Abstract
Adaptive responses in glutamate and opioid receptor systems in limbic circuits are emerging as a critical component of the neural plasticity induced by chronic use of abused substances. The present commentary reviews findings from neuroanatomical studies, with superior spatial resolution, that support a cellular basis for prominent interactions of glutamate and opioid receptor systems in preclinical models of drug addiction. The review begins by highlighting the advantages of high-resolution electron microscopic immunohistochemistry for unraveling receptor interactions at the synapse. With an emphasis on a recent publication describing the anatomical relationship between the μ-opioid receptor (MOR) and the AMPA-GluR2 subunit by Beckerman and Glass (2010), we review the anatomical evidence for opioid-induced neural plasticity of glutamate receptors in selected brain circuits that are key integrative substrates in the brain's motivational system. The findings stress the importance of glutamate-opioid interactions as important neural mediators of adaptations to chronic use of abused drugs, particularly within the amygdaloid complex.
The Role of Glutamate in Opiate Addiction
The importance of glutamate signaling in drug addiction has been extensively characterized for numerous drugs of abuse, including amphetamines, benzodiazepines, cocaine, ethanol, nicotine and opiates [for reviews see, (Cunha-Oliveira, et al., 2008, Kalivas, et al., 2009, Koob, 2003)]. Disruption of normal glutamate transmission has been implicated in drug-dependent excitotoxicity, drug seeking and reinstatement, as well as reward and reinforcement (Kalivas, et al., 2009, Kalivas, et al., 2006, Knackstedt and Kalivas, 2009). For many drugs of abuse, glutamate-opioid interactions are important determinants of addictive processes in the brain, and both stress and exposure to drugs of abuse engage the glutamatergic system at common neural sites (Fitzgerald, et al., 1996). As with many addictive compounds, opiate abuse can lead to disturbances in excitatory amino acid signaling, particularly through homeostatic disruption of glutamate, a ubiquitous excitatory neurotransmitter in the nervous system (Cunha-Oliveira, et al., 2008). As a result, numerous investigators have explored the nature of glutamate-opioid interactions in drug abuse in the hopes of better identifying potential therapeutic targets for the treatment of addiction.
The amygdaloid complex, in particular, serves as a critical site where glutamate and opioid signaling intersect, and the role of this limbic structure has been the subject of intensive investigation (Koob, 2003). The impact of the opioid system on glutamate regulation of synaptic plasticity will be reviewed in light of recently published findings from Beckerman and Glass in a recent issue of the journal. Glass's group has examined the cellular basis for glutamate-opioid interactions in the amygdala using high-resolution electron microscopy and this work provides an important anatomical substrate for proposed interactions between the two systems.
Benefits of a High Resolution Immuno-Electron Microscopy Approach
In order to understand how drugs of abuse may impact G protein-coupled receptor or ionotropic receptor dynamics and contribute to synaptic plasticity, it is essential to achieve superior anatomical resolution to unravel the complexities of their proposed interactions at the synaptic level. In situations in which light and fluorescence microscopy techniques may provide only limited resolution, electron microscopy provides enhanced subcellular resolution. Dual labeling immunohistochemistry employing visually distinct immunoperoxidase and immunogold markers has been an effective approach for elucidating complex receptor profiles at the synapse and to definitively establish the localization of individual receptors and ligands to common cellular profiles. The immuno-electron microscopy approach offers the potential for determining membrane versus intracellular protein localization (Bangasser, et al., 2010, Reyes, et al., 2010, Reyes, et al., 2006, Reyes, et al., 2008), as well as the association with various identifiable cellular organelles. For both G-protein coupled receptors and ionotropic receptors, resolving the subcellular distribution of these proteins allows predictions to be made regarding potential receptor interactions and the ability to test specific synaptic models of interaction between related receptor systems.
