Abstract
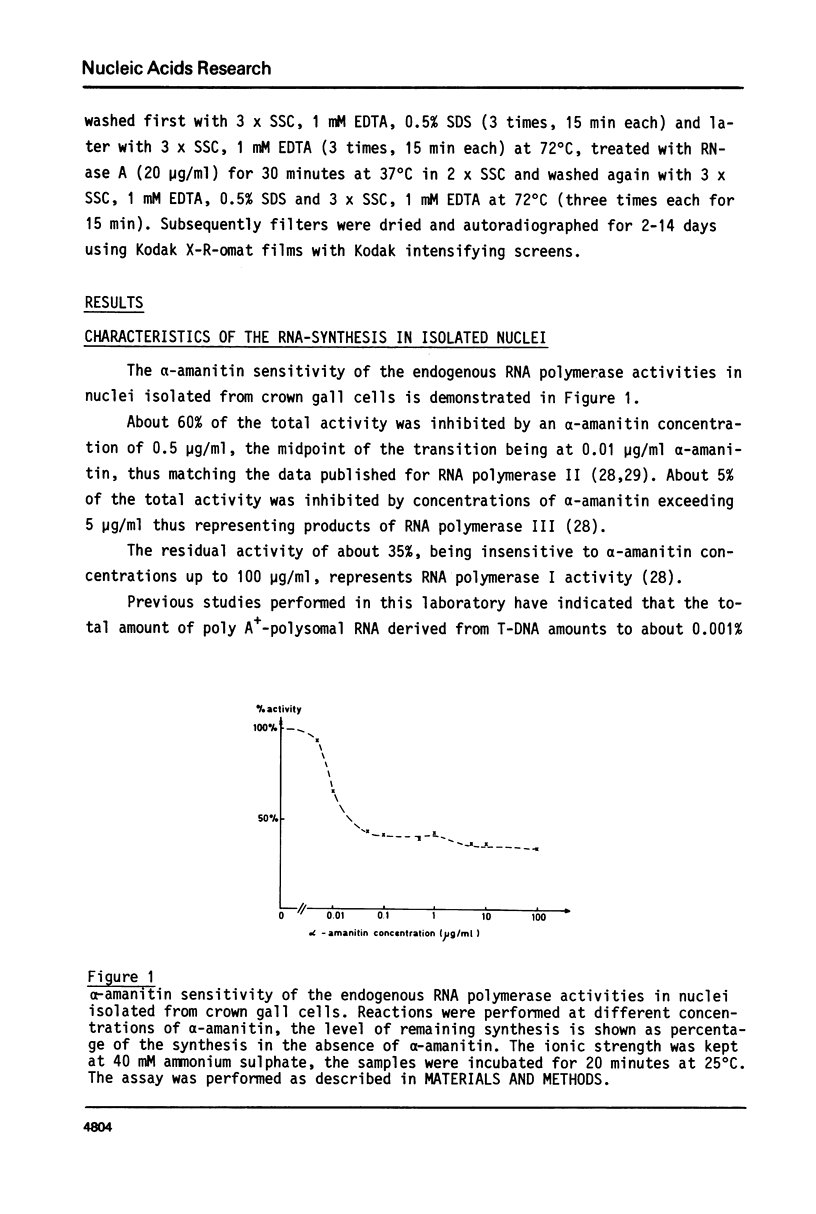
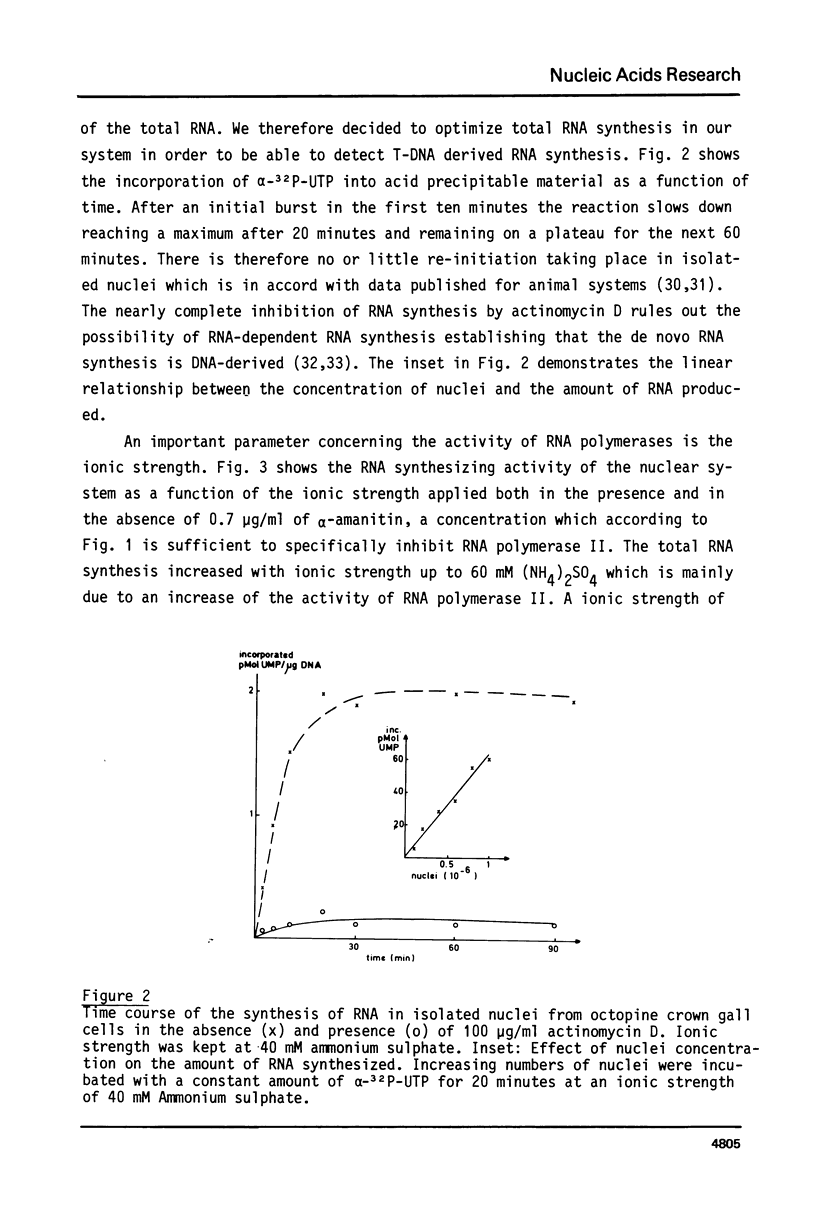
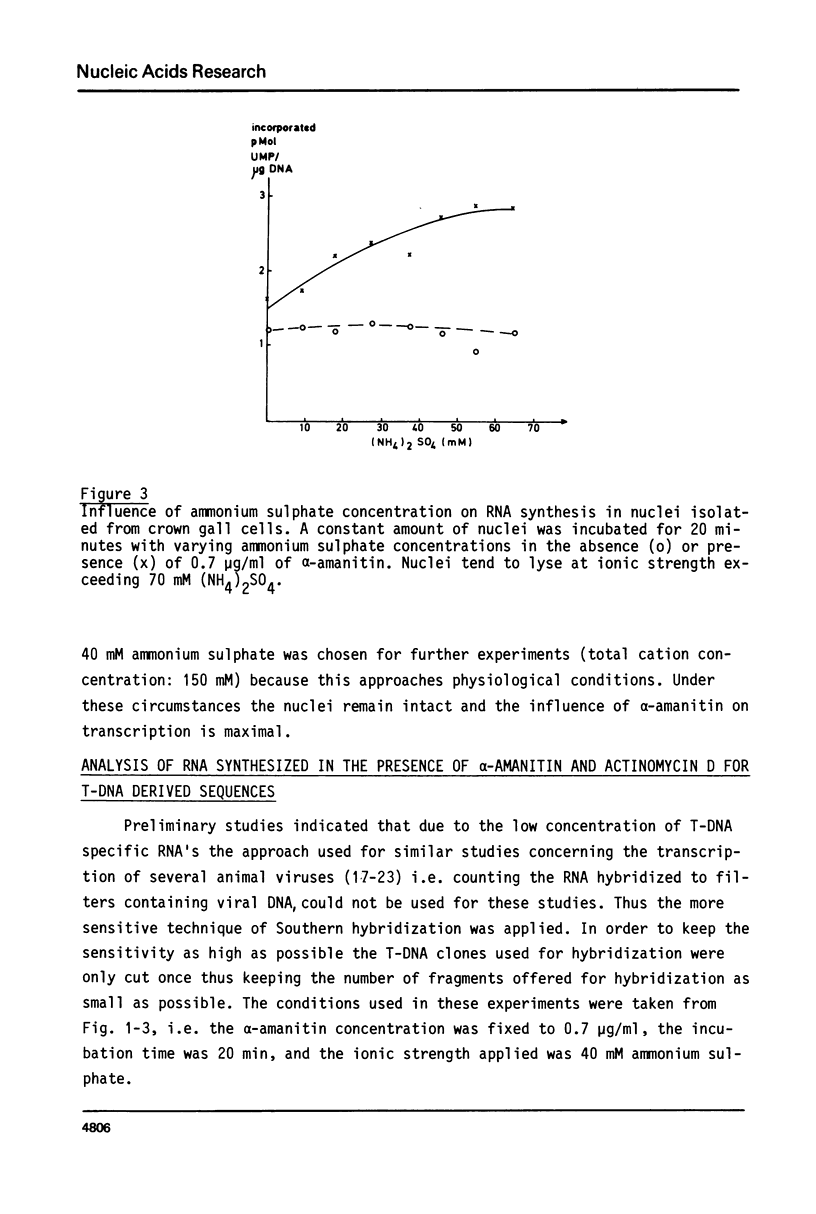
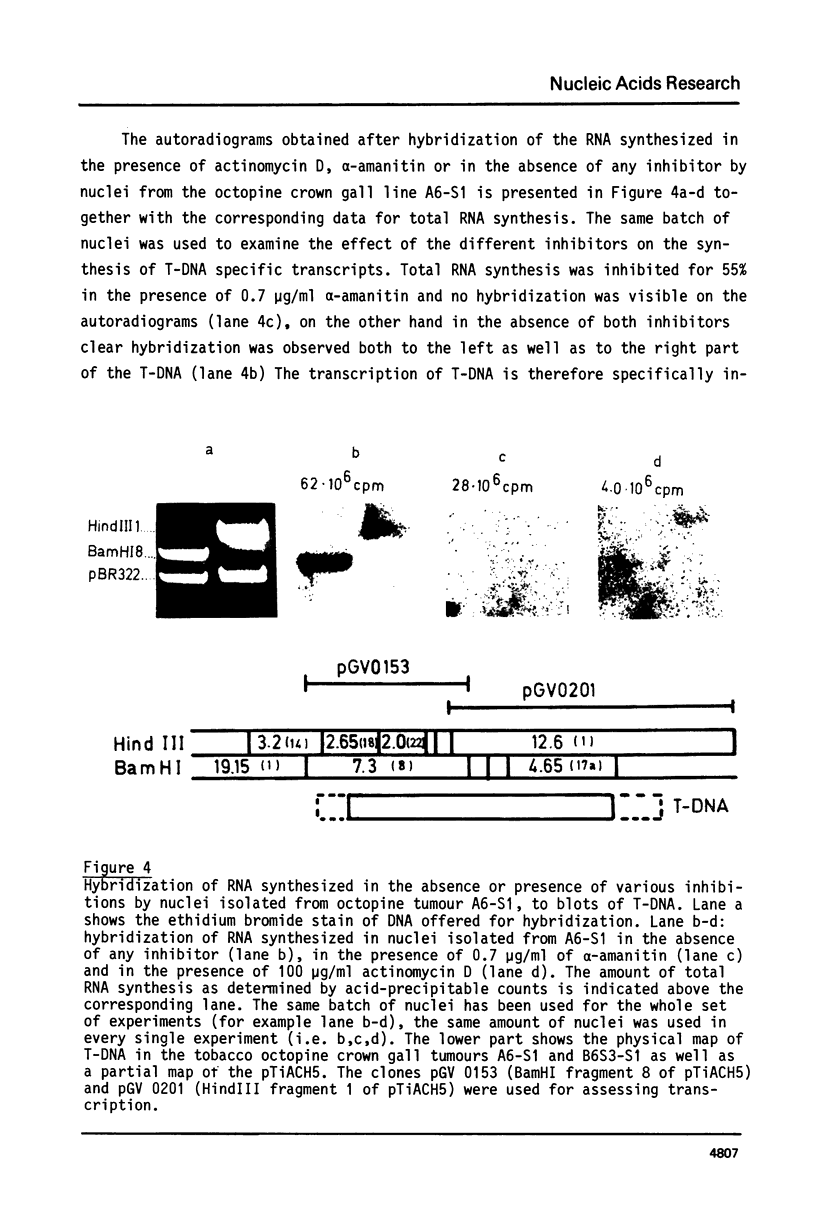
Highly purified and physiologically active nuclei were isolated from four different octopine and nopaline crown gall lines. These nuclei exhibited a high endogenous RNA synthesizing activity involving all three RNA-polymerases I, II and III. Isolated nuclei were shown by Southern blotting to synthesize T-DNA specific RNA. This synthesis was shown to be sensitive to actinomycin D and therefore to be DNA-dependent. The transcription of the T-DNA was also inhibited for more than 90% by low concentrations of alpha-amanitin (0.7 micrograms/ml) indicating that the T-DNA, although from bacterial origin, is transcribed by the host RNA polymerase II.