefly prior to capture (as evidenced by their estimated age when collected from the wild and their ability to fly). The only juvenile in the experimental group that did not emit buzzes towards the mealworm was also the only captive-born bat in this group. In addition, all three bats in the control group that buzzed at the mealworm were captured when already volant. These observations suggest that prior experience might be useful in learning a new foraging task; however, it does not appear to be essential. Of the two experimental juveniles captured when they were a few days old (pre-volant), one emitted buzzes towards the mealworm, and the other attacked it.
The three bats that repeatedly attacked the mealworm while directing buzzes towards it (but did not successfully capture it) were all juveniles, indicating that developmental abilities likely played a role in performance. In addition, because young bats were not food-deprived during the experiment, if a young bat began detecting the mealworm as a prey item, but was repeatedly unsuccessful at capture, it could have given up and waited to be fed later in the day. That juvenile bats, but not adults, in the control group emitted buzzes towards the mealworm may indicate that newly volant bats are more likely to investigate a novel item as a potential food source. This has been seen in other species as well; for example, Biondi et al. (2010) found that juvenile raptors (Milvago chimango) outperformed adults and were quicker to investigate a box containing food in a social learning experiment. This suggests that a tendency to explore and individual learning are also important in the process by which young animals, including insectivorous bats, learn to forage. The result that no bat in the control group, compared with a majority of juveniles in the experimental group, ever attacked the mealworm signifies that social learning can be an integral part of the process as well. While a young bat may have an innate tendency to investigate an item, hearing an experienced conspecific track, capture, and consume the prey item may both confirm that the object is edible and provide information about where and how to obtain the prey item.
Although many studies addressing social learning by juveniles focus on transmission of information or skills from parent to offspring, our findings demonstrate that young animals that commonly forage in the vicinity of unrelated adults can learn from non-kin. This result is consistent with findings from previous studies of other animals that forage in similar social settings, such as birds that scramble-compete for food (Hatch & Lefebvre 1997) and young perch that acquire information about appropriate prey (Magnhagen & Staffan 2003).
Previous studies have demonstrated social learning in a foraging setting for a variety of species. However, the mechanism by which such learning occurs is often unknown, only anecdotally described, or poorly understood. Analysis of high-speed video interactions and audio files allowed us to quantitatively examine interactions between observer and demonstrator bats and reach the conclusion that increased in-flight interaction, as measured by smaller inter-bat distances and greater likelihood of following/chasing behaviour, is positively associated with social learning, a finding not previously reported for any bat species. In addition, we showed quantitatively that bats displaying evidence of learning (buzzing and/or attacking the mealworm) fly closer to demonstrator bats during feeding buzzes only after showing buzzing or attacking behaviour (indicating that they have begun to learn the task). In conclusion, our results indicate that juvenile E. fuscus learn about where and how to capture prey by interacting with experienced conspecifics and that this learning behaviour is not limited to young bats. Bats that learned to attack the mealworm interacted more with demonstrator bats, and appeared to learn via feeding-related auditory cues from conspecifics. Further research could determine whether other bat species learn to forage in a similar way.
Supplementary Material
Video S1 (2006.07.18.04). Animation of a recording from a session on the day before the observer bat present (learning category 3) first emitted buzzes towards the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. Based upon behaviour and call characteristics, the red bat is the demonstrator (Y31) in this trial, and the blue bat is the observer (B47). When the blue bat first appears, note the close following behaviour of the blue bat (naïve juvenile observer) behind the red bat (trained demonstrator), who emits a feeding buzz later in the trial. The portion of the video in which the bats disappear then reappear from another location is due to the bats temporarily leaving the field of view of both cameras.
Video S2 (2006.07.14.10). Animation of a recording from a session the first day the observer bat present (B60) captured the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. In this animation, it is not known which bat is which (because both bats may have emitted feeding buzzes during this session). Note the close following behaviour of the red bat by the blue bat as the red bat hones in on the prey item and emits a feeding buzz.
Video S3 (2007.07.31.04). Animation of a recording from a session from the first day the observer bat present (OR48) first attacked the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. In this animation, it is not known which bat is which (because both bats may have emitted feeding buzzes during this session). Note the following behaviour of the red bat by the blue bat as the red bat hones in on the prey item and emits a feeding buzz.
Highlights.
We assessed social learning of a foraging task in juvenile and adult big brown bats.
Naïve bats exposed to knowledgeable demonstrators displayed learning.
Bats that learned the task flew closer to demonstrators than did nonlearning bats.
Increased following/chasing behaviour was associated with social learning.
Smaller inter-bat distances during feeding buzzes were associated with social learning.