The pre-embedding immunogold-silver technique used in the study by Beckerman and Glass (2010) has several advantages. This approach maintains morphological preservation while preserving discrete subcellular localization of the antigen of interest (Leranth and Pickel, 1989). Furthermore, pre-embedding immunogold-silver labeling can be more appropriate than post-embedding immunogold labeling for localizing immunoreactivity at extra-synaptic sites, and therefore, is particularly useful for determining the regional distribution of receptors (Lujan, et al., 1996). Post-embedding immunogold labeling, although useful for some antigens, can yield a high degree of non-specific adhesion of the gold particles, making specificity of the reaction deposit difficult to discern. In combination with peroxidase detection of a second antigen, which appears as a dense homogeneous precipitate within cellular compartments, the dual labeling approach can unequivocally establish co-existence of distinct receptor proteins in common cellular elements. Although the pre-embedding immunogold-silver approach may produce lower estimates of receptor number than immunoperoxidase labeling due to differences in reagent penetration (Leranth and Pickel, 1989), limitations of the experimental approach are lessened by restricting analysis of sections to the outer surface of the tissue where penetration of reagents is optimal. Furthermore, whenever penetration is considered more important than preservation of fine structure of the neuropil, enhancement methods (such as increased detergents i.e. Triton X-100) can be considered (Leranth and Pickel, 1989). In summary, the quantitative approach used by Beckerman and Glass (2010) has been extensively validated (Leranth and Pickel, 1989) and, under carefully controlled conditions, can allow for the quantitative evaluation of the co-localization of neurotransmitters and receptors within the neuropil.
Techniques used to discern the cellular distribution of glutamate and opioid receptor/ligand constituents have provided critical information pertaining to functional implications of interactions between these systems (Glass, et al., 2009). Using immunohistochemistry combined with electron microscopy, such high-resolution neuroanatomical studies have revealed sites of receptor interaction that can inform experimental approaches using pharmacological manipulation to target these systems under conditions of chronic use of abused substances versus control conditions.
The amygdala: an integrator of emotion and behavior
Due to its documented role in fear conditioning and emotional reactions to pain, the CeA has been suggested to form a focal point for the convergence of emotional stimuli to produce emotional responses (Koob, 2003). Moreover, the CeA is a key component of the extended amygdala, a macrostructure composed of basal forebrain areas involved in mediating both reward and stress, and is emerging as a critical neuroanatomical substrate of drug abuse and addiction (Koob, 2003, Koob, 2008, Koob, 2008, Lang and Davis, 2006).
Increasing evidence indicates that glutamate transmission in the CeA plays a central role in addiction-related processes (Bie, et al., 2009, Bie, et al., 2009, Glass, et al., 2008, Glass, et al., 2009, Good and Lupica, 2010, Li, et al., 2007, Pollandt, et al., 2006, Zhu, et al., 2007, Zhu and Pan, 2005). Glutamatergic afferents to the CeA originate from the lateral and BLA nuclei (Pitkanen, et al., 1997, Zhu and Pan, 2004). Additionally, thalamic and limbic cortical areas project to CeA subdivisions (McDonald, et al., 1999, Turner and Herkenham, 1991, Vertes and Hoover, 2008). Information relating to sensory experiences and emotional states relayed by these inputs are integrated with mnemonic systems via afferents from the hippocampal formation (Pitkanen, et al., 2000) and processing of this information in the CeA is modulated by dense monoaminergic innervation (Asan, 1998, Asan, et al., 2005, Eliava, et al., 2003). The CeA, in turn, modulates autonomic, endocrine, and behavioral processes through its projections to other components of the extended amygdala, the hypothalamus, and the brainstem (Koob, 2008).
The CeA may be an important neuroanatomical substrate for glutamate-opioid receptor interactions in opioid addictive behaviors. Most, if not all of the CeA neurons are GABAergic and, in subpopulations, produce neuropeptides including opioid peptides and corticotropin releasing factor (CRF), a stress-related peptide. Increased release of CRF in the CeA is thought to mediate stress and anxiety associated with withdrawal from drugs of abuse, including opioids (Asan, 1998, Asan, et al., 2005, Cassell and Gray, 1989, Fallon and Leslie, 1986, Koob, 2008). Opioid and other peptides are also found in CeA axon terminals (Eliava, et al., 2003, Poulin, et al., 2006). Moreover, the MOR is expressed in neurons in the CeA (Mansour, et al., 1995, Mansour, et al., 1987, Mansour, et al., 1986, Poulin, et al., 2006), and dual immuno-EM labeling for the MOR and the CRF receptor-1 (CRFr-1; (Jaferi and Pickel, 2009)) in the lateral CeA documented that both proteins were colocalized in somata, dendrites, and dendritic spines with over 50% or the CRFr-1-reactive dendritic profiles co-labeled for MOR and 25% of MOR-labeled dendritic profiles colocalizing CRFr1. Dendritic profiles containing CRFr1 and/or MOR received asymmetric synapses from unlabeled or CRFr-1-reactive terminals, while MOR was found in terminals forming symmetric, inhibitory-type synapses. Electrophysiological evidence of an interaction of the drug withdrawal-associated CRF system with glutamatergic transmission in the CeA was suggested by the finding that CRF–dependent LTP in the BLA–CeA pathway was potentiated 2 weeks after cocaine withdrawal, a process possibly involving postsynaptic NMDA receptors (Pollandt, et al., 2006).
