Abstract
The asymmetric unit of the title compound, C17H16O4, contains two crystallographically independent molecules with different absolute configurations.
Related literature
Flavonoids are thought to have protective effects against cardiovascular diseases, cancers and other age-related diseases due to their high antioxidant capacity, see: Zhang et al. (2008 ▶). For our efforts to synthesize derivatives of flavonoids for urease inhibitors and antibacterial activity screening, see: Xiao et al. (2010 ▶, 2011 ▶).
Experimental
Crystal data
C17H16O4
M r = 284.30
Orthorhombic,
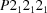
a = 10.601 (2) Å
b = 15.762 (4) Å
c = 16.793 (4) Å
V = 2805.9 (11) Å3
Z = 8
Mo Kα radiation
μ = 0.10 mm−1
T = 296 K
0.20 × 0.20 × 0.10 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.981, T max = 0.991
14633 measured reflections
5752 independent reflections
3699 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.053
wR(F 2) = 0.141
S = 1.03
5752 reflections
383 parameters
H-atom parameters constrained
Δρmax = 0.41 e Å−3
Δρmin = −0.16 e Å−3
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811055048/aa2036sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055048/aa2036Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811055048/aa2036Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C33—H33B⋯O6i | 0.96 | 2.47 | 3.278 (4) | 141 |
Symmetry code: (i) 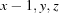 .
.
Acknowledgments
The work was financed by a project supported by the Hunan Provincial Natural Science Foundation of China (grant No. 11 J J3113) and the Key Laboratory of Hunan Forest Products and Chemical Industry Engineering of Hunan Province (grant No. JDZ201102).
supplementary crystallographic information
Comment
Flavonoids are thought to have protective effects against cardiovascular diseases, cancers, and other age-related diseases due to their high antioxidant capacity both in vivo and in vitro systems (Zhang, et al., 2008). Recently, we focused our efforts to synthesize derivatives of flavonoids for urease inhibitors and antibacterial activity screening (Xiao, et al., 2010 and 2011).
The crystal structure of the title compound, 7-methoxy-3-(4-methoxyphenyl)chroman-4-one, contains two crystallographically independent molecules (Fig. 1) in the asymmetric unit. They are a pair of enantiomers, and we define C1 to C17 as molecule A, while C18 to C34 as molecule B. In molecule A, the ring C4/C5/C6/C7/C8/C9 makes a dihedral angle of 72.74 (9) ° with the ring C10/C11/C12/C13/C14/C15. However, in molecule B, the corresponding dihedral angle is 83.32 (11) °.
Experimental
LiAlH4 (5.0 mmol) and AlCl3 (6 mmol) was dissolved in THF (10 ml), and 6 mmol of 7-methoxy-3-(4-methoxyphenyl)-4H-chromen-4-one was subsequently added. The mixture was stirred at 273 K for 3 h, and extracted with ethylacetate followed by addition of water (5 ml). After removal of the solvent, the resulting residue was purified over a silica gel column eluting with etrylacetate-petroleum ether (1:1). The crystals suitable for single-crystal structure determination of the title compound were grown from ethylacetate-petroleum ether at room temperature by slow evaporation.
Refinement
All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H = 0.93 Å for aromatic H atoms, 0.96 Å for CH3 type H atoms, 0.97 Å for CH2 type H atoms and 0.98 Å for CH type H atom, respectively. Uiso(H) values were set at 1.5 times Ueq(C) for methyl H atoms, and 1.2 times for the rest H atoms.
Figures
Fig. 1.
Molecular structure of the title compound. Thermal displacement ellipsoids are drawn at the 30% probability level.
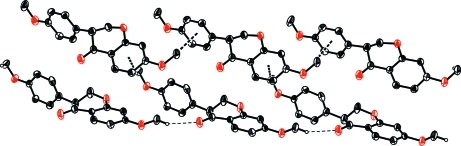
Fig. 2.
Packing diagram.