Acknowledgments
We thank J. Finder, N. Luciano, R. Yu, W. Law, M. Chavis, S. Ball, J. Botvinick, A. Murti, C. Atekwana, N. Destler, K. Isgrig, J. Kalkavage, and C. Seo for assistance in collecting and analyzing data. C. Chiu, W. Xian, B. Falk A. Perez, H. Xi, M. Chadha, and J. Wright also assisted. We are grateful to the Demery family and others who gave us access to the bats at their homes. Members of the Wilkinson and Moss labs provided useful discussions about this research, and along with two anonymous reviewers, helpful comments on earlier drafts of the manuscript. P. Blank and B. Momen provided guidance regarding statistical analyses. This research was conducted while G.S. Wright was supported by training grant DC-00046 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Barclay RMR. Interindividual use of echolocation calls: eavesdropping by bats. Behavioral Ecology Sociobiology. 1982;10:271–275. [Google Scholar]
- Biondi LM, Garcia GO, Bo MS, Vassallo AI. Social learning in the Caracara Chimango, Milvago chimango (Aves: Falconiformes): an age comparison. Ethology. 2010;116:722–735. [Google Scholar]
- Bonnie KE, Earley RL. Expanding the scope for social information use. Animal Behaviour. 2007;74:171–181. [Google Scholar]
- Boyd R, Richerson PJ. Culture and the evolutionary process. Chicago: University of Chicago Press; 1985. [Google Scholar]
- Brigham RM, Brigham AC. Evidence for association between a mother bat and its young during and after foraging. American Midland Naturalist. 1989;121:205–207. [Google Scholar]
- Burnett CD, Kunz TH. Growth rates and age estimation in Eptesicus fuscus and comparison with Myotis lucifugus. Journal of Mammalogy. 1982;63:33–41. [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Cultural versus genetic adaptation. Proceedings of the National Academy of Sciences, USA. 1983;80:4993–4996. doi: 10.1073/pnas.80.16.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Xian W, Moss CF. Flying in silence: echolocating bats cease vocalizing to avoid sonar jamming. Proceedings of the National Academy of Sciences. 2008;105:13115–13120. doi: 10.1073/pnas.0804408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Reddy PV, Xian W, Krishnaprasad PS, Moss CF. Effects of competitive prey capture on flight behavior and sonar beam pattern in paired big brown bats, Eptesicus fuscus. The Journal of Experimental Biology. 2010;213:3348–3356. doi: 10.1242/jeb.044818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft TJ, Edmonson MI, Agee R. A comparative study of the mechanics of flying and swimming in some common brown bats. Ohio Journal of Science. 1958;58:245–249. [Google Scholar]
- Davis WH, Hitchcok HB. Biology and migration of the bat, Myotis lucifugus, in New England. Journal of Mammalogy. 1965;46:296–313. [Google Scholar]
- Dechmann DKN, Heucke SL, Giuggioli L, Safi K, Voigt CC, Wikelski M. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proceedings of the Royal Society B. 2009;276:2721–2728. doi: 10.1098/rspb.2009.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MB, Gaudet CL, Leonard ML. Feeding behaviour of the bats Nycteris grandis and Nycteris thebaica (Nycteridae) in captivity. Journal of Zoology (London) 1983;200:347–354. [Google Scholar]
- Galef BG., Jr . Communication of information concerning distant diets in a social, central-place foraging species: Rattus norvegicus. In: Zentall TR, Galef BG Jr, editors. Social Learning: Psychological and Biological Perspectives. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. pp. 119–139. [Google Scholar]
- Galef BG, Jr, Laland KN. Social learning in animals: empirical studies and theoretical models. Bioscience. 2005;55:489–499. [Google Scholar]
- Gaudet CL, Fenton MB. Observational learning in three species of insectivorous bats (Chiroptera) Animal Behaviour. 1984;32:385–388. [Google Scholar]
- Griffin DR, Webster FA, Michael CR. The echolocation of flying insects by bats. Animal Behaviour. 1960;8:141–154. [Google Scholar]
- Guthrie MJ. Notes on the seasonal movements and habits of some cave bats. Journal of Mammalogy. 1933;14:1–19. [Google Scholar]
- Hatch KK, Lefebvre L. Does father know best? Social learning from kin and non-kin in juvenile ringdoves. Behavioural Processes. 1997;41:1–10. doi: 10.1016/s0376-6357(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Hickey MBC, Fenton MB. Foraging by red bats (Lasiurus borealis): do intraspecific chases mean territoriality? Canadian Journal of Zoology. 1990;68:2477–2482. [Google Scholar]
- Hope GM, Bhatnagar KP. Electrical response of bat retina to spectral stimulation: comparison of four microchiropteran species. Experientia. 1979;35:1189–1191. doi: 10.1007/BF01963279. [DOI] [PubMed] [Google Scholar]
- Kerth G, Reckardt K. Information transfer about roosts in female Bechstein’s bats: an experimental field study. Proceedings of the Royal Society B. 2003;270:511–515. doi: 10.1098/rspb.2002.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz TH. Reproduction, growth, and mortality of the vespertilionid bat, Eptesicus fuscus, in Kansas. Journal of Mammalogy. 1974;55:1–13. [PubMed] [Google Scholar]