Concerning MOR-glutamate receptor interactions, electrophysiological data has shown that stimulation of NMDA and MORs can have significant interactive effects on neuronal activity in the CeA, but suggested somewhat diverse and competing synaptic models of NMDA and MOR signaling (Glass, et al., 2009, Zhu and Pan, 2004, Zhu and Pan, 2005). Moreover, pharmacological antagonists of AMPA and NMDA receptors (Watanabe, et al., 2002), as well as spatial-temporal deletion of NR1 (Glass, et al., 2008) were documented to inhibit the conditioned aversive properties of opioid withdrawal.
Two recent ultrastructural studies by Glass et al. (2009) and Beckerman and Glass (2010) have now provided conclusive morphological proof for association of ionotropic glutamate receptor (iGluR) subunits, namely the NMDA-NR1 and the AMPA GluR2 subunits, with MOR in the mouse CeA (Beckerman and Glass, 2010, Glass, et al., 2009). Their studies corroborated the ultrastructural localization of MOR described for the mouse lateral amygdala (Jaferi and Pickel, 2009), and documented that both glutamate receptor subunits were frequently found to co-localize with MOR in postsynaptic structures of the CeA which were contacted by terminals forming asymmetric, excitatory synapses. The findings were taken to indicate that postsynaptic co-modulation of central amygdala neurons may be a key cellular substrate mediating glutamate and opioid interactions of the neural signaling and plasticity, underlying both normal and pathological emotional processes associated with addictive behavior (Glass, et al., 2009). Interestingly, MOR and the AMPA-GluR2 subunit were frequently associated with common intracellular organelles, as well as adjacent areas of the surface membrane, indicating the possibility that both proteins can form larger macromolecular complexes (Beckerman and Glass, 2010). The NMDA-NR1 subunit and MOR were typically present in non-overlapping subcellular compartments, suggesting distinct synthesis and trafficking of these receptors (Glass, et al., 2009).
Anatomical Evidence for Glutamate-Opioid Interactions
Cellular substrates for glutamate and opioid system interactions have been demonstrated through the co-localization of various glutamate and opioid receptor system constituents in several brain regions known to play a role in opiate addiction. A host of studies of glutamate-opioid interactions have focused upon the iGluRs such as N-methyl D-aspartate receptors (NMDARs) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs). These ion channel receptors allow for the flow of cations such as Ca2+, Na+ and K+, and mediate fast excitatory neurotransmission in the nervous system. The NMDARs are transmembrane ionotropic receptors that require the presence of the NR1 subunit for functionality and, by regulating synaptic plasticity, play an essential role in learning and memory processes [reviewed in (Brown, et al., 1988, Cull-Candy, et al., 2001, Maren, 1999, Rebola, et al., 2010)]. AMPARs are transmembrane ionotropic receptors that mediate fast excitatory synaptic transmission in the nervous system, named for AMPA, a glutamate analogue that binds specifically to these receptors (Cull-Candy, et al., 2006, Kessels and Malinow, 2009, Tanaka, et al., 2000). These receptors are often heterotetramers comprised of combinations of the four AMPAR subunits: GluR1 GluR2, GluR3, and GluR4. The subunit composition of these receptors determines cation permeability, with Ca2+ impermeability conferred by the presence of the GluR2 subunit (Tanaka, et al., 2000). Activity-dependent cellular regulation of the subunit composition of both AMPAR and NMDARs determine both their functionality and the amount of current transmitted. Within the amygdala, NMDA receptor subunits have differential localization in comparison to the AMPA receptor subunits (Farb, et al., 1995, Radley, et al., 2007).