Crystal data
| C17H16O4 | F(000) = 1200 |
| Mr = 284.30 | Dx = 1.346 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 3901 reflections |
| a = 10.601 (2) Å | θ = 2.5–26.0° |
| b = 15.762 (4) Å | µ = 0.10 mm−1 |
| c = 16.793 (4) Å | T = 296 K |
| V = 2805.9 (11) Å3 | Block, colorless |
| Z = 8 | 0.20 × 0.20 × 0.10 mm |
Data collection
| Bruker SMART APEX CCD diffractometer | 5752 independent reflections |
| Radiation source: fine-focus sealed tube | 3699 reflections with I > 2σ(I) |
| graphite | Rint = 0.034 |
| φ and ω scan | θmax = 26.5°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −12→13 |
| Tmin = 0.981, Tmax = 0.991 | k = −19→19 |
| 14633 measured reflections | l = −14→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.053 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.141 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0682P)2 + 0.1397P] where P = (Fo2 + 2Fc2)/3 |
| 5752 reflections | (Δ/σ)max < 0.001 |
| 383 parameters | Δρmax = 0.41 e Å−3 |
| 0 restraints | Δρmin = −0.16 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.5166 (3) | 0.7672 (2) | 0.2776 (2) | 0.0665 (10) | |
| H1A | 0.5125 | 0.7484 | 0.2226 | 0.080* | |
| H1B | 0.5336 | 0.7178 | 0.3103 | 0.080* | |
| C2 | 0.6228 (3) | 0.8276 (2) | 0.2860 (2) | 0.0613 (9) | |
| H2 | 0.6243 | 0.8451 | 0.3420 | 0.074* | |
| C3 | 0.5957 (3) | 0.90714 (18) | 0.2376 (2) | 0.0508 (8) | |
| C4 | 0.4627 (3) | 0.93083 (18) | 0.23531 (18) | 0.0456 (7) | |
| C5 | 0.4228 (3) | 1.00835 (18) | 0.20398 (19) | 0.0536 (8) | |
| H5 | 0.4821 | 1.0445 | 0.1812 | 0.064* | |
| C6 | 0.2990 (3) | 1.03259 (19) | 0.20583 (19) | 0.0557 (8) | |
| H6 | 0.2750 | 1.0847 | 0.1848 | 0.067* | |
| C7 | 0.2092 (3) | 0.97925 (19) | 0.23918 (18) | 0.0491 (7) | |
| C8 | 0.2442 (3) | 0.90182 (19) | 0.27018 (19) | 0.0515 (8) | |
| H8 | 0.1842 | 0.8660 | 0.2925 | 0.062* | |
| C9 | 0.3703 (3) | 0.87803 (18) | 0.26759 (18) | 0.0458 (7) | |
| C10 | 0.7500 (3) | 0.78672 (18) | 0.2689 (2) | 0.0544 (8) | |
| C11 | 0.7875 (3) | 0.75939 (19) | 0.1948 (2) | 0.0568 (8) | |
| H11 | 0.7347 | 0.7685 | 0.1513 | 0.068* | |
| C12 | 0.9017 (3) | 0.71863 (19) | 0.1831 (2) | 0.0584 (8) | |
| H12 | 0.9246 | 0.6999 | 0.1326 | 0.070* | |
| C13 | 0.9818 (3) | 0.70588 (19) | 0.2476 (2) | 0.0554 (9) | |
| C14 | 0.9440 (3) | 0.73214 (19) | 0.3223 (2) | 0.0568 (8) | |
| H14 | 0.9961 | 0.7232 | 0.3661 | 0.068* | |
| C15 | 0.8298 (3) | 0.7713 (2) | 0.33210 (19) | 0.0556 (8) | |
| H15 | 0.8053 | 0.7880 | 0.3829 | 0.067* | |
