Abstract
Evidence has accumulated that the voltage-dependent anion channel (VDAC), located on the outer membrane of mitochondria, plays a central role in apoptosis. The involvement of VDAC oligomerization in apoptosis has been suggested in various studies. However, it still remains unknown how exactly VDAC supramolecular assembly can be regulated in the membrane. This study addresses the role of lipids in this process. We investigate the effect of cardiolipin (CL) and phosphatidylglycerol (PG), anionic lipids important for mitochondria metabolism and apoptosis, on VDAC oligomerization. By applying fluorescence cross-correlation spectroscopy to VDAC reconstituted into giant unilamellar vesicles, we demonstrate that PG significantly enhances VDAC oligomerization in the membrane, whereas cardiolipin disrupts VDAC supramolecular assemblies. During apoptosis, the level of PG in mitochondria increases, whereas the CL level decreases. We suggest that the specific lipid composition of the outer mitochondrial membrane might be of crucial relevance and, thus, a potential cue for regulating the oligomeric state of VDAC.
Introduction
The mitochondrial voltage-dependent anion channel (VDAC) is the most abundant channel located at the interface between mitochondria and the cytoplasm. VDAC is supposedly of a crucial importance for the homeostasis of living cells, as it provides the main pathway for the exchange of metabolites, such as ATP and ADP, between the mitochondria and the cytosol (1).
Recent studies, however, have shown that this channel also plays an important role in cell death: accumulating evidence indicates that VDAC is involved in mitochondria-mediated apoptosis (2–4). In particular, apoptosis induction was found to be associated with increased VDAC oligomerization (5–7). The existence of VDAC dimers and higher oligomers was mentioned for the first time almost 30 years ago (8). Recent AFM studies on native mitochondrial membranes, as well as NMR studies on VDAC in detergent solution, confirm the ability of VDAC to form dimers, trimers, tetramers, and higher oligomers (9–11).
VDAC oligomerization during apoptosis has been suggested to result in the formation of large pores, allowing for release of apoptogenic factors including cytochrome c from mitochondria, and thus inducing cell death. In particular, the above-mentioned pores are believed to be created by VDAC homo-oligomers (6,12,13) or hetero-oligomers of VDAC with apoptotic proteins of the Bcl-2 family (9). Formation of these oligomers was suggested to be regulated not only by Bcl-2 family apoptotic proteins, but also by hexokinase (6) and cytochrome c (13), although no direct evidence has been presented so far to show that these proteins have a direct influence on VDAC oligomerization.
On the other hand, it is known that membrane protein function and structure can be regulated by lipids (14). Protein-lipid interactions can be either specific or nonspecific; in particular, they can depend on the properties of the lipid bilayer, including the lipid charge, hydrophobic mismatch, and the presence of nonbilayer lipids (for review, see Raja (15)). Protein oligomerization can thus also be significantly influenced by the lipid environment. In particular, it was shown that negatively charged lipids are important for the stability of the quaternary structure of ADP-ATP carrier (16) and potassium channel KcsA (17).
The anionic lipid cardiolipin (CL) is found almost exclusively in mitochondria (18). CL is considered as the major negatively charged lipid in the inner mitochondrial membrane (18 wt % of the total lipid content). The amount of CL in the outer mitochondrial membrane is lower (∼4 wt %); however, it is found in high concentrations at the contact sites between the inner and outer mitochondrial membrane (27 wt %) (19) where VDAC was shown to primarily localize (20). CL is synthesized from phosphatidylglycerol (PG) by means of cardiolipin synthetase (21). Under normal physiological conditions, the amount of PG in mitochondria is relatively low; during apoptosis, however, it can increase as much as twofold, whereas the opposite effect is observed for CL, the amount of which is significantly decreased (22,23). It was also shown that a knockdown of cardiolipin synthetase increases the level of PG and decreases the level of CL in HeLa cells, leading to an acceleration of stimuli-elicited apoptosis (24). The influence of CL on VDAC channel activity has been previously demonstrated by electrophysiology measurements (25). However, the interaction of VDAC with PG was not addressed before. In addition, whereas the role of CL in apoptosis is emphasized in the literature, the role of PG in the cell death is largely unknown.
Because of the tight relationship between CL and PG, and the important role that CL plays in apoptosis, it is reasonable to suggest that PG can also be involved in controlled cell death by participating in the regulation of VDAC oligomerization. To test this hypothesis, a chemically well-defined system that avoids unknown factors and biological off-target effects is required. To this end, we here employ a cell-free minimal model system (26)—giant unilamellar vesicles (GUVs) with incorporated VDAC. To characterize the degree of VDAC oligomerization in various lipid environments, fluorescence cross-correlation spectroscopy (FCCS) measurements were carried out to characterize VDAC oligomerization in GUV membranes containing CL, PG, and two other anionic lipids. Based on our results, we suggest that VDAC oligomerization in the membrane can be tuned by up- or downregulation of CL and PG levels in mitochondria during apoptosis, respectively.
Materials and Methods
Materials
DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DOPG (1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt)), DOPS (1,2-dioleoyl-sn-glycero-3-phospho-L-serine (sodium salt)), DOPI (1,2-dioleoyl-sn-glycero-3-phospho-(1′-myo-inositol) (ammonium salt)), CL (cardiolipin (heart, bovine) (sodium salt)), and DPhPC (1,2-diphytanoyl-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids (Alabaster, AL). Alexa Fluor 488 C5-maleimide was obtained from Invitrogen (Darmstadt, Germany). ATTO 655-maleimide was purchased from ATTO-TEC (Siegen, Germany). Detergent LDAO (N,N-dimethylododecilamine N-oxide) 30% in H2O was obtained from Sigma (St. Louis, MO). SM-2 Bio-Beads were purchased from Bio-Rad Laboratories (Hercules, CA).