Evidence for co-existence of NMDA and opioid receptors
Because of its well-known role in synaptic plasticity, opioid influences on the NMDAR have received considerable attention. Within the central nucleus of the amygdala (CeA), Glass et al. (2009) provided an anatomical substrate for NMDA-NR1 subunit co-existence with the μ-opioid receptor (MOR) subtype in primarily dendritic profiles that often received asymmetric excitatory-type synaptic input. Within the nucleus of the solitary tract (NTS), a major afferent to the CeA, Huang, et al. (2000) detected the functional NR1 subunit of the NMDAR along organelle membranes within the soma and proximal dendrites of NTS neurons that also exhibited MOR labeling along extrasynaptic membranes. Co-localization of presynaptic MOR and NMDA-NR1 was also detected, to a lesser extent, within axon terminals forming asymmetric-type synaptic junctions (Huang, et al., 2000). Within the nucleus of the solitary tract (NTS), MOR immunoreactivity has been demonstrated within postsynaptic targets of axon terminal projections from the CeA (Pickel and Colago, 1999). Furthermore, within the NTS, small axons containing MOR were identified apposed to anterogradely-labeled axons from the CeA, suggesting that MOR agonists may modulate the postsynaptic inhibition produced by CeA afferents, but may also play a role in the presynaptic release of other neurotransmitters (Aicher, et al., 2000). Though MOR-immunoreactive terminals formed primarily inhibitory-type synaptic junctions in the NTS, asymmetric excitatory-type junctions were also occasionally observed to contain plasmalemmal immunogold labeling for MOR (Cheng, et al., 1996). In rats self-administering morphine, Glass et al. (2004) examined the subcellular targeting of the NMDA-NR1 receptor subunit in the NTS. Compared to saline-treated controls, self-administration of escalating doses of morphine led to a reduction in plasmalemmal NMDA-NR1 with concurrent increases in intracellular NMDA-NR1 localization. This shift in the distribution of NMDA-NR1 was primarily within smaller, presumably more distal dendritic profiles of medial NTS neurons (Glass, et al., 2004).
In other key brain regions implicated in opioid-mediated behaviors, prominent co-existence between NMDAR and MOR has been reported. Within the shell of the nucleus accumbens (NAc), a subset of dendritic profiles was shown to contain both MOR and NMDA-NR1 immunoreactivities. Additionally, presynaptic NMDARs were observed in direct apposition with MOR-labeled dendrites. These dual anatomical substrates provide multiple potential substrates for glutamate-opioid interactions in the NAc, a region critical to the regulation of the motivational and motor effects of opioids (Gracy, et al., 1997). MOR-enriched regions within the caudate-putamen were often observed to exhibit labeling for NMDA-NR1 within dendrites and dendritic spines, and asymmetric synapses accounted for the majority of synaptic complexes targeting MOR and NR1-labeled profiles (Wang, et al., 1999).
Evidence for co-existence of AMPA and opioid receptors
More recently, evidence for AMPAR-opioid interactions within brain regions critical to opioid addiction is emerging. In one of the first studies to examine the ultrastructural relationship of Ca2+ impermeable AMPARs with MOR, Beckerman and Glass (2010) have recently described evidence of AMPA-GluR2 co-localization with MOR within the CeA. This builds on the group's prior studies showing that self-administration of morphine alters the distribution of the AMPA-GluR1 subunit in the amygdala (Glass, et al., 2005). In that study, Glass demonstrated that the Ca2+ sensitive AMPA-GluR1 subunit was recruited to extrasynaptic plasmalemmal sites of larger dendrites in response to morphine self-administration in the basolateral amygdaloid nuclei (BLA). However, similar observations were not made within the central nucleus, where AMPA-GluR1 distribution was unchanged in response to morphine self-administration (Glass, et al., 2005). The limited observation of co-localized AMPA-GluR1 and MOR in these regions suggested that direct opioid-AMPA-GluR1 interactions within the amygdala may not underlie the observed shift in the distribution of AMPA-GluR1 to the plasma membrane in response to morphine administration (Glass, et al., 2005). Similarly to that reported for MOR and AMPA-GluR2 in the CeA (Beckerman and Glass, 2010), these receptors co-localized within dendritic profiles that often received asymmetric excitatory-type synaptic input (Glass, et al., 2009).