| C16 | −0.0074 (3) | 0.9543 (2) | 0.2701 (2) | 0.0706 (10) | |
| H16A | −0.0069 | 0.9000 | 0.2441 | 0.106* | |
| H16B | −0.0882 | 0.9807 | 0.2628 | 0.106* | |
| H16C | 0.0084 | 0.9468 | 0.3259 | 0.106* | |
| C17 | 1.1718 (3) | 0.6448 (3) | 0.2977 (3) | 0.0954 (14) | |
| H17A | 1.1985 | 0.6952 | 0.3249 | 0.143* | |
| H17B | 1.2444 | 0.6142 | 0.2792 | 0.143* | |
| H17C | 1.1243 | 0.6097 | 0.3335 | 0.143* | |
| C18 | 0.1377 (3) | 0.5956 (2) | 1.0117 (3) | 0.0792 (12) | |
| H18A | 0.1493 | 0.6508 | 1.0362 | 0.095* | |
| H18B | 0.1475 | 0.6027 | 0.9547 | 0.095* | |
| C19 | 0.2374 (3) | 0.5387 (2) | 1.0404 (3) | 0.0736 (11) | |
| H19 | 0.2248 | 0.5342 | 1.0980 | 0.088* | |
| C20 | 0.2169 (3) | 0.45049 (19) | 1.0078 (2) | 0.0563 (8) | |
| C21 | 0.0856 (3) | 0.42614 (17) | 0.99789 (17) | 0.0421 (7) | |
| C22 | 0.0498 (3) | 0.34237 (18) | 0.98230 (19) | 0.0518 (8) | |
| H22 | 0.1118 | 0.3023 | 0.9711 | 0.062* | |
| C23 | −0.0736 (3) | 0.31797 (17) | 0.9831 (2) | 0.0525 (8) | |
| H23 | −0.0951 | 0.2618 | 0.9730 | 0.063* | |
| C24 | −0.1670 (3) | 0.37726 (17) | 0.99896 (17) | 0.0440 (7) | |
| C25 | −0.1365 (3) | 0.46103 (17) | 1.01252 (18) | 0.0461 (7) | |
| H25 | −0.1994 | 0.5010 | 1.0218 | 0.055* | |
| C26 | −0.0111 (3) | 0.48479 (16) | 1.01209 (17) | 0.0450 (7) | |
| C27 | 0.3690 (3) | 0.5740 (2) | 1.0297 (2) | 0.0600 (9) | |
| C28 | 0.4459 (3) | 0.58703 (19) | 1.0935 (2) | 0.0600 (9) | |
| H28 | 0.4169 | 0.5732 | 1.1442 | 0.072* | |
| C29 | 0.5642 (3) | 0.6198 (2) | 1.0852 (2) | 0.0539 (8) | |
| H29 | 0.6145 | 0.6277 | 1.1299 | 0.065* | |
| C30 | 0.6100 (3) | 0.64138 (16) | 1.01120 (18) | 0.0449 (7) | |
| C31 | 0.5366 (3) | 0.6276 (2) | 0.9448 (2) | 0.0580 (8) | |
| H31 | 0.5664 | 0.6409 | 0.8942 | 0.070* | |
| C32 | 0.4158 (3) | 0.5929 (2) | 0.9551 (2) | 0.0674 (10) | |
| H32 | 0.3661 | 0.5824 | 0.9106 | 0.081* | |
| C33 | −0.3882 (3) | 0.4047 (2) | 1.0076 (3) | 0.0741 (11) | |
| H33A | −0.3813 | 0.4334 | 1.0579 | 0.111* | |
| H33B | −0.4667 | 0.3744 | 1.0054 | 0.111* | |
| H33C | −0.3854 | 0.4456 | 0.9652 | 0.111* | |
| C34 | 0.7894 (3) | 0.6869 (2) | 0.9356 (2) | 0.0702 (10) | |
| H34A | 0.7964 | 0.6330 | 0.9092 | 0.105* | |
| H34B | 0.8720 | 0.7102 | 0.9438 | 0.105* | |
| H34C | 0.7407 | 0.7249 | 0.9032 | 0.105* | |
| O1 | 0.39795 (19) | 0.80063 (12) | 0.29927 (15) | 0.0646 (7) | |
| O2 | 0.6782 (2) | 0.95173 (15) | 0.21016 (18) | 0.0815 (8) | |
| O3 | 0.0888 (2) | 1.00702 (14) | 0.23637 (15) | 0.0665 (6) | |
| O4 | 1.0946 (2) | 0.66753 (16) | 0.23146 (17) | 0.0768 (7) | |