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter G. A., Roeder R. G. Transcription of viral genes by RNA polymerase II in nuclei isolated from adenovirus 2 transformed cells. Biochemistry. 1978 May 30;17(11):2198–2205. doi: 10.1021/bi00604a028. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Saiki R. K., Yadav N., Gordon M. P., Quetier F. T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4060–4064. doi: 10.1073/pnas.77.7.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke K., Sippel A. E., Nguyen-Huu M. C., Groner B., Hynes N. E., Wurtz T., Schütz G. A RNA-dependent RNA polymerase activity: implications for chromatin transcription experiments. Nucleic Acids Res. 1977 Nov;4(11):3943–3958. doi: 10.1093/nar/4.11.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Sugden B. Inhibition by -amanitin of simian virus 40-specific ribonucleic acid synthesis in nuclei of infected monkey cells. J Virol. 1972 Nov;10(5):1086–1089. doi: 10.1128/jvi.10.5.1086-1089.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrisak J., Guilfoyle T. J. Eukaryotic RNA polymerase: comparative subunit structures, immunological properties, and alpha-amanitin sensitivities of the class II enzymes from higher plants. Biochemistry. 1978 Apr 4;17(7):1322–1327. doi: 10.1021/bi00600a029. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Parsons J. T., Coffin J. M., Weissmann C. In vitro synthesis of Rous sarcoma virus-specific RNA is catalyzed by a DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2782–2786. doi: 10.1073/pnas.71.7.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell J., Van Montagu M., De Beuckeleer M., De Block M., Depicker A., De Wilde M., Engler G., Genetello C., Hernalsteens J. P., Holsters M. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):251–266. doi: 10.1098/rspb.1979.0026. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Otten L., Simons G., Schmalenbach W., Schröder J., Schröder G., Van Montagu M., de Vos G., Schell J. Nuclear and polysomal transcripts of T-DNA in octopine crown gall suspension and callus cultures. Mol Gen Genet. 1981;182(2):255–262. doi: 10.1007/BF00269667. [DOI] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Holsters M., Kruger K., Depicker A., Schell J., Van Montagu M., Goodman H. M. Tumor DNA structure in plant cells transformed by A. tumefaciens. Science. 1980 Sep 19;209(4463):1385–1391. doi: 10.1126/science.6251546. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Analysis of in vitro transcription of duck reticulocyte chromatin using mercury-substituted ribonucleoside triphosphates. Biochemistry. 1977 Nov 15;16(23):5135–5145. doi: 10.1021/bi00642a029. [DOI] [PubMed] [Google Scholar]