Whereas repeated exposure to morphine led to an increased presence of AMPA-GluR1 along the membrane of neurons in the BLA (Glass, et al., 2005), opposite findings were reported in the NAc. Chronic exposure to escalating morphine doses led to a decreased plasmalemmal presence of the AMPA-GluR1 in medium-large dopamine D1 receptor (D1R) labeled dendrites in the NAc shell subregion. In the NAc core subregion, decreased plasmalemmal AMPA-GluR1 was observed in smaller dendritic profiles that were found to lack dopamine D1Rs. The findings suggested differential sensitivity to dopamine in NAc core versus shell dendrites that exhibit decreased plasmalemmal GluR1 in response to chronic, intermittent morphine exposure (Glass, et al., 2008). Additionally, Lane, et al. (2008) described alterations in AMPA-GluR1 in response to both acute and chronic morphine exposure in the ventral tegmental area (VTA), a midbrain dopaminergic region integral to reward and reinforcement(Lane, et al., 2008). Chronic opioid exposure induced an increased presence of AMPA-GluR1 immunoreactivity at the plasma membrane and post-synaptic densities of forebrain-projecting (motivation and drug-seeking) and of limbic-structure projecting (locomotor and reward) VTA neurons (Lane, et al., 2008).
Significance of subcellular localization
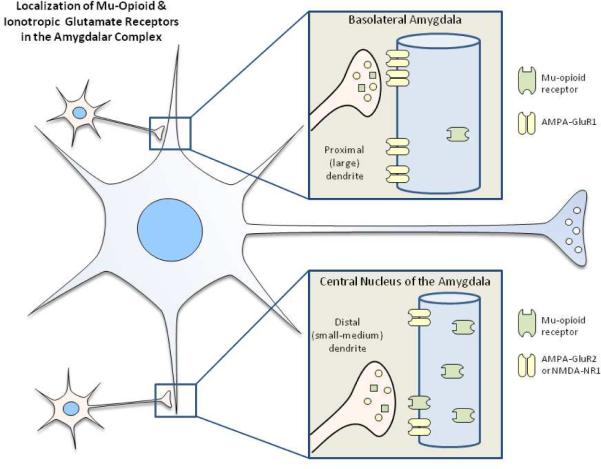
The functional implications of the neuroanatomical findings may depend, in part, on the subcellular site at which MORs and iGluRs co-localize. Two potential issues for consideration include dendritic size and localization relative to the synapse. Because functional input to neurons may be quite different in proximal versus distal dendrites, the implications of glutamate-opioid interactions may differ between smaller (distal) and larger (proximal) profiles. Glass et al. (2005) described a significant increase in the proportion of AMPA GluR1 on the plasma membrane of large profile (2–4 micron) dendrites in the basolateral amygdala following morphine self-administration (Glass, et al., 2005). This result differs from observations within the CeA where morphine self-administering and control animals exhibited similar proportions of surface and intracellular GluR1 receptors in dendrites that exhibited abundant co-expression of MORs. Interestingly, BLA neurons with increased surface GluR1 receptors did not frequently co-express MORs. With respect to the non-calcium-permeable AMPA receptor, GluR2, in the CeA, MOR was frequently co-localized within small to medium dendrites providing a neuronal substrate for opioid-mediated plasticity (Glass, 2010). This prominent co-localization is also consistent with the distribution of NMDA R1 and MORs in CeA neurons which was also observed in small to medium CeA dendrites (Glass, et al., 2009). Irrespective of the size of the postsynaptic targets in either the BLA or the CeA, synaptic inputs to dual-labeled dendrites were frequently from unlabeled axon terminals forming asymmetric excitatory-type synapses. In summary, these results suggest that trafficking of GluR1, GluR2 and NMDA NR1 in response to opiate exposure is not only regionally specific within amygdalar subdivisions but differs with respect to proximal versus distal portions of dendrites (Figure 1). Furthermore, these findings indicate that dendrites are a critical site of integration for agonists of NMDA, AMPA receptors and MORs that may contribute to neural plasticity associated with pathological emotional processes, particularly those associated with substance abuse.
Figure 1.
Summary diagram of results obtained from high resolution electron microscopy studies of MORS and iGluRs. Glass et al. (2005) described a significant increase in the proportion of AMPA GluR1 on the plasma membrane of large profile (2–4 micron) dendrites in the basolateral amygdala following morphine self-administration, an effect not observed in the CeA. With respect to GluR2 or NMDA NR1 in the CeA, MOR was frequently co-localized with MORS within small to medium dendrites providing a neuronal substrate for opioid-mediated plasticity (Glass, 2010; Glass, et al., 2009). These results suggest that trafficking of GluR1, GluR2 and NMDA NR1 in response to opiate exposure is not only regionally specific within amygdalar subdivisions but differs with respect to proximal versus distal portions of dendrites and may contribute to neural plasticity associated with pathological emotional processes, particularly those associated with drug abuse.