The plasmid pTMVDAC1 containing the gene for hVDAC1 expression was a generous gift of Dr. Jörg H. Kleinschmidt (University of Konstanz, Konstanz, Germany).
Methods
VDAC purification, labeling, and refolding
A single cysteine mutant of hVDAC1 was generated using a PCR Quik-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA). The cysteine at position 232 was replaced by serine using the sense strand primer GCACATTAACCTGGGCagcGACATGGATTTCGACATTGC and anti-sense strand primer GCAATGTCGAAATCCATGTCgctGCCCAGGTTAATGTGC (lowercase letters show mismatched codons). The protein was expressed in Escherichia coli, purified from inclusion bodies, and refolded in LDAO detergent using the protocol described by Schanmugavadivu et al. (27). The protein was labeled in the unfolded state at the single cysteine by thiol-reactive dyes Alexa 488 and ATTO 655. The single-cysteine mutation of VDAC was shown not to affect VDAC oligomerization (28). To separate the labeled protein from free dye, a Sephadex G-25 gel filtration column (GE Healthcare, Waukesha, WI) was used. The protein concentration was determined by measuring absorbance at 280 nm using the extinction coefficient of 1.25 L/(g·cm) or by the Lowry method (both methods gave close values). The degree of labeling was determined by measuring absorption spectra of the protein and dye, and was found to be 90% for VDAC-Alexa 488 (VDACgreen) and 70% for VDAC-ATTO 655 (VDACred).
Reconstitution of VDAC into giant unilamellar vesicles
VDAC was reconstituted into GUVs using the protocol described by Girard et al. (29) with minor modifications of the GUV electroformation procedure. Small unilamellar vesicles were prepared from DOPC, DOPC/CL, DOPC/PG, DOPC/PI, DOPC/PS, and DOPC/PG/CL at various lipid mixture ratios at a total lipid concentration of 4 mg/ml by sonication in 2 mM MOPS-Tris buffer, pH 7. Mixed micelles were prepared by solubilizing small unilamellar vesicles in detergent at a lipid/detergent (LDAO) ratio of 2 w/w. VDAC in LDAO was added to the mixed micelles mixture at the lipid/protein ratio 40 w/w (which corresponds to the lipid/protein molar ratio of ∼1500:1) and incubated for 30 min. To remove the detergent, 30 mg of Bio-Beads (Bio-Rad Laboratories) per 1 mg of detergent were added to the mixture followed by incubation for 4 h. Proteoliposomes obtained in the above procedure at a concentration of 2 mg/ml were deposited on indium tin oxide-coated glass slides (GeSiM, Grosserkmannsdorf, Germany) in the form of several 2 μL droplets and dried for 30 min in a desiccator. GUVs were produced in a custom-made perfusion chamber by the electroformation method (30) in a buffer containing 300 mM sucrose, 1 mM MOPS-Tris, 2 mM KCl, pH 7. A simpler electroformation procedure compared to the one described in Girard et al. (29) was found to be sufficient for producing good-quality GUV samples with GUV diameters in the range of 10–100 μm. In particular, an alternating-current electric field with the root-mean-square field strength of 400 V/m was applied across the chamber at a frequency of 12 Hz for 3 h. After preparation, the buffer surrounding the vesicles was exchanged to 10 mM Tris, 150 mM KCl, pH 7.2.
Representative confocal fluorescent images of GUVs containing fluorescently labeled VDAC are shown in Fig. 1. As can be seen from the image, the protein was homogeneously distributed in the membrane, showing no significant aggregation, which allowed us to carry out measurements of protein diffusion and oligomerization using fluorescence cross-correlation spectroscopy (see below).
Figure 1.
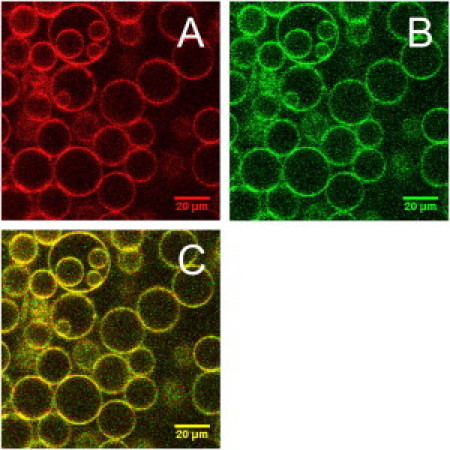
Representative confocal fluorescence microscopy images of GUVs with reconstituted red- and green-labeled VDAC: (A) red (ATTO 655) channel, (B) green (Alexa 488) channel, and (C) merge of panels A and B. GUV composition: DOPC/DOPG/CL 75:15:5.
VDAC activity measurements
To determine the structure of VDAC refolded in detergent, circular dichroism (CD) spectroscopy was used. Spectra were recorded by a model No. 715 CD spectrometer (JASCO, Tokyo, Japan) using a 0.5-mm cuvette. Far-UV CD spectra of VDAC in LDAO matches the one reported previously (27) (data not shown).
To check the functionality of the protein refolded in LDAO and incorporated into proteoliposomes, electrophysiology measurements were carried out (for details, see Supporting Material). Activity of refolded VDAC was studied in black lipid membranes prepared from DPhPC at a concentration of 5 mg/ml dissolved in pentane. For the electrophysiology studies on VDAC incorporated from proteoliposomes, black lipid membranes were made of DOPC, instead of DPhPC.