| O5 | 0.01306 (19) | 0.56791 (13) | 1.02714 (16) | 0.0678 (7) | |
| O6 | 0.3028 (2) | 0.39876 (14) | 1.00363 (17) | 0.0798 (8) | |
| O7 | −0.28651 (18) | 0.34673 (12) | 0.99912 (14) | 0.0607 (6) | |
| O8 | 0.72870 (19) | 0.67584 (14) | 1.01015 (13) | 0.0604 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.049 (2) | 0.0531 (18) | 0.098 (3) | 0.0031 (16) | 0.010 (2) | 0.0168 (18) |
| C2 | 0.053 (2) | 0.0519 (17) | 0.079 (2) | 0.0071 (16) | −0.0011 (18) | 0.0017 (17) |
| C3 | 0.0434 (19) | 0.0398 (15) | 0.069 (2) | −0.0033 (14) | 0.0028 (16) | −0.0044 (14) |
| C4 | 0.0432 (19) | 0.0422 (15) | 0.0514 (18) | −0.0008 (13) | −0.0010 (14) | −0.0022 (13) |
| C5 | 0.0491 (19) | 0.0462 (16) | 0.065 (2) | −0.0066 (15) | 0.0050 (16) | 0.0071 (15) |
| C6 | 0.054 (2) | 0.0427 (16) | 0.071 (2) | 0.0057 (15) | 0.0004 (17) | 0.0035 (15) |
| C7 | 0.0407 (18) | 0.0502 (18) | 0.0564 (18) | 0.0023 (14) | −0.0006 (15) | −0.0048 (14) |
| C8 | 0.0416 (18) | 0.0538 (18) | 0.0592 (19) | −0.0020 (14) | 0.0056 (15) | 0.0022 (15) |
| C9 | 0.0477 (19) | 0.0412 (15) | 0.0484 (17) | −0.0004 (13) | 0.0015 (14) | 0.0009 (13) |
| C10 | 0.0461 (19) | 0.0424 (16) | 0.075 (2) | −0.0022 (14) | 0.0049 (17) | 0.0001 (16) |
| C11 | 0.054 (2) | 0.0546 (18) | 0.062 (2) | −0.0024 (16) | −0.0137 (18) | 0.0050 (16) |
| C12 | 0.067 (2) | 0.0531 (17) | 0.055 (2) | −0.0020 (17) | 0.0034 (18) | −0.0044 (15) |
| C13 | 0.0430 (19) | 0.0464 (17) | 0.077 (3) | −0.0015 (15) | 0.0007 (17) | 0.0008 (16) |
| C14 | 0.055 (2) | 0.0577 (18) | 0.058 (2) | 0.0026 (16) | −0.0096 (17) | 0.0028 (16) |
| C15 | 0.057 (2) | 0.0580 (18) | 0.0517 (19) | 0.0033 (16) | 0.0005 (17) | −0.0013 (15) |
| C16 | 0.0416 (19) | 0.075 (2) | 0.095 (3) | 0.0097 (18) | 0.010 (2) | −0.004 (2) |
| C17 | 0.055 (2) | 0.081 (3) | 0.150 (4) | 0.017 (2) | −0.024 (3) | 0.009 (3) |
| C18 | 0.044 (2) | 0.0441 (17) | 0.149 (4) | −0.0088 (15) | 0.001 (2) | −0.013 (2) |
| C19 | 0.046 (2) | 0.057 (2) | 0.118 (3) | −0.0070 (17) | 0.000 (2) | −0.009 (2) |
| C20 | 0.0426 (19) | 0.0464 (16) | 0.080 (2) | 0.0013 (15) | −0.0040 (18) | 0.0017 (16) |
| C21 | 0.0363 (16) | 0.0410 (14) | 0.0489 (17) | 0.0024 (12) | −0.0016 (14) | 0.0010 (12) |
| C22 | 0.0475 (19) | 0.0409 (16) | 0.067 (2) | 0.0088 (14) | −0.0038 (16) | −0.0035 (14) |
| C23 | 0.0496 (19) | 0.0340 (14) | 0.074 (2) | −0.0020 (13) | −0.0088 (17) | −0.0042 (14) |
| C24 | 0.0354 (16) | 0.0421 (14) | 0.0545 (18) | −0.0003 (12) | −0.0093 (14) | 0.0032 (14) |