Convergent lines of evidence indicate that glutamate receptor localization is a highly dynamic process that involves complex regulation of receptor trafficking and lateral diffusion between synaptic and extrasynaptic sites (Groc and Choquet, 2006, Groc, et al., 2004, Newpher and Ehlers, 2008, Triller and Choquet, 2005). Extrasynaptic glutamate receptors can both regulate the equilibrium of synaptic receptor exchange in addition to activation of independent signaling pathways (Newpher and Ehlers, 2008). Accordingly, examination of the subcellular localization of MOR and iGluR labeling relative to excitatory synapses in the amygdala may help to elucidate glutamate-involvement in opioid-related behaviors such as conditioned place preference, withdrawal-related place aversion, and behavioral sensitization to drug exposure. Neuroanatomical and electrophysiological findings suggest a significant extrasynaptic presence of both iGluR and MORs. These receptors commonly co-localize in cellular profiles in which one or both receptors may be extrasynaptic. Extrasynaptic NMDAR or MOR have been detected in the CeA (NR2B- Lopez de Armentia and Sah, 2003), NTS (MOR: Huang, et al., 2000), PAG (MOR: Commons, et al., 1999), and in the NAc (NR1, MOR: Gracy, et al., 1997). AMPAR and MOR co-localization has also been detected in the BLA (GluR1, MOR: Glass, et al., 2005), hippocampus (GluR1: Billa, et al., 2010), and most recently, in the CeA by Beckerman and Glass (2010). Their findings provide evidence for both synaptic and extrasynaptic localization of AMPA GluR2 and MOR in CeA dendrites. This body of neuroanatomical data is indicative of the importance of iGluR and MOR interactions not only at the excitatory synapse, but also within the numerous sites of extrasynaptic co-localization throughout the brain. Moreover, a growing body of electrophysiological data is suggestive of the functionality of extrasynaptic iGluRs (Jonas and Sakmann, 1992, Spruston, et al., 1995, Spruston, et al., 1995).
Functional Consequences of Glutamate-Opioid Interactions in the Amygdala
Glutamate-opioid interactions have direct implications for glutamate synaptic transmission. Whole-cell recordings from CeA neurons in brain slices reveal that presynaptic activation of MOR significantly attenuated glutamate synaptic transmission (Zhu and Pan, 2005), and similar findings were reported in neocortical neurons (Ostermeier, et al., 2000). Within cerebrocortical synaptosomes, acute morphine was shown to inhibit glutamate release through the reduction of Ca2+ influx into the terminal (Yang, et al., 2004). The mechanisms underlying these physiological changes have also been explored. While acute morphine attenuates prefrontal cortical cell activation by excitatory afferents (Giacchino and Henriksen, 1998), following chronic exposure, morphine withdrawal significantly upregulates glutamatergic synaptic transmission via presynaptic mechanisms involving the cAMP pathway (Bie, et al., 2005, Moron, et al., 2010), PKC activation (Bie, et al., 2005, Chen and Huang, 1991). Ionotropic glutamate receptors have important regulatory roles related to opioid system function in the amygdala, particularly through their contribution to learning and memory. Opioid exposure leads to definitive effects on both the AMPAR and NMDAR systems that can be observed at multiple different levels within the nervous system.
NMDAR-specific effects
Narita, et al. (2000) have documented that morphine-conditioning leads to up-regulation of the NMDAR subunit NR2b specifically within the limbic forebrain. This phenomenon of morphine-conditioned place preference was abolished through blockade of NR1 or NR2 activation without affecting the drug's rewarding effects (Narita, et al., 2000). Similar effects have been noted specifically within the amygdala. Microinjection of the NMDAR antagonist MK-801 (Ishida, et al., 2008, Rezayof, et al., 2007, Watanabe, et al., 2002) or DCPPene (Watanabe, et al., 2002) in to the CeA significantly attenuated morphine-withdrawal-induced place aversion. Furthermore, deletion of the NR1 subunit within the CeA preserved the morphine withdrawal syndrome, but eliminated the ability of mice to exhibit conditioned-place aversion following naloxone-precipitated withdrawal (Glass, 2010).