Experiments have shown that wild-type VDAC and its labeled single cysteine mutants are electrophysiologically active. Our results are in agreement with the previous studies, showing that VDAC cysteine residues do not influence protein function (28). In addition, our findings indicate that attachment of fluorescent dyes Alexa 488 and ATTO 655 to the cysteine residue at the position 127 does not affect VDAC channel activity.
We also found that upon reconstitution of VDAC from proteoliposomes, the channel shows the characteristic voltage-dependent behavior, indicating proper folding of VDAC in proteoliposomes.
FCCS measurements
FCCS measurements were carried out on a LSM 510 Meta system (Zeiss, Jena, Germany) using a home-built detection unit at the optical fiber output channel. All experiments were carried out at 21 ± 0.5°C. The light was collected using a 40× NA 1.2 UV-VIS-IR C-Apochromat water immersion objective (Zeiss, Jena, Germany). The sample was excited by the 488-nm line of an Argon-ion laser (6 μW) and the 633-nm line of a He-Ne laser (15 μW). A 570DCXRUV dichroic mirror and HQ 520/40 and HQ700/75 band-pass filters (AHF Analysentechnik, Tübingen, Germany) were positioned behind a collimating achromat to split the emission for the dual-color detection and to reject the residual laser light. Fluorescence was detected by avalanche photodiodes (PerkinElmer, San Jose, CA). Photon arrival times were recorded in the photon mode of a Flex 02-01D hardware correlator (Correlator.com, Bridgewater, NJ).
Two-focus, two-color scanning FCCS measurements across the GUV equator were carried out, and the resulting sets of auto- and cross-correlation curves of fluorescence intensity fluctuations were analyzed by a global nonlinear least-squares fitting software developed in-house in MATLAB v. R2007b (The MathWorks, Natick, MA). Detailed description of the experimental method and the model used in the data analysis is given in the Supporting Material. When analyzing the data, we assumed that diffusion coefficients of the red-labeled, green-labeled, and red-green-labeled species are all equal and independent of the protein oligomerization degree. The latter assumption is justified because of the very weak dependence of the translational diffusion coefficient on the membrane inclusion size for the expected monomer and oligomer sizes (31).
As a result of the analysis of FCCS data, surface concentrations of single-color (Cr and Cg) and two-color particles (Crg), as well as their diffusion coefficient, were determined.
Oligomerization of VDAC should not depend on whether it is labeled with a red or green fluorophore, and therefore formation of two-color (red-green) and single-color (red or green) oligomers is possible. Cr (Cg) will account not only for red (green) monomers, but also for the corresponding single-color oligomers. At the same time, Crg will describe the concentration of oligomers containing both red- and green-labeled molecules. As a result, Crg will generally underestimate the total concentration of oligomers (a fraction of which will be single-color), but this discrepancy vanishes fast upon an increase in the degree of oligomerization.
To quantitatively characterize oligomerization of VDAC in the membrane, we therefore define the (approximate) oligomer fraction as
| (1) |
Here, Cmin = min(Cr,Cg) is the minimum of the concentrations of red (Cr) and green (Cg) particles measured in the FCCS experiment. The representative auto- and cross-correlation curves obtained by two-color, two-focus FCCS measurements for GUV reconstituted VDAC along with the corresponding fit curves are presented in Fig. 2.
Figure 2.
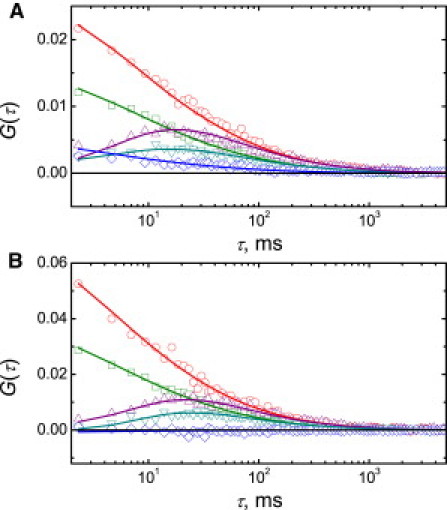
Representative scanning FCCS auto- and cross-correlation curves of fluorescence intensity fluctuations of red- and green-labeled VDAC reconstituted into GUVs. Experimental FCS data (symbols) are shown along with the corresponding least-squares fits: single-focus, single-color, red-channel autocorrelation (circles); single-focus, single-color, green-channel autocorrelation (squares); two-focus, single-color, red channel cross-correlation (up-triangles); two-focus, single-color, green-channel cross-correlation (down-triangles); and one-focus, two-color, red-green cross-correlation (diamonds). GUV composition: (A) DOPC/DOPG 80:20, (B) DOPC/CL 80:20. Temperature, 21 ± 0.5°C.
Results
VDAC reconstitution into giant unilamellar vesicles
To investigate the oligomeric properties of VDAC in the membrane under controlled conditions, purified fluorescently labeled VDAC was reconstituted into chemically well-defined model systems represented by GUVs with different lipid compositions. We found that the protein was uniformly distributed in GUVs (Fig. 1) and showed free diffusion in the membrane with the diffusion coefficient in a DOPC environment similar to the one reported previously for the trimeric glutamate transporter (32). We found, however, that the diffusion coefficient showed slight variations depending on the lipid composition of the membrane of GUVs (Table 1). Typical surface densities of fluorescent VDACgreen and VDACred particles in the membrane as determined by FCCS measurements were in the range of 5–70 μm−2.