| C25 | 0.0409 (17) | 0.0384 (14) | 0.0590 (19) | 0.0038 (12) | −0.0020 (14) | −0.0049 (13) |
| C26 | 0.0454 (18) | 0.0374 (14) | 0.0521 (18) | 0.0012 (13) | −0.0018 (14) | −0.0023 (13) |
| C27 | 0.045 (2) | 0.0467 (18) | 0.088 (3) | −0.0048 (15) | 0.0068 (19) | −0.0004 (18) |
| C28 | 0.056 (2) | 0.055 (2) | 0.069 (2) | −0.0074 (17) | 0.0047 (18) | −0.0027 (16) |
| C29 | 0.0466 (19) | 0.0557 (18) | 0.059 (2) | −0.0058 (15) | 0.0018 (16) | −0.0064 (15) |
| C30 | 0.0397 (17) | 0.0380 (14) | 0.057 (2) | −0.0044 (12) | 0.0017 (15) | −0.0064 (14) |
| C31 | 0.055 (2) | 0.064 (2) | 0.055 (2) | −0.0058 (17) | −0.0069 (17) | 0.0038 (16) |
| C32 | 0.049 (2) | 0.078 (2) | 0.076 (3) | 0.0067 (19) | −0.023 (2) | −0.014 (2) |
| C33 | 0.0402 (19) | 0.061 (2) | 0.121 (3) | −0.0022 (16) | 0.005 (2) | 0.002 (2) |
| C34 | 0.060 (2) | 0.069 (2) | 0.082 (3) | −0.0110 (19) | 0.023 (2) | −0.0022 (18) |
| O1 | 0.0452 (13) | 0.0520 (12) | 0.0967 (18) | 0.0035 (10) | 0.0122 (12) | 0.0219 (12) |
| O2 | 0.0483 (14) | 0.0606 (14) | 0.136 (2) | −0.0077 (12) | 0.0038 (15) | 0.0222 (15) |
| O3 | 0.0434 (13) | 0.0552 (12) | 0.1010 (18) | 0.0060 (11) | 0.0061 (13) | 0.0001 (12) |
| O4 | 0.0524 (14) | 0.0700 (15) | 0.108 (2) | 0.0136 (13) | 0.0108 (15) | 0.0035 (14) |
| O5 | 0.0392 (13) | 0.0409 (11) | 0.123 (2) | −0.0001 (9) | 0.0027 (13) | −0.0177 (12) |
| O6 | 0.0435 (14) | 0.0604 (14) | 0.135 (2) | 0.0086 (11) | −0.0034 (16) | 0.0031 (15) |
| O7 | 0.0391 (12) | 0.0472 (11) | 0.0958 (17) | −0.0052 (10) | −0.0051 (12) | −0.0040 (11) |
| O8 | 0.0471 (13) | 0.0733 (13) | 0.0607 (14) | −0.0151 (11) | 0.0063 (11) | −0.0113 (11) |
Geometric parameters (Å, °)
| C1—O1 | 1.411 (4) | C18—O5 | 1.415 (4) |
| C1—C2 | 1.482 (4) | C18—C19 | 1.467 (5) |
| C1—H1A | 0.9700 | C18—H18A | 0.9700 |
| C1—H1B | 0.9700 | C18—H18B | 0.9700 |
| C2—C3 | 1.521 (5) | C19—C20 | 1.511 (5) |
| C2—C10 | 1.523 (4) | C19—C27 | 1.512 (5) |
| C2—H2 | 0.9800 | C19—H19 | 0.9800 |
| C3—O2 | 1.213 (4) | C20—O6 | 1.224 (3) |
| C3—C4 | 1.459 (4) | C20—C21 | 1.453 (4) |
| C4—C9 | 1.395 (4) | C21—C22 | 1.399 (4) |
| C4—C5 | 1.396 (4) | C21—C26 | 1.401 (4) |
| C5—C6 | 1.368 (4) | C22—C23 | 1.364 (4) |
| C5—H5 | 0.9300 | C22—H22 | 0.9300 |
| C6—C7 | 1.388 (4) | C23—C24 | 1.388 (4) |
| C6—H6 | 0.9300 | C23—H23 | 0.9300 |
| C7—O3 | 1.350 (3) | C24—O7 | 1.355 (3) |
| C7—C8 | 1.378 (4) | C24—C25 | 1.378 (4) |
| C8—C9 | 1.389 (4) | C25—C26 | 1.381 (4) |
| C8—H8 | 0.9300 | C25—H25 | 0.9300 |
| C9—O1 | 1.363 (3) | C26—O5 | 1.359 (3) |
| C10—C11 | 1.376 (5) | C27—C28 | 1.362 (5) |
| C10—C15 | 1.379 (4) | C27—C32 | 1.380 (5) |
| C11—C12 | 1.384 (4) | C28—C29 | 1.364 (4) |
| C11—H11 | 0.9300 | C28—H28 | 0.9300 |
| C12—C13 | 1.391 (5) | C29—C30 | 1.376 (4) |
| C12—H12 | 0.9300 | C29—H29 | 0.9300 |
| C13—O4 | 1.366 (4) | C30—O8 | 1.370 (3) |
| C13—C14 | 1.381 (5) | C30—C31 | 1.377 (4) |
| C14—C15 | 1.368 (4) | C31—C32 | 1.403 (4) |
| C14—H14 | 0.9300 | C31—H31 | 0.9300 |
| C15—H15 | 0.9300 | C32—H32 | 0.9300 |
| C16—O3 | 1.432 (4) | C33—O7 | 1.421 (4) |
| C16—H16A | 0.9600 | C33—H33A | 0.9600 |
| C16—H16B | 0.9600 | C33—H33B | 0.9600 |
| C16—H16C | 0.9600 | C33—H33C | 0.9600 |
| C17—O4 | 1.427 (5) | C34—O8 | 1.419 (4) |
| C17—H17A | 0.9600 | C34—H34A | 0.9600 |
| C17—H17B | 0.9600 | C34—H34B | 0.9600 |
| C17—H17C | 0.9600 | C34—H34C | 0.9600 |
| O1—C1—C2 | 114.3 (3) | O5—C18—H18B | 108.5 |
| O1—C1—H1A | 108.7 | C19—C18—H18B | 108.5 |
| C2—C1—H1A | 108.7 | H18A—C18—H18B | 107.5 |
| O1—C1—H1B | 108.7 | C18—C19—C20 | 109.8 (3) |
| C2—C1—H1B | 108.7 | C18—C19—C27 | 113.7 (3) |
| H1A—C1—H1B | 107.6 | C20—C19—C27 | 115.4 (3) |
| C1—C2—C3 | 109.6 (3) | C18—C19—H19 | 105.7 |
| C1—C2—C10 | 112.5 (3) | C20—C19—H19 | 105.7 |
| C3—C2—C10 | 114.5 (3) | C27—C19—H19 | 105.7 |
| C1—C2—H2 | 106.6 | O6—C20—C21 | 122.0 (3) |
| C3—C2—H2 | 106.6 | O6—C20—C19 | 121.8 (3) |
| C10—C2—H2 | 106.6 | C21—C20—C19 | 115.0 (3) |
| O2—C3—C4 | 122.6 (3) | C22—C21—C26 | 117.2 (3) |
| O2—C3—C2 | 123.0 (3) | C22—C21—C20 | 122.0 (3) |
| C4—C3—C2 | 114.0 (3) | C26—C21—C20 | 120.5 (2) |
| C9—C4—C5 | 117.1 (3) | C23—C22—C21 | 121.6 (3) |
| C9—C4—C3 | 121.0 (3) | C23—C22—H22 | 119.2 |
| C5—C4—C3 | 121.8 (3) | C21—C22—H22 | 119.2 |
| C6—C5—C4 | 121.8 (3) | C22—C23—C24 | 119.8 (3) |
| C6—C5—H5 | 119.1 | C22—C23—H23 | 120.1 |
| C4—C5—H5 | 119.1 | C24—C23—H23 | 120.1 |
| C5—C6—C7 | 119.9 (3) | O7—C24—C25 | 124.0 (3) |
| C5—C6—H6 | 120.1 | O7—C24—C23 | 115.4 (2) |
| C7—C6—H6 | 120.1 | C25—C24—C23 | 120.6 (3) |
| O3—C7—C8 | 123.7 (3) | C24—C25—C26 | 119.0 (3) |
| O3—C7—C6 | 116.0 (3) | C24—C25—H25 | 120.5 |
| C8—C7—C6 | 120.3 (3) | C26—C25—H25 | 120.5 |
| C7—C8—C9 | 119.1 (3) | O5—C26—C25 | 116.2 (2) |
| C7—C8—H8 | 120.4 | O5—C26—C21 | 122.0 (3) |
| C9—C8—H8 | 120.4 | C25—C26—C21 | 121.8 (2) |
| O1—C9—C8 | 115.9 (3) | C28—C27—C32 | 117.8 (3) |
| O1—C9—C4 | 122.4 (3) | C28—C27—C19 | 121.0 (3) |
| C8—C9—C4 | 121.8 (3) | C32—C27—C19 | 121.2 (4) |
| C11—C10—C15 | 117.7 (3) | C27—C28—C29 | 121.8 (3) |
| C11—C10—C2 | 124.0 (3) | C27—C28—H28 | 119.1 |
| C15—C10—C2 | 118.3 (3) | C29—C28—H28 | 119.1 |
| C10—C11—C12 | 121.7 (3) | C28—C29—C30 | 120.8 (3) |
| C10—C11—H11 | 119.2 | C28—C29—H29 | 119.6 |
| C12—C11—H11 | 119.2 | C30—C29—H29 | 119.6 |
| C11—C12—C13 | 119.4 (3) | O8—C30—C29 | 115.7 (3) |
| C11—C12—H12 | 120.3 | O8—C30—C31 | 124.8 (3) |
| C13—C12—H12 | 120.3 | C29—C30—C31 | 119.5 (3) |
| O4—C13—C14 | 124.5 (3) | C30—C31—C32 | 118.5 (3) |
| O4—C13—C12 | 116.3 (3) | C30—C31—H31 | 120.8 |
| C14—C13—C12 | 119.1 (3) | C32—C31—H31 | 120.8 |
| C15—C14—C13 | 120.1 (3) | C27—C32—C31 | 121.7 (3) |
| C15—C14—H14 | 120.0 | C27—C32—H32 | 119.2 |
| C13—C14—H14 | 120.0 | C31—C32—H32 | 119.2 |
| C14—C15—C10 | 122.0 (3) | O7—C33—H33A | 109.5 |
| C14—C15—H15 | 119.0 | O7—C33—H33B | 109.5 |
| C10—C15—H15 | 119.0 | H33A—C33—H33B | 109.5 |
| O3—C16—H16A | 109.5 | O7—C33—H33C | 109.5 |
| O3—C16—H16B | 109.5 | H33A—C33—H33C | 109.5 |
| H16A—C16—H16B | 109.5 | H33B—C33—H33C | 109.5 |
| O3—C16—H16C | 109.5 | O8—C34—H34A | 109.5 |
| H16A—C16—H16C | 109.5 | O8—C34—H34B | 109.5 |
| H16B—C16—H16C | 109.5 | H34A—C34—H34B | 109.5 |
| O4—C17—H17A | 109.5 | O8—C34—H34C | 109.5 |
| O4—C17—H17B | 109.5 | H34A—C34—H34C | 109.5 |
| H17A—C17—H17B | 109.5 | H34B—C34—H34C | 109.5 |
| O4—C17—H17C | 109.5 | C9—O1—C1 | 115.2 (2) |
| H17A—C17—H17C | 109.5 | C7—O3—C16 | 118.1 (2) |
| H17B—C17—H17C | 109.5 | C13—O4—C17 | 117.3 (3) |
| O5—C18—C19 | 115.1 (3) | C26—O5—C18 | 116.1 (2) |
| O5—C18—H18A | 108.5 | C24—O7—C33 | 118.8 (2) |
| C19—C18—H18A | 108.5 | C30—O8—C34 | 118.4 (2) |
| O1—C1—C2—C3 | −56.3 (4) | C19—C20—C21—C22 | −168.2 (3) |
| O1—C1—C2—C10 | 175.0 (3) | O6—C20—C21—C26 | 172.5 (3) |
| C1—C2—C3—O2 | −152.6 (3) | C19—C20—C21—C26 | 5.0 (5) |
| C10—C2—C3—O2 | −25.1 (5) | C26—C21—C22—C23 | −1.8 (5) |
| C1—C2—C3—C4 | 34.6 (4) | C20—C21—C22—C23 | 171.6 (3) |
| C10—C2—C3—C4 | 162.1 (3) | C21—C22—C23—C24 | 0.6 (5) |
| O2—C3—C4—C9 | −179.9 (3) | C22—C23—C24—O7 | −179.4 (3) |
| C2—C3—C4—C9 | −7.0 (4) | C22—C23—C24—C25 | 1.2 (5) |
| O2—C3—C4—C5 | −2.5 (5) | O7—C24—C25—C26 | 179.1 (3) |
| C2—C3—C4—C5 | 170.3 (3) | C23—C24—C25—C26 | −1.6 (5) |
| C9—C4—C5—C6 | 1.1 (4) | C24—C25—C26—O5 | −178.9 (3) |
| C3—C4—C5—C6 | −176.3 (3) | C24—C25—C26—C21 | 0.3 (5) |
| C4—C5—C6—C7 | −0.3 (5) | C22—C21—C26—O5 | −179.4 (3) |
| C5—C6—C7—O3 | −178.1 (3) | C20—C21—C26—O5 | 7.1 (5) |
| C5—C6—C7—C8 | −0.2 (5) | C22—C21—C26—C25 | 1.4 (4) |
| O3—C7—C8—C9 | 177.7 (3) | C20—C21—C26—C25 | −172.1 (3) |
| C6—C7—C8—C9 | 0.0 (5) | C18—C19—C27—C28 | 119.8 (4) |