AMPAR-specific effects
Similar to findings within the NMDAR system, local blockade of AMPAR activation in the CeA effectively attenuated or eliminated opiate withdrawal-conditioned behaviors. Microinjection of the AMPAR antagonist CNQX (Watanabe, et al., 2002) or GYK152466 (Ishida, et al., 2008) directly into the CeA attenuated the conditioned-place aversion induced by precipitated opiate withdrawal. Behavioral sensitization to morphine was associated with decreased AMPA-GluR2 mRNA expression in the amygdala, suggesting a potential role for enhanced Ca2+ permeability of iGluRs in this opioid-conditioned behavioral response (Sepehrizadeh, et al., 2008). Whereas AMPA-GluR2 expression decreased with behavioral sensitization to morphine, NMDA mRNA expression in the amygdala was increased (Sepehrizadeh, et al., 2008). Similarly, Billa, et al. (2010) reported that repeated morphine administration also increased the abundance of GluR2-lacking, GluR1-containing AMPARS at both synaptic and extrasynaptic sites (Billa, et al., 2010). Corresponding electrophysiological studies have demonstrated increased Ca2+ permeability of synaptic AMPARs following repeated morphine exposure (Billa, et al., 2010). Collectively, these data suggest that glutamate-opioid interactions may result in a chronic opioid exposure dependent shift in AMPAR composition and associated Ca2+ permeability that impacts the excitability of neurons in the CeA and beyond.
Neuroanatomical findings such as those presented by Beckerman and Glass (2010) provide a high-resolution snap-shot of iGluRs and MOR localization. While these data are strongly supportive of glutamate-opioid interactions in the CeA and beyond, regulation of excitatory synaptic transmission is a highly dynamic process that is dependent upon the presence of the necessary synaptic players. With exposure to opioids, rapid adaptations to excitatory transmission occur that regulate the presence of iGluR subunits both along the membrane and at the synapse. For instance, an increased cell-surface presence of AMPARs lacking the GluR2 subunit was noted 12 hours following repeated morphine exposure at both extrasynaptic sites and within post-synaptic densities (Billa, et al., 2010). Repeated morphine effects on the surface expression of AMPAR subunits were also examined throughout the rat limbic system by Mickiewicz and Napier (2011). Surprisingly, surface GluR1 and GluR2 presence was not altered by morphine exposure in several regions with known involvement in opiate-mediated behaviors, including the NAc and ventral pallidum. However, GluR1 surface expression was decreased in the medial PFC in response to repeated opiate exposure, indicating that the functional consequences of glutamate-opioid interactions appear to be highly region-specific (Mickiewicz and Napier, 2011).
Recent studies may shed some light on the mechanism whereby AMPA receptors exhibit differential trafficking as an adaptive response. Pacchioni and Kalivas (2009) have described a role for neuronal pentraxins in AMPA receptor clustering. Of the three neuronal pentraxins described Narp, NP1 and NPR, Narp and NP1 appear to cluster AMPA receptors, while NPR contributes to removing AMPA receptors during mGluR-dependent long-term depression (Pacchioni and Kalivas, 2009). Further studies are required to determine whether a similar mechanism applies to morphine-induce adaptations in amygdalar subregions.
Non-Opiate Drugs of Abuse: Potential Involvement of Glutamate-Opioid Interactions?
A plethora of evidence provides support for the critical importance of glutamate-opioid system interactions in opiate exposure, abuse, and addiction. Beckerman and Glass (2010) emphasize the importance of glutamate-opioid receptor relationships in the CeA- a region critical to the integration of learning and emotion associated with goal-directed behaviors involved in opiate addiction. However, glutamatergic signaling has been shown to be intricately linked to the addictive processes associated with other drugs of abuse such as cocaine, benzodiazepines, ethanol and nicotine [for general reviews, see (Cunha-Oliveira, et al., 2008, Kalivas, et al., 2009, Koob, 2003)]. Cocaine has been shown to alter the expression, abundance and trafficking of various NMDAR and AMPAR subunits in the striatum (Ghasemzadeh, et al., 2009, Ghasemzadeh, et al., 2009, Ross and Peselow, 2009) and NAc (Famous, et al., 2008, Good and Lupica, 2010). The role of glutamate in cocaine seeking has received attention from numerous groups [for selected publications, see (Anderson, et al., 2008, Famous, et al., 2008, Famous, et al., 2007, Ghasemzadeh, et al., 2009, Ghasemzadeh, et al., 2009, Hearing, et al., 2010, Knackstedt and Kalivas, 2009, Kumaresan, et al., 2009, Schmidt, et al., 2005)]. Important glutamatergic mