Abstract
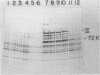
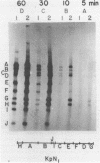
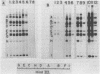
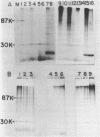
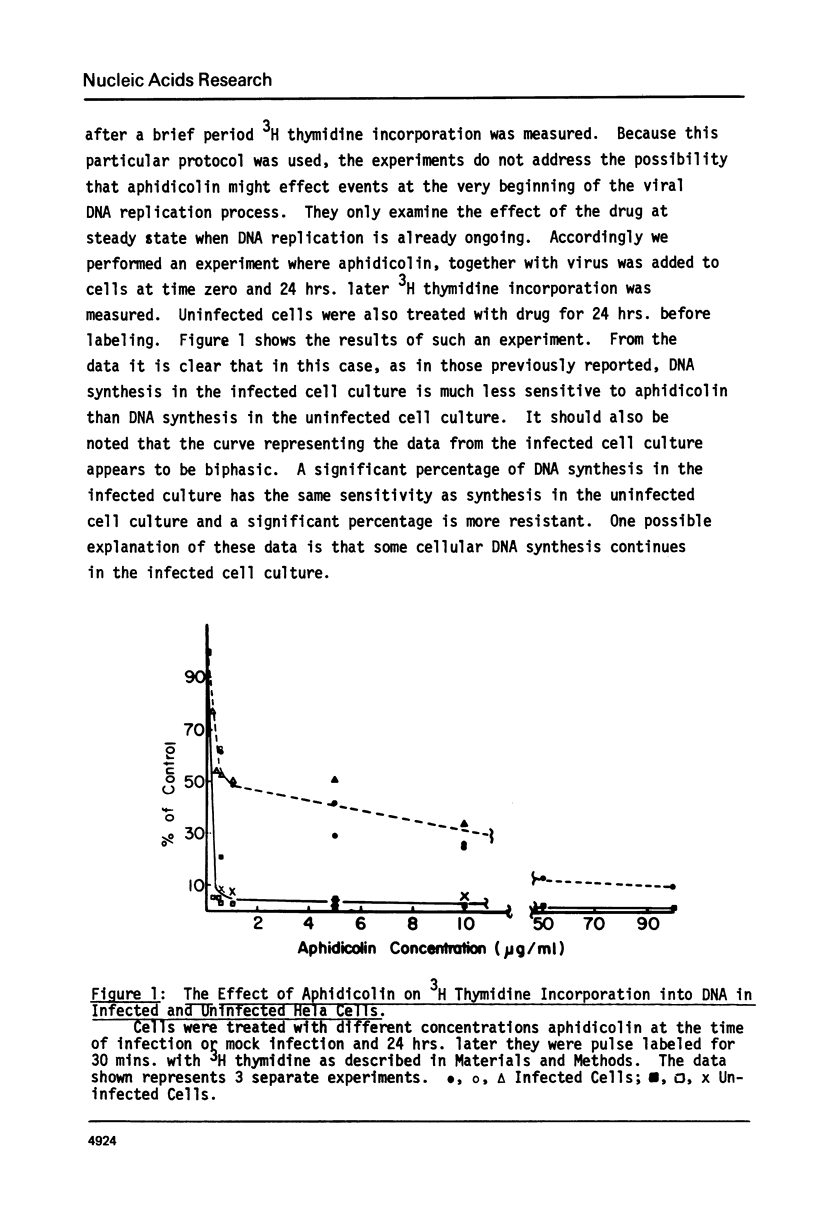
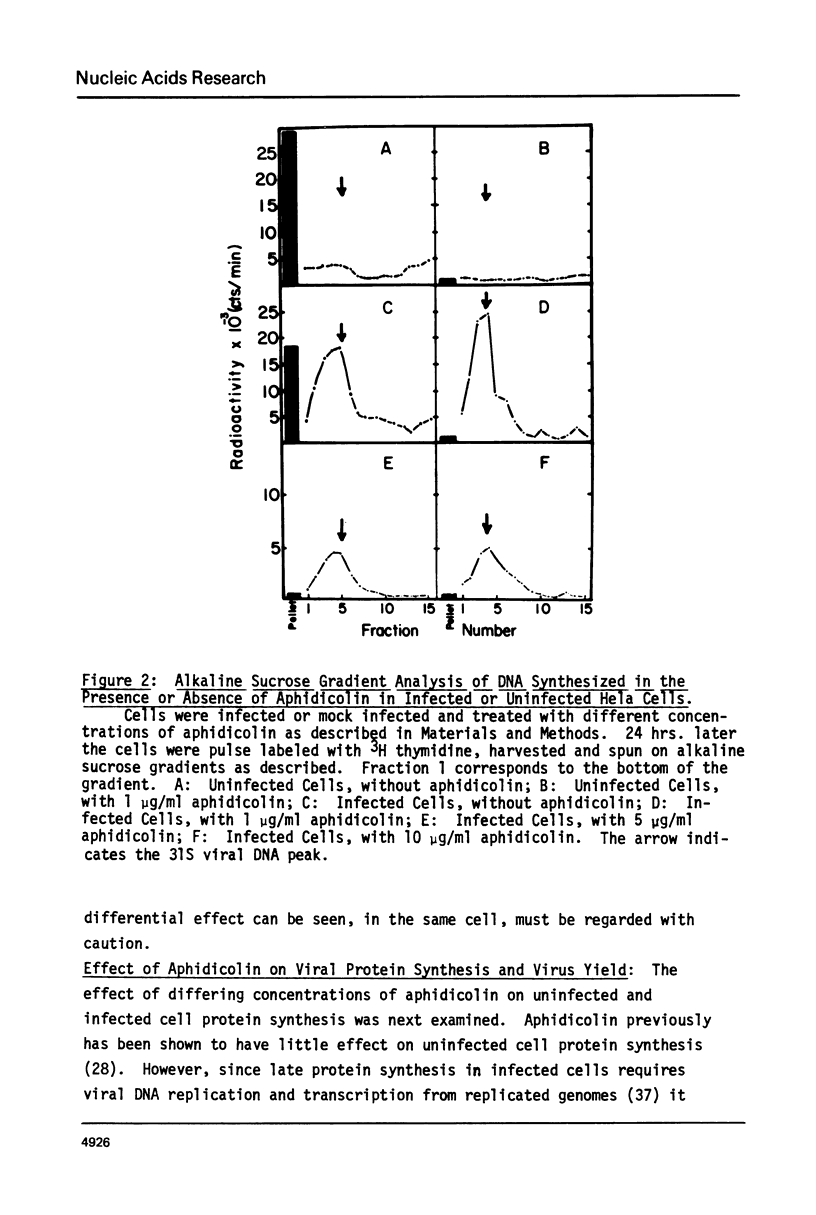
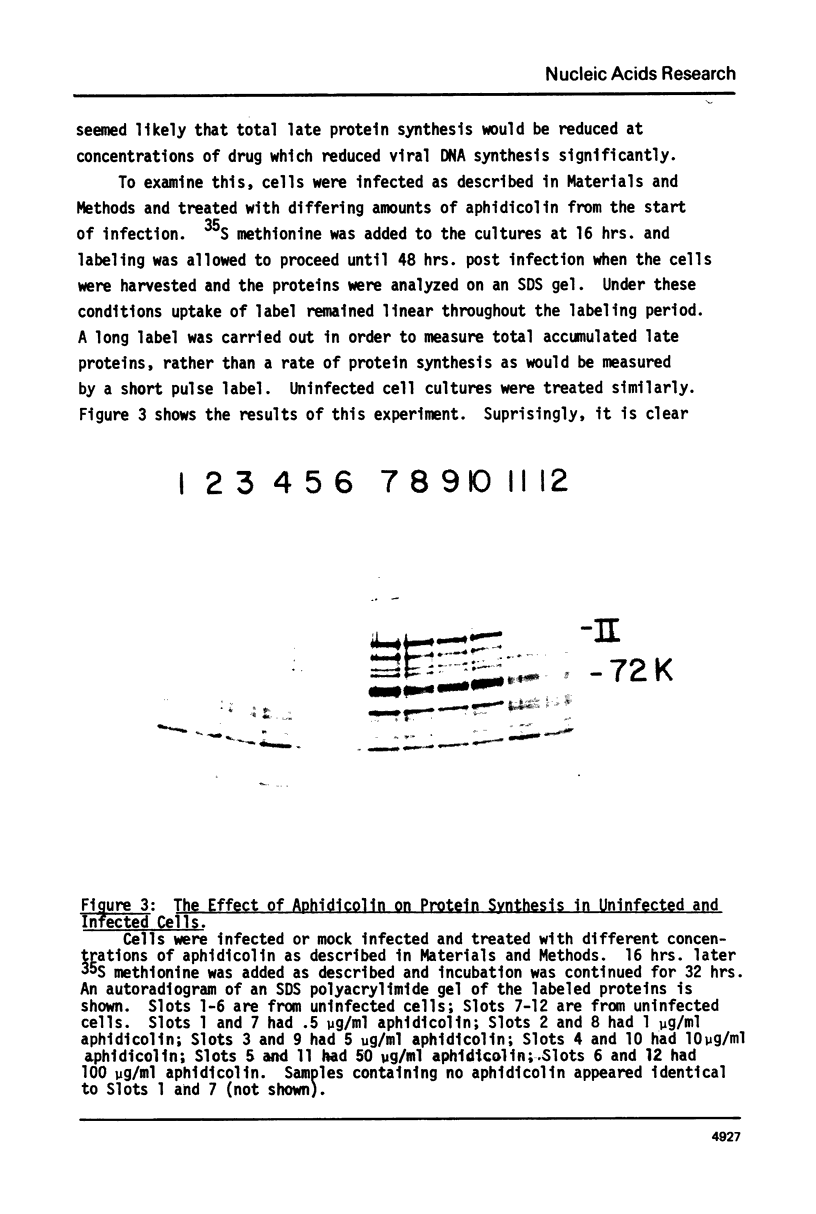
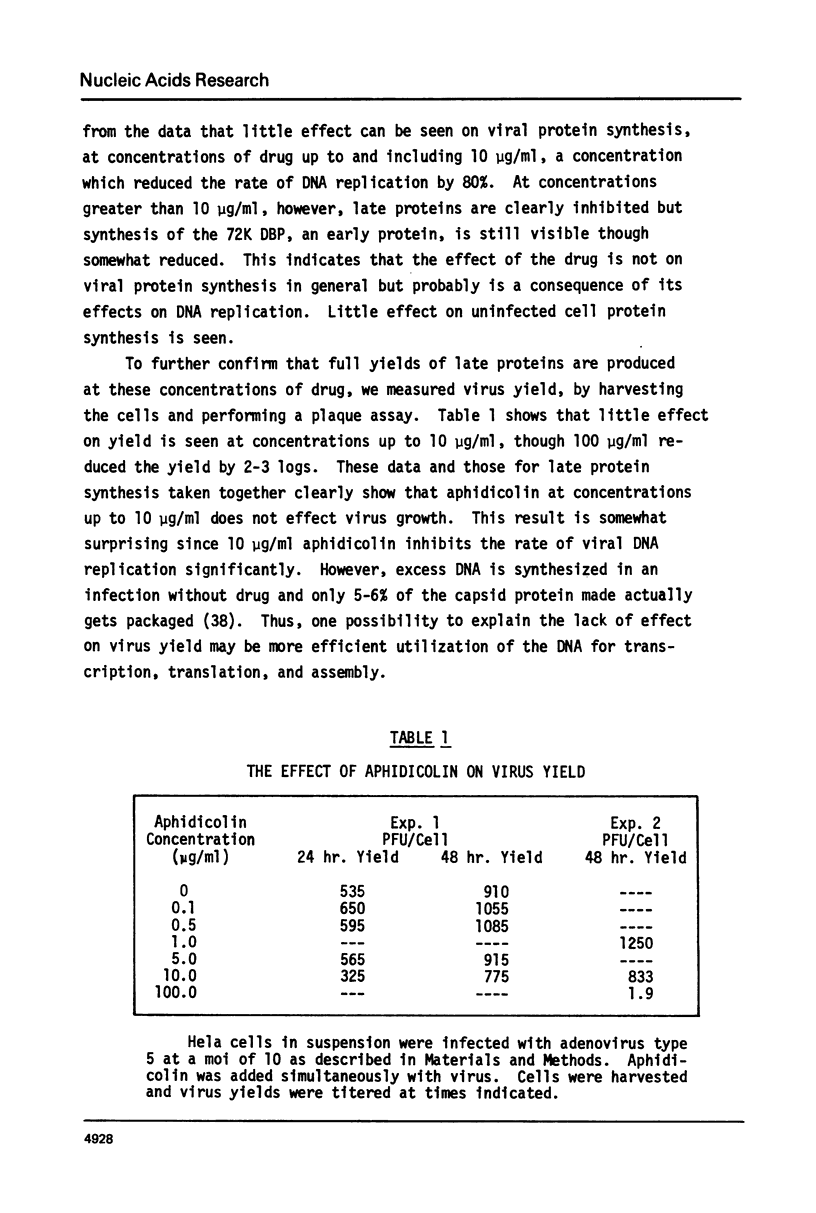
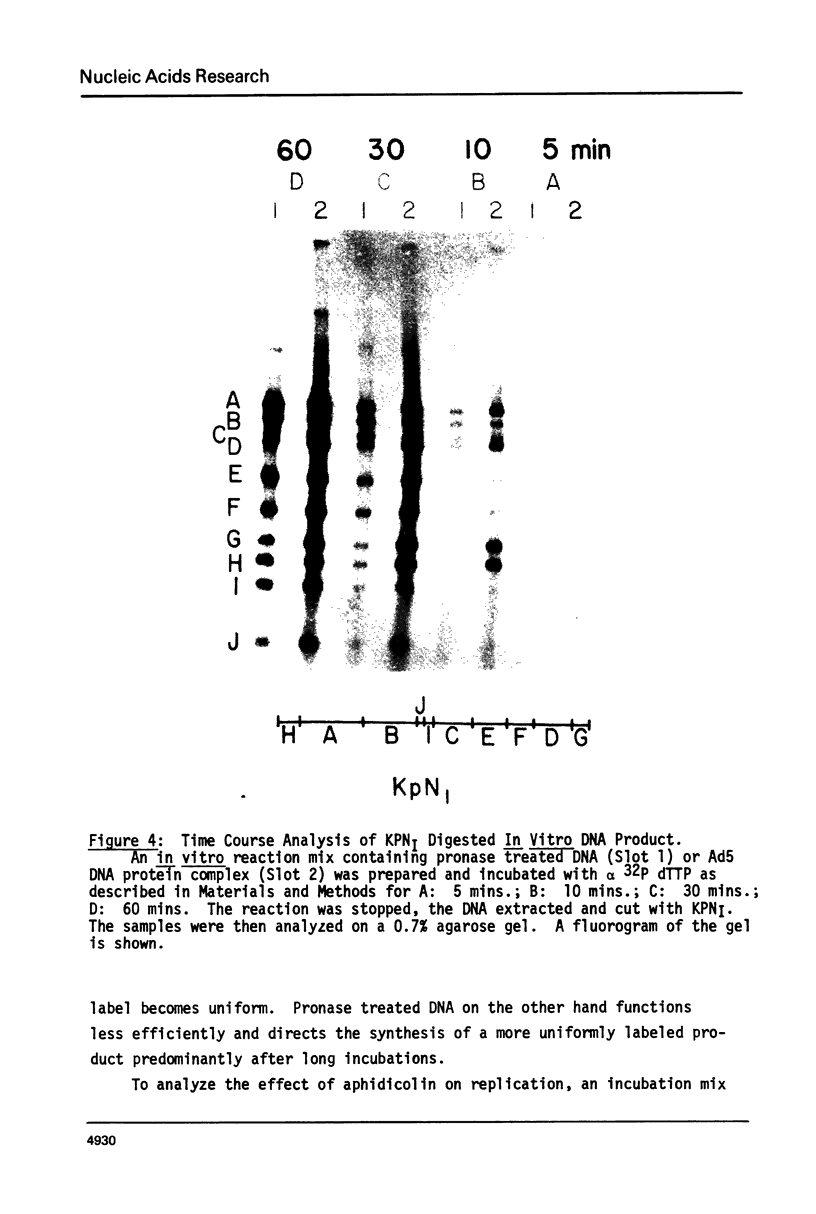
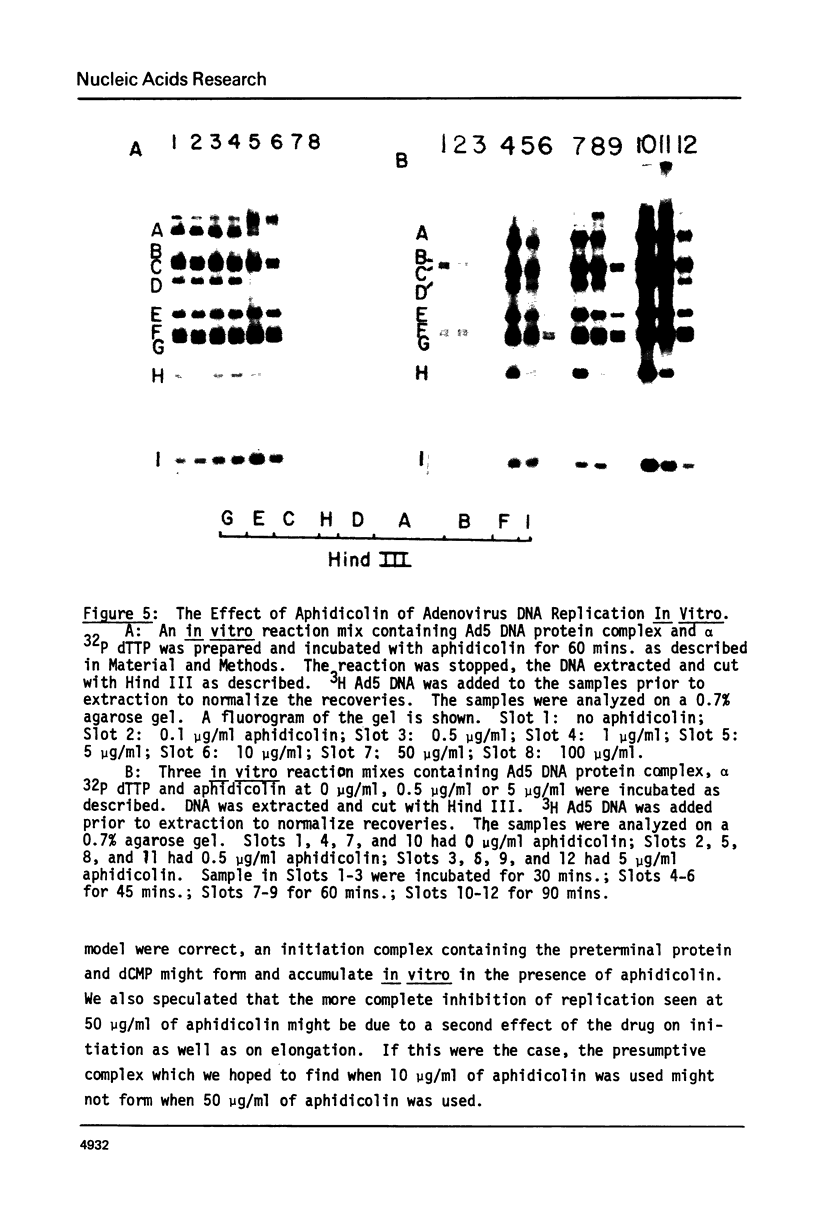
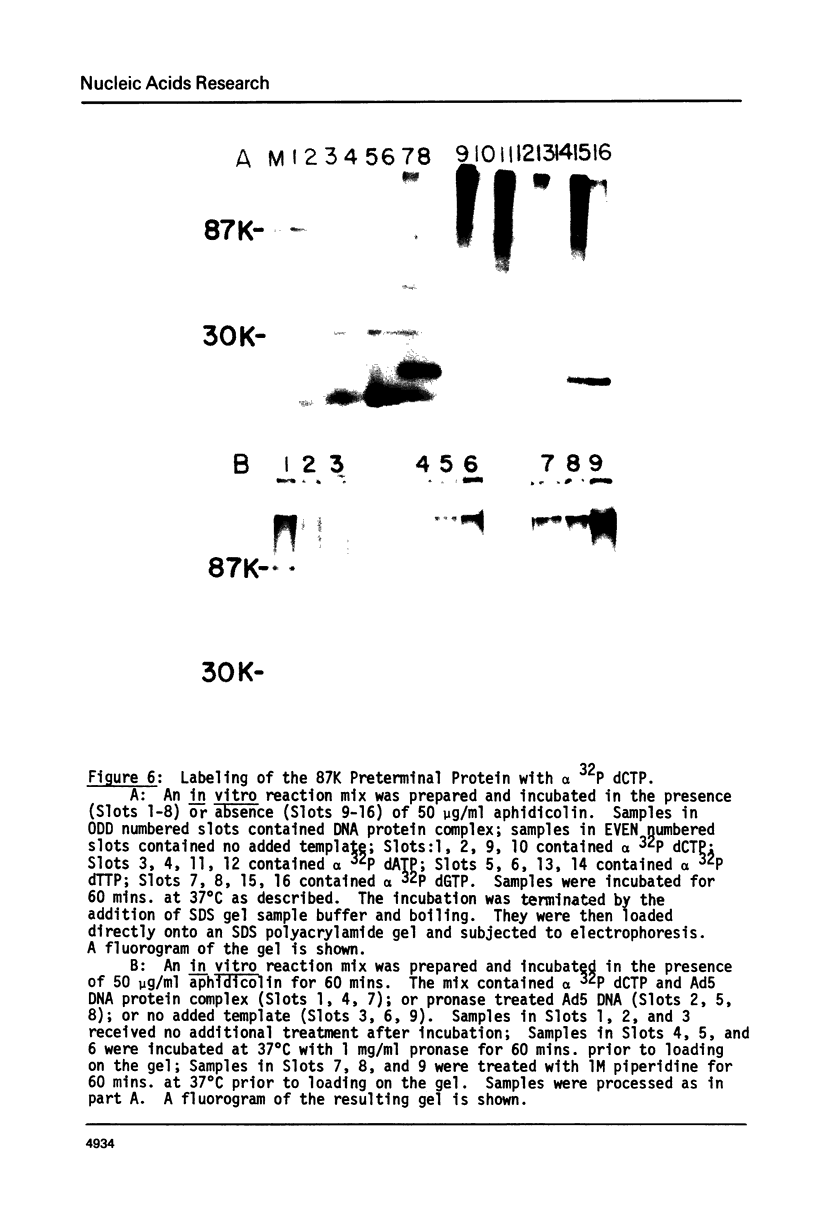
Adenovirus DNA replication is inhibited by aphidicolin but the inhibition clearly has different parameters than the inhibition of purified DNA polymerase alpha. In adenovirus infected Hela cells, 10 micrograms/ml of aphidicolin reduced viral DNA synthesis by 80%. Cellular DNA synthesis was inhibited by 97% at 0.1 microgram/ml. 10 micrograms/ml of drug had no effect on virus yield or late protein synthesis though higher concentrations of drug (50 micrograms/ml) caused an abrupt cessation of late protein synthesis and 100 micrograms/ml reduced virus yield by 3 logs. Concentrations of the drug from 0.5 microgram/ml to 10 micrograms/ml were found to dramatically slow the rate of DNA chain elongation in vitro but not stop it completely, so that over a long period of time net incorporation was reduced only slightly compared to the control. 50 micrograms/ml or 100 micrograms/ml of drug completely inhibited incorporation in vitro. Initiation of viral DNA replication - covalent attachment of dCMP to the preterminal protein - occurs in vitro. This reaction was found to be insensitive to inhibition by aphidicolin. We thus conclude that aphidicolin exerts its effect on adenovirus DNA chain elongation, but not on the primary initiation event of protein priming.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Cells inhibited by n-butyrate support adenovirus replication. Virology. 1980 Dec;107(2):514–519. doi: 10.1016/0042-6822(80)90318-9. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Habara A., Kano K., Nagano H., Mano Y., Ikegami S., Yamashita T. Inhibition of DNA synthesis in the adenovirus DNA replication complex by aphidicolin and 2',3'-dideoxythymidine triphosphate. Biochem Biophys Res Commun. 1980 Jan 15;92(1):8–12. doi: 10.1016/0006-291x(80)91511-9. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Jeng Y. H., Wold W. S., Sugawara K., Gilead Z., Green M. Adenovirus type 2 coded single-stranded DNA binding protein: in vivo phosphorylation and modification. J Virol. 1977 May;22(2):402–411. doi: 10.1128/jvi.22.2.402-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Postel E. H., Levine A. J. In vivo and in vitro phosphorylation of the adenovirus type 5 single strand-specific DNA-binding protein. Virology. 1977 Jun 1;79(1):144–159. doi: 10.1016/0042-6822(77)90341-5. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linné T., Jörnvall H., Philipson L. Purification and characterization of the phosphorylated DNA-binding protein from adenovirus-type-2-infected cells. Eur J Biochem. 1977 Jun 15;76(2):481–490. doi: 10.1111/j.1432-1033.1977.tb11618.x. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Horwitz M. S. Chinese hamster ovary cells replicate adenovirus deoxyribonucleic acid. Mol Cell Biol. 1981 Mar;1(3):208–215. doi: 10.1128/mcb.1.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro M., Shioda M., Nagano H., Mano Y., Hanaoka F., Yamada M. The mode of action of aphidicolin on DNA synthesis in isolated nuclei. Biochem Biophys Res Commun. 1980 Jan 15;92(1):13–19. doi: 10.1016/0006-291x(80)91512-0. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bodnar J. W., Coombs D. H., Pearson G. D. Replicating adenovirus 2 DNA molecules contain terminal protein. Virology. 1979 Jul 15;96(1):143–158. doi: 10.1016/0042-6822(79)90180-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Shaw C. H., Rekosh D. M., Russell W. C. Catalysis of adenovirus DNA synthesis in vitro by DNA polymerase gamma. J Gen Virol. 1980 May;48(1):231–236. doi: 10.1099/0022-1317-48-1-231. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S. The nucleotide sequence of the right-hand terminus of adenovirus type 5 DNA: implications for the mechanism of DNA replication. Gene. 1979 Aug;6(4):307–318. doi: 10.1016/0378-1119(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. Adenovirus DNA replication in vitro: a protein linked to the 5' end of nascent DNA strands. J Virol. 