Table 1.
Translational diffusion coefficients of VDAC in GUVs with different lipid compositions obtained from the analysis of FCCS data
| Lipid composition | D, μm2/s |
|---|---|
| DOPC | 4.6 ± 0.5 |
| DOPC/CL 80:20 | 5.4 ± 0.4 |
| DOPC/DOPG 90:10 | 5.2 ± 1.0 |
| DOPC/DOPG 80:20 | 5.8 ± 0.8 |
| DOPC/DOPI 90:10 | 6.1 ± 0.4 |
| DOPC/DOPI 80:20 | 5.2 ± 0.5 |
| DOPC/DOPS 90:10 | 5.0 ± 0.7 |
| DOPC/DOPS 90:10 | 5.0 ± 0.7 |
| DOPC/DOPS 80:20 | 4.8 ± 1.0 |
| DOPC/DOPG/CL 80:15:5 | 6.3 ± 0.7 |
| DOPC/DOPG/CL 80:10:10 | 5.4 ± 0.8 |
Measurements were carried out at 21 ± 0.5°C. Mean values and standard deviations obtained from measurements on 10–30 GUVs.
VDAC forms oligomers in detergent that are stable even after reconstitution into the membrane
Previous experiments had shown that VDAC from different species forms dimers and higher order oligomers in detergent and in the membranes of living cells (9–12,33,34).
To check the oligomeric state of refolded VDAC in the detergent solution, VDACred and VDACgreen in LDAO micelles were mixed together (see the upper part of Fig. 3 A) and FCCS measurements were carried out on this sample. The estimated oligomer fraction for VDAC in detergent solution clearly indicates the formation of VDACred-VDACgreen complexes (Fig. 4).
Figure 3.
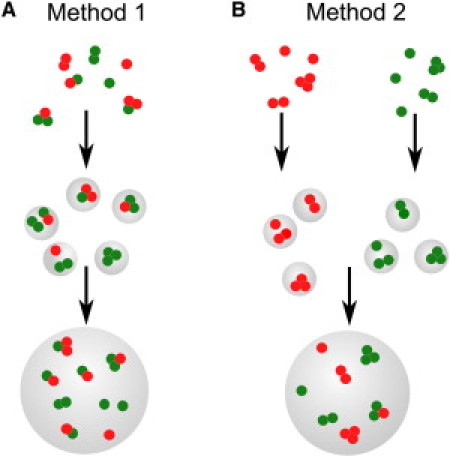
Two different methods of reconstitution of fluorescently labeled VDAC into GUVs. (A) VDACred and VDACgreen are mixed in detergent solution and incorporated into proteoliposomes, which are further used to form GUVs. (B) VDACred and VDACgreen are incorporated into proteoliposomes separately, and the proteoliposomes are mixed only immediately before the formation of GUVs.
Figure 4.
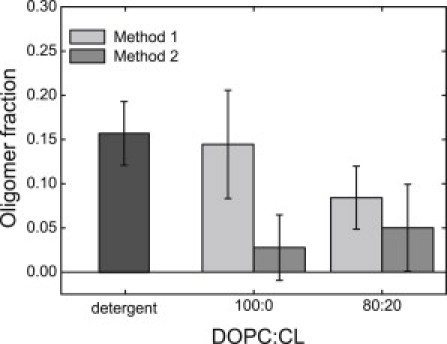
Oligomer fraction of fluorescently labeled VDAC in detergent solution (dark shaded) and upon reconstitution into GUVs with two lipid compositions: pure DOPC and DOPC/CL 80:20. VDACred and VDACgreen were incorporated into GUVs using the two different methods shown in Fig. 3: Method 1 (light shaded) or Method 2 (medium shaded). Data were obtained in three independent experiments with three independently prepared samples; each point is an average of values measured on 10–40 vesicles.
To monitor the oligomeric state of VDAC in the membranes, the same solution was used further to incorporate VDAC into proteoliposomes made of DOPC (Fig. 3 A). GUVs were grown from these proteoliposomes and two-focus, two-color FCCS measurements in the membrane were performed to check whether VDAC oligomers were present in the membrane. The oligomerization level of VDAC in the membrane appeared to be similar to the one observed in detergent solution (Fig. 4). This clearly shows that the VDAC oligomers preexisting in detergent solution are preserved after reconstitution into the membrane.
The crucial question now is whether the detected oligomers were first formed in detergent and subsequently transferred to the membrane during reconstitution, or if they were formed in the membrane after protein reconstitution. To distinguish between these two scenarios, proteoliposomes containing VDACred and VDACgreen were prepared separately and mixed just before the formation of GUVs to ensure that only oligomeric complexes newly formed in the membrane are detected by FCCS (Fig. 3 B). As can be seen from Fig. 4, very few oligomeric complexes were observed in DOPC lipid membrane in this case. Therefore, we conclude that VDAC forms stable oligomers already in detergent, which are preserved even after incorporation into the membrane.
These results confirm our suspicions that a particular protein incorporation procedure can strongly affect subsequent experiments and conclusions made on their basis. The main goal of this article was to study the stability of existing VDAC oligomers once transferred to the membrane, and their dependence on varying lipid composition; to this end, the preparation procedure depicted in Fig. 3 A was applied. In addition, the ability of VDAC to form oligomers after reconstitution to the membrane was also studied, using the procedure depicted in Fig. 3 B.