- Kurta A, Baker RH. Eptesicus fuscus. Mammalian Species. 1990;356:1–10. [Google Scholar]
- Lawrence BD, Simmons JA. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation. Journal of the Acoustical Society of America. 1982;71:585–590. doi: 10.1121/1.387529. [DOI] [PubMed] [Google Scholar]
- Magnhagen C, Staffan F. Social learning in young-of-the-year perch encountering a novel food type. Journal of Fish Biology. 2003;63:824–829. [Google Scholar]
- Page RA, Ryan MJ. Social transmission of novel foraging behavior in bats: frog calls and their referents. Current Biology. 2006;16:1201–1205. doi: 10.1016/j.cub.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Previde EP, Poli MD. Mother-pup transmission of a feeding technique in the golden hamster (Mesocricetus auratus) In: Gardner RA, Gardner BT, Chiarelli B, Plooji FX, editors. The Ethological Roots of Culture. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 125–142. [Google Scholar]
- Ratcliffe JM, ter Hofstede HM. Roosts as information centres: social learning of food preference in bats. Biology Letters. 2005;1:72–74. doi: 10.1098/rsbl.2004.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruczynski I, Kalko EKV, Siemers BM. The sensory basis of roost finding in a forest bat, Nyctalus noctula. The Journal of Experimental Biology. 2007;210:3607–3615. doi: 10.1242/jeb.009837. [DOI] [PubMed] [Google Scholar]
- Rydell J. Feeding territoriality in female northern bats, Eptesicus nilssoni. Ethology. 1986;72:329–337. [Google Scholar]
- Seppanen JT, Forman JT, Monkkonen M, Thomson RL. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology. 2007;88:1622–1633. doi: 10.1890/06-1757.1. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Eastman KM, Horowitz SS, O’Farrell MJ, Lee DN. Versatility of biosonar in the big brown bat, Eptesicus fuscus. Acoustic Research Letters Online. 2001;2:43–48. [Google Scholar]
- Surlykke A, Moss CF. Echolocation behavior of the big brown bat, Eptesicus fuscus, in the field and the laboratory. Journal of the Acoustical Society of America. 2000;108:2419–2429. doi: 10.1121/1.1315295. [DOI] [PubMed] [Google Scholar]
- Terkel J. Cultural transmission of feeding behavior in the black rat (Rattus rattus) In: Hayes CM, Galef BG Jr, editors. Social Learning in Animals: The Roots of Culture. San Diego: Academic Press; 1996. pp. 17–48. [Google Scholar]
- Thornton A. Social learning about novel foods in young meerkats. Animal Behaviour. 2008;76:1411–1421. [Google Scholar]
- Wilkinson GS. Altruism and co-operation in bats. In: Racey PA, Fenton MB, Rayner JMV, editors. Recent advances in the study of bats. Cambridge: Cambridge University Press; 1987. pp. 299–323. [Google Scholar]
- Wilkinson GS. Information transfer at evening bat colonies. Animal Behaviour. 1992;44:501–518. [Google Scholar]
- Wilkinson GS, Boughman JW. Social influences on foraging in bats. In: Box HO, Gibson KR, editors. Mammalian Social Learning: Comparative and Ecological Perspectives. Cambridge: Cambridge University Press; 1999. pp. 188–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 (2006.07.18.04). Animation of a recording from a session on the day before the observer bat present (learning category 3) first emitted buzzes towards the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. Based upon behaviour and call characteristics, the red bat is the demonstrator (Y31) in this trial, and the blue bat is the observer (B47). When the blue bat first appears, note the close following behaviour of the blue bat (naïve juvenile observer) behind the red bat (trained demonstrator), who emits a feeding buzz later in the trial. The portion of the video in which the bats disappear then reappear from another location is due to the bats temporarily leaving the field of view of both cameras.
Video S2 (2006.07.14.10). Animation of a recording from a session the first day the observer bat present (B60) captured the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. In this animation, it is not known which bat is which (because both bats may have emitted feeding buzzes during this session). Note the close following behaviour of the red bat by the blue bat as the red bat hones in on the prey item and emits a feeding buzz.
Video S3 (2007.07.31.04). Animation of a recording from a session from the first day the observer bat present (OR48) first attacked the mealworm. Red and blue each represent a bat, and the synchronized audio and video data have been slowed 10x. Each circle represents an echolocation pulse emitted by that bat. In this animation, it is not known which bat is which (because both bats may have emitted feeding buzzes during this session). Note the following behaviour of the red bat by the blue bat as the red bat hones in on the prey item and emits a feeding buzz.