| C7—C8—C9—O1 | −179.9 (3) | C20—C19—C27—C28 | −112.0 (4) |
| C7—C8—C9—C4 | 0.8 (5) | C18—C19—C27—C32 | −61.1 (5) |
| C5—C4—C9—O1 | 179.5 (3) | C20—C19—C27—C32 | 67.1 (5) |
| C3—C4—C9—O1 | −3.1 (5) | C32—C27—C28—C29 | 1.8 (5) |
| C5—C4—C9—C8 | −1.3 (4) | C19—C27—C28—C29 | −179.1 (3) |
| C3—C4—C9—C8 | 176.1 (3) | C27—C28—C29—C30 | 0.3 (5) |
| C1—C2—C10—C11 | 68.4 (4) | C28—C29—C30—O8 | 178.3 (3) |
| C3—C2—C10—C11 | −57.7 (4) | C28—C29—C30—C31 | −1.7 (4) |
| C1—C2—C10—C15 | −107.5 (4) | O8—C30—C31—C32 | −179.0 (3) |
| C3—C2—C10—C15 | 126.4 (3) | C29—C30—C31—C32 | 1.0 (4) |
| C15—C10—C11—C12 | −0.7 (4) | C28—C27—C32—C31 | −2.5 (5) |
| C2—C10—C11—C12 | −176.6 (3) | C19—C27—C32—C31 | 178.4 (3) |
| C10—C11—C12—C13 | −0.9 (4) | C30—C31—C32—C27 | 1.1 (5) |
| C11—C12—C13—O4 | −178.1 (3) | C8—C9—O1—C1 | 163.5 (3) |
| C11—C12—C13—C14 | 1.8 (4) | C4—C9—O1—C1 | −17.2 (4) |
| O4—C13—C14—C15 | 178.9 (3) | C2—C1—O1—C9 | 48.1 (4) |
| C12—C13—C14—C15 | −1.0 (5) | C8—C7—O3—C16 | 2.3 (4) |
| C13—C14—C15—C10 | −0.7 (5) | C6—C7—O3—C16 | −179.9 (3) |
| C11—C10—C15—C14 | 1.6 (5) | C14—C13—O4—C17 | 7.3 (5) |
| C2—C10—C15—C14 | 177.7 (3) | C12—C13—O4—C17 | −172.8 (3) |
| O5—C18—C19—C20 | 54.4 (5) | C25—C26—O5—C18 | −168.2 (3) |
| O5—C18—C19—C27 | −174.6 (3) | C21—C26—O5—C18 | 12.5 (4) |
| C18—C19—C20—O6 | 158.8 (4) | C19—C18—O5—C26 | −44.7 (5) |
| C27—C19—C20—O6 | 28.7 (5) | C25—C24—O7—C33 | 5.1 (5) |
| C18—C19—C20—C21 | −33.6 (4) | C23—C24—O7—C33 | −174.3 (3) |
| C27—C19—C20—C21 | −163.7 (3) | C29—C30—O8—C34 | 170.1 (3) |
| O6—C20—C21—C22 | −0.7 (5) | C31—C30—O8—C34 | −9.9 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C33—H33B···O6i | 0.96 | 2.47 | 3.278 (4) | 141 |
Symmetry codes: (i) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AA2036).
References
- Bruker (2007). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Xiao, Z.-P., Ma, T.-W., Fu, W.-C., Peng, X.-C., Zhang, A.-H. & Zhu, H.-L. (2010). Eur. J. Med. Chem. 45, 5064–5070. [DOI] [PubMed]
- Xiao, Z.-P., Peng, Z.-Y., Peng, M.-J., Yan, W.-B., Ouyang, Y.-Z. & Zhu, H.-L. (2011). Mini-Rev. Med. Chem. 11, 169–177. [DOI] [PubMed]
- Zhang, W. S., Li, X., Zheng, J. T., Wang, G. Y. & Sun, C. D. (2008). Eur. Food Res. Technol. 227, 1091–1097.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811055048/aa2036sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055048/aa2036Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811055048/aa2036Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report