Cardiolipin disrupts VDAC oligomeric complexes in the membrane
Cardiolipin, a negatively charged lipid with four acyl chains, is an important constituent of the mitochondrial membrane known to be related to mitochondrial metabolism and the process of apoptosis (35). Cardiolipin is enriched in the contact sites between the inner and outer mitochondrial membrane (19) where VDAC is localized (20). Therefore, it was straightforward to check whether the VDAC oligomerization state can be affected by this lipid. As can be seen from Fig. 4, very few VDAC oligomers were found in the membrane at the composition DOPC/CL 80:20, similar to the case of the pure DOPC membrane.
To check whether CL influences the stability of VDAC oligomers preformed in detergent, we carried out experiments on GUVs containing VDACred and VDACgreen incorporated following the procedure depicted in Fig. 3 A. As it is clear from Fig. 4, the presence of cardiolipin substantially reduced the amount of VDACred-VDACgreen oligomers compared to the pure DOPC membrane. We therefore conclude that cardiolipin is able to disrupt supramolecular assemblies of VDAC in the membrane.
Electrophysiology studies suggest that VDAC can partition into CL-enriched domains (25) formed on mitochondrial contact sites. In view of this model, the implication of our finding is that under normal (nonapoptotic) conditions the relatively high CL level on the mitochondrial membrane precludes VDAC oligomerization.
PG induces VDAC oligomerization
PG is a precursor of CL in mitochondria. Similar to cardiolipin, it carries a negative charge, but, unlike CL, has only two acyl chains. During the programmed cell death, an increase in PG concentration by ∼30–50% of the control and the decrease in the CL levels by ∼15–50% have been reported (22,23).
To investigate whether the elevated PG levels can influence VDAC oligomerization, we have incorporated VDAC into DOPC/DOPG GUVs with the composition DOPC/DOPG 80:20 and monitored the formation of oligomers in the membrane by means of FCCS (here and elsewhere, if not specified otherwise, VDACred and VDACgreen were reconstituted into GUVs using Method 2 (Fig. 3 B)). Interestingly, we observed a significantly enhanced level of VDAC oligomerization in DOPC/DOPG membranes compared to pure DOPC membrane. Moreover, an increase in the PG concentration in GUVs leads to a gradual increase in the number of VDAC oligomeric complexes formed in the membrane (Fig. 5).
Figure 5.
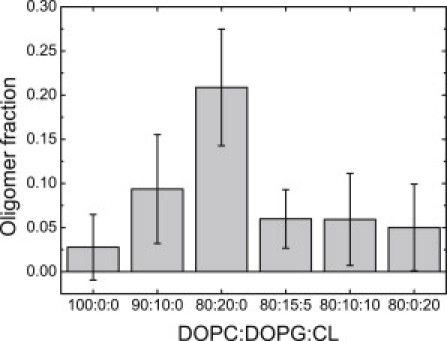
Oligomer fraction of fluorescently labeled VDAC in GUVs. GUV composition: DOPC, DOPC/DOPG 90:10, DOPC/DOPG 80:20, DOPC/DOPG/CL 80:15:5, DOPC/DOPG/CL 80:10:10, and DOPC/CL 80:20. Data were obtained in three independent experiments with three independently prepared samples; each point is an average of values measured on 13–39 vesicles.
These conclusions are based on the FCCS measurements that provide information on the amount of red-green complexes formed in the membrane. The experiment can be modified such that only one-color species are present in the membrane, and oligomerization of VDAC is followed by the so-called specific brightness of fluorescent particles measured in photon counts per particle per second, which can be extracted from the fluorescence fluctuation data. If single-color particles cluster and diffuse together, the detected specific brightness should increase. Our experiments show that the mean specific brightness of fluorescent particles increases roughly by a factor of two in membranes containing PG compared to membranes with CL (Fig. 6), which is additional evidence supporting the formation of oligomeric complexes. The fact that VDAC is able to form not only dimers, but also higher-order oligomers in the membrane (9–11), does not allow us to draw more quantitative conclusions from these data.
Figure 6.
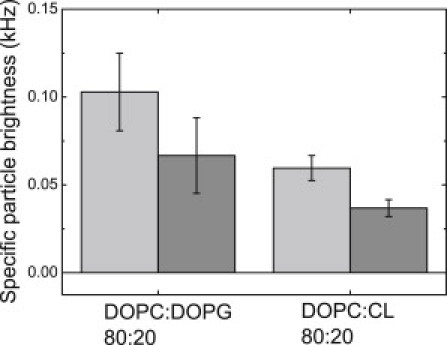
Specific particle brightness of fluorescently labeled VDACgreen in GUVs. GUV composition: DOPC/DOPG 80:20 or DOPC/CL 80:20. (Light-shaded and medium-shaded) Data depict two independent experiments carried out with two independently prepared samples. Each point is an average of values measured on 5–7 vesicles.
The results of the above experiments allow us to conclude that the negatively charged lipid PG induces formation of VDAC supramolecular assemblies, in contrast to its four-acyl chain analog CL that, inversely, prevents oligomerization in the membrane and even disrupts the oligomers originally formed in solution after they attach to the membrane.
Effect of PG on VDAC assembly vanishes in presence of CL
In view of the above findings, it was interesting to check whether the effect of CL on VDAC oligomers would also be detected in the presence of PG in the membrane, and vice versa. The results presented in Fig. 5 demonstrate that addition of CL to the lipid mixture disrupts oligomeric complexes of VDAC formed in presence of PG. Virtually no oligomers were detected in GUVs with the compositions DOPC/DOPG/CL 80:15:5 or 80:10:10, similar to what was observed for the pure DOPC membrane (Fig. 4).