1981 Jan;37(1):139–147. doi: 10.1128/jvi.37.1.139-147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Green M. Purification and molecular characterization of adenovirus type 2 DNA-binding protein. J Virol. 1977 Jan;21(1):338–346. doi: 10.1128/jvi.21.1.338-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. The mechanism of replication of adenovirus DNA. VI. Localization of the origins of the displacement synthesis. Virology. 1978 Feb;84(2):509–517. doi: 10.1016/0042-6822(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingärtner B., Winnacker E. L., Tolun A., Pettersson U. Two complementary strand-specific termination sites for adenovirus DNA replication. Cell. 1976 Oct;9(2):259–268. doi: 10.1016/0092-8674(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- Wist E., Prydz H. The effect of aphidicolin on DNA synthesis in isolated HeLa cell nuclei. Nucleic Acids Res. 1979 Apr;6(4):1583–1590. doi: 10.1093/nar/6.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., van der Vliet P. C., Jansz H. S. DNA polymerases in adenovirus type 5-infected and uninfected KB cells. Induction of an alpha-type DNA polymerase in adenovirus type 5-infected and in fast growing cells. Biochim Biophys Acta. 1977 May 17;476(2):156–165. doi: 10.1016/0005-2787(77)90092-2. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bouché J. P., Méchali M., Girard M. Involvement of both DNA polymerases alpha and gamma in the replication of adenovirus deoxyribonucleic acid in vitro. Virology. 1980 Jul 15;104(1):56–72. doi: 10.1016/0042-6822(80)90365-7. [DOI] [PubMed] [Google Scholar]