Thus, we conclude that CL reduces the number of supramolecular complexes of VDAC that can be formed in the membrane in presence of PG. We suggest that under the normal cell conditions, one of the functions of CL in mitochondria consists in keeping VDAC in the monomeric state. During apoptosis, cardiolipin levels in mitochondria decrease (36), the cristae are rearranged, and contact sites between the inner and outer membrane, where VDAC is colocalized with CL, are disrupted (37). These conditions favor the interaction of VDAC with PG and subsequent VDAC oligomerization.
Other anionic lipids with two acyl chains also increase the level of VDAC oligomerization
To find out how specific is the interaction of VDAC with DOPG, oligomerization of the protein in membranes containing other negatively charged lipids with two acyl chains, PI and PS, was tested. Fig. 7 summarizes the results obtained with FCCS. An increase in PI and PS concentrations in the membrane clearly leads to an increase in the amount of oligomeric complexes. Thus, anionic phospholipids PI and PS also induce VDAC oligomerization, although their effect is weaker compared to PG (see Fig. 5).
Figure 7.
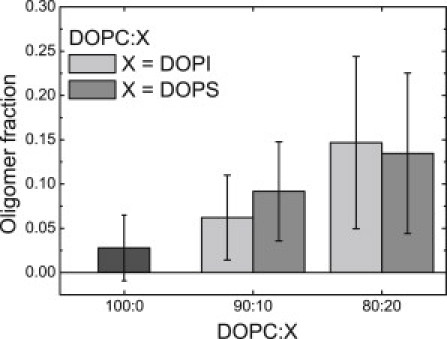
Oligomer fraction of fluorescently labeled VDAC in GUVs. GUV composition: DOPC (dark shaded), DOPC/DOPI 90:10, DOPC/DOPI 80:20 (light shaded) or DOPC/DOPS 90:10, and DOPC/DOPS 80:20 (medium shaded). Data were obtained in three independent experiments with three independently prepared samples; each point is an average of values measured on 13–29 vesicles.
Discussion
To summarize, we have found that PG, PI, and PS induce VDAC oligomerization, whereas CL disrupts VDAC supramolecular assemblies in the membrane. We believe that these results can be related to VDAC oligomerization observed during programmed cell death (5–7). Indeed, experimental studies show that apoptosis is associated with the suppressed CL synthesis and an increase in the level of PG (22,23). On the other hand, CL deficiency and PG accumulation in cardiolipin-synthetase-deficient yeast cells were shown to lead to cytochrome c release from mitochondria followed by apoptosis (24). A decrease in the amount of CL, which interacts with VDAC in the mitochondria contact sites, can also occur as a consequence of rearrangements of cristae observed during cell death (37) and possible disruption of the contact sites. As we know from our results, a decrease in the amount of CL and increase of PG levels in the membrane can lead to efficient formation of VDAC oligomers. Interestingly, in previous studies (22,24), CL deficiency was stressed as the most important factor for apoptosis, which was connected only with disruption of interactions between CL and cytochrome c, whereas PG was not even considered as a component playing any significant role in cell death.
On the other hand, a recent study (38) has shown that cell lines from patients with the Barth syndrome, which also exhibit low levels of CL, but no increase in PG, are not susceptible to apoptosis. This observation suggests that the level of PG in mitochondria may be a crucial factor regulating apoptosis. As we have demonstrated, an increase in the PG level in the membrane induces VDAC oligomerization that, in turn, is believed to be an important step in programmed cell death. It is known that, in general, lipids can regulate protein oligomerization in different ways, and the literature on this topic is extensive (see, e.g., (15,39)). Most of the research in this field is concerned with the influence of lipid charge and hydrophobic mismatch on protein association. The importance of negatively charged lipids for protein oligomerization has been discussed widely in the literature. For example, it was shown by in vivo studies that PG is required for the formation and stability of supramolecular assembly of the photosystem I reaction center (40). Another negatively charged lipid DOPA (dioleoyl phosphatidic acid) was shown to stabilize the tetrameric assembly of KcsA channel (17), whereas DMPS (dimyristoyl phosphatidylserine) and DMPG (dimyristoyl phosphatidylglycerol) were reported to be involved in aggregation of the AβP(25–35) peptide (41). Other studies also indicate that CL enhances the formation and stability of oligomers of KcsA channel (17) and mitochondrial respiratory chain supercomplexes (42).
Protein-lipid interactions on membranes containing anionic lipids have also been addressed in theoretical studies (43,44). The general conclusion is that negatively charged lipids in the membrane migrate toward the positively charged protein, which leads to electrostatics-induced local demixing of charged and neutral lipids, which produces a varying lipid composition profile around the charged protein (44). In this way lipid-protein complexes can be produced in the form of domains enriched in both positively charged protein and anionic lipids.
Our results are generally in line with the above-mentioned experimental and theoretical studies (40,41,44). VDAC is positively charged at neutral pH (45) and therefore anionic PG, PS, PI, and CL interact with VDAC, which should result in their local demixing. We found that in agreement with previous findings for other proteins interacting with anionic lipids (17,40,41), PG, PI, and PS induce oligomerization of VDAC in the membrane.
On the other hand, we found that—surprisingly—CL, which is also negatively charged, does not induce VDAC oligomerization and moreover, precludes the formation of oligomeric complexes in the membrane. The question arises why CL has an opposite effect on VDAC oligomerization compared to the other negatively charged lipids PG, PS, and PI.
In our opinion, this difference is most probably related to the different structure of the hydrophobic part of these lipids (CL has four acyl chains compared to two acyl chains in PG, PS, and PI), as well as with the character of protein-protein interactions responsible for VDAC oligomerization. The crystal packing analysis of murine VDAC suggests that oligomerization of VDAC is mostly driven by van der Waals interactions (46). Using the data from the Protein Data Bank (PDB ID:2JK4 (33)), we reconstructed the three-dimensional view of the VDAC protein with the help of the software PyMOL 1.3 (DeLano Scientific, www.pymol.org), and the interaction surface responsible for VDAC oligomer formation (46) was highlighted.
One can see that the interaction surface is characterized by areas with a strong positive charge (Fig. 8 A). Negatively charged lipids can interact with the positively charged surface of VDAC, resulting in charge screening, which decreases the distance between VDAC monomers and facilitates the formation of stable complexes due to van der Waals attraction (Fig. 8 B). If, instead of two-acyl chain lipid (e.g., PG), a substantially more bulky CL is present in the membrane, its local electrostatics-induced demixing should, in contrast, preclude the close approach of VDAC molecules and thus prevent the formation of VDAC complexes (Fig. 8 C). In addition, we observed that CL eliminates the effect of PG on VDAC oligomerization. This means that CL competes with PG for binding with VDAC, and interaction of CL with VDAC seems to be energetically more favorable.
Figure 8.
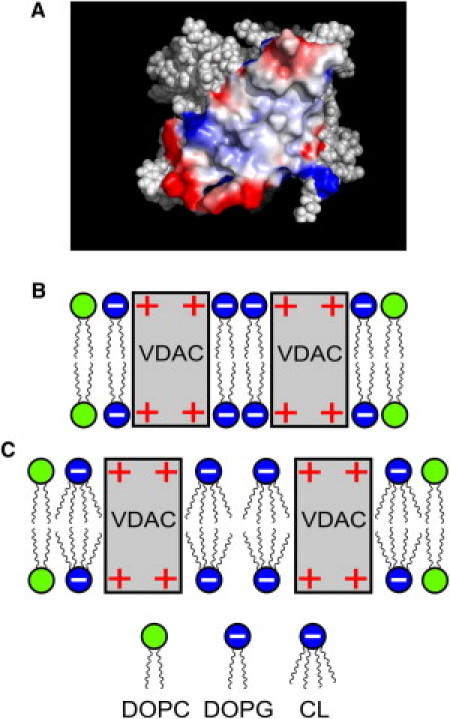
Charge-surface (positive, red, and negative, blue) of VDAC monomer responsible for the dimer formation generated using the software PyMOL 1.3 (DeLano Scientific) (A) and schematic representation of the interaction of VDAC with anionic lipids (B and C). (B) Formation of VDAC dimers in presence of PG. (C) Interaction of VDAC with CL.
Several previous studies suggested that, in general, negatively charged lipids induce aggregation or clustering of transmembrane proteins (17,41). Although the dependence of the stability of oligomers on the lipid charge and specific properties of the headgroup was emphasized (17), the role of the hydrophobic part of the lipid was usually not taken into consideration in these studies. Our experiments demonstrate that the shape of hydrophobic part of lipids can play a crucial role in the formation and stability of protein supramolecular assemblies in the membrane. We believe that our results shed light on the role played by the anionic lipids PG and CL in the regulation of VDAC oligomerization during apoptosis, and thus provide additional information of the molecular mechanisms of the programmed cell death.
Acknowledgments
We thank Dr. Jörg H. Kleinschmidt (University of Konstanz, Germany) for pTMVDAC1 plasmid and help with VDAC refolding. We are also grateful to Dr. Roland Hemmler (Ionovation, Osnabrück, Germany) for the introduction of the Bilayer Explorer system and important advices on proteoliposomes fusion.
This work was supported by Dresden International Graduate School for Biomedicine and Bioengineering.
Supporting Material
References
- 1.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004;256-257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 2.Tajeddine N., Galluzzi L., Kroemer G. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene. 2008;27:4221–4232. doi: 10.1038/onc.2008.63. [DOI] [PubMed] [Google Scholar]
- 3.Madesh M., Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu S., Matsuoka Y., Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J. Cell Biol. 2001;152:237–250. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keinan N., Tyomkin D., Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol. Cell. Biol. 2010;30:5698–5709. doi: 10.1128/MCB.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Hamad S., Arbel N., Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 7.Mader A., Abu-Hamad S., Shoshan-Barmatz V. Dominant-negative VDAC1 mutants reveal oligomeric VDAC1 to be the active unit in mitochondria-mediated apoptosis. Biochem. J. 2010;429:147–155. doi: 10.1042/BJ20091338. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller R., Freitag H., Neupert W. A water-soluble form of porin from the mitochondrial outer membrane of Neurospora crassa. Properties and relationship to the biosynthetic precursor form. J. Biol. Chem. 1985;260:8188–8193. [PubMed] [Google Scholar]
- 9.Malia T.J., Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46:514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogenboom B.W., Suda K., Fotiadis D. The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J. Mol. Biol. 2007;370:246–255. doi: 10.1016/j.jmb.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves R.P., Buzhynskyy N., Scheuring S. Supramolecular assembly of VDAC in native mitochondrial outer membranes. J. Mol. Biol. 2007;369:413–418. doi: 10.1016/j.jmb.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Zalk R., Israelson A., Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 2005;386:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y., Shi Y., Chen Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouritsen O.G. Springer; Berlin, Germany: 2005. Life as a Matter of Fat: The Emerging Science of Lipidomics. [Google Scholar]
- 15.Raja M. The potassium channel KcsA: a model protein in studying membrane protein oligomerization and stability of oligomeric assembly? Arch. Biochem. Biophys. 2011;510:1–10. doi: 10.1016/j.abb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Beyer K., Nuscher B. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 1996;35:15784–15790. doi: 10.1021/bi9610055. [DOI] [PubMed] [Google Scholar]
- 17.Raja M. The role of phosphatidic acid and cardiolipin in stability of the tetrameric assembly of potassium channel KcsA. J. Membr. Biol. 2010;234:235–240. doi: 10.1007/s00232-010-9251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardail D., Privat J.P., Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- 20.Shoshan-Barmatz V., Zalk R., Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Schlame M., Rua D., Greenberg M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 22.Ostrander D.B., Sparagna G.C., Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 23.Matsko C.M., Hunter O.C., Amoscato A.A. Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem. Biophys. Res. Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- 24.Choi S.-Y., Gonzalvez F., Frohman M.A. Cardiolipin deficiency releases cytochrome c from the inner mitochondrial membrane and accelerates stimuli-elicited apoptosis. Cell Death Differ. 2007;14:597–606. doi: 10.1038/sj.cdd.4402020. [DOI] [PubMed] [Google Scholar]
- 25.Rostovtseva T.K., Kazemi N., Bezrukov S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 26.Schwille P., Diez S. Synthetic biology of minimal systems. Crit. Rev. Biochem. Mol. Biol. 2009;44:223–242. doi: 10.1080/10409230903074549. [DOI] [PubMed] [Google Scholar]
- 27.Shanmugavadivu B., Apell H.-J., Kleinschmidt J.H. Correct folding of the β-barrel of the human membrane protein VDAC requires a lipid bilayer. J. Mol. Biol. 2007;368:66–78. doi: 10.1016/j.jmb.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 28.Aram L., Geula S., Shoshan-Barmatz V. VDAC1 cysteine residues: topology and function in channel activity and apoptosis. Biochem. J. 2010;427:445–454. doi: 10.1042/BJ20091690. [DOI] [PubMed] [Google Scholar]
- 29.Girard P., Pécréaux J., Bassereau P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 2004;87:419–429. doi: 10.1529/biophysj.104.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelova M.I., Soléau S., Bothorel P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Prog. Colloid Polym. Sci. 1992;89:161–176. [Google Scholar]
- 31.Petrov E.P., Schwille P. Translational diffusion in lipid membranes beyond the Saffman-Delbrück approximation. Biophys. J. 2008;94:L41–L43. doi: 10.1529/biophysj.107.126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramadurai S., Duurkens R., Poolman B. Lateral diffusion of membrane proteins: consequences of hydrophobic mismatch and lipid composition. Biophys. J. 2010;99:1482–1489. doi: 10.1016/j.bpj.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayrhuber M., Meins T., Zeth K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoshan-Barmatz V., Keinan N., Aram L. Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: release of cytochrome c, AIF and Smac/Diablo. Biochim. Biophys. Acta. 2010;1797:1281–1291. doi: 10.1016/j.bbabio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Wright M.M., Howe A.G., Zaremberg V. Cell membranes and apoptosis: role of cardiolipin, phosphatidylcholine, and anticancer lipid analogues. Biochem. Cell Biol. 2004;82:18–26. doi: 10.1139/o03-092. [DOI] [PubMed] [Google Scholar]
- 36.Crimi M., Esposti M.D. Apoptosis-induced changes in mitochondrial lipids. Biochim. Biophys. Acta. 2011;1813:551–557. doi: 10.1016/j.bbamcr.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi R., Perkins G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim. Biophys. Acta. 2009;1787:963–972. doi: 10.1016/j.bbabio.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valianpour F., Mitsakos V., Vaz F.M. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 2005;46:1182–1195. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Botelho A.V., Huber T., Brown M.F. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys. J. 2006;91:4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domonkos I., Malec P., Gombos Z. Phosphatidylglycerol is essential for oligomerization of photosystem I reaction center. Plant Physiol. 2004;134:1471–1478. doi: 10.1104/pp.103.037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Mar Martínez-Senac M., Villalaín J., Gómez-Fernández J.C. Structure of the Alzheimer β-amyloid peptide (25–35) and its interaction with negatively charged phospholipid vesicles. Eur. J. Biochem. 1999;265:744–753. doi: 10.1046/j.1432-1327.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 43.Khelashvili G., Harries D., Weinstein H. Modeling membrane deformations and lipid demixing upon protein-membrane interaction: the BAR dimer adsorption. Biophys. J. 2009;97:1626–1635. doi: 10.1016/j.bpj.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.May S., Harries D., Ben-Shaul A. Lipid demixing and protein-protein interactions in the adsorption of charged proteins on mixed membranes. Biophys. J. 2000;79:1747–1760. doi: 10.1016/S0006-3495(00)76427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasteiger E., Gattiker A., Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucl. Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ujwal R., Cascio D., Abramson J. Crystal packing analysis of murine VDAC1 crystals in a lipidic environment reveals novel insights on oligomerization and orientation. Channels (Austin) 2009;3:167–170. doi: 10.4161/chan.3.3.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrov E.P., Schwille P. State of the art and novel trends in fluorescence correlation spectroscopy. In: Resch-Genger U., editor. Standardization and Quality Assurance in Fluorescence Measurements II. Vol. 6. Springer; Berlin, Heidelberg, New York: 2008. pp. 145–197. (Springer Series on Fluorescence). [Google Scholar]
- 48.Ries J., Petrášek Z., Schwille P. A comprehensive framework for fluorescence cross-correlation spectroscopy. New J. Phys. 2010;12:113009–113041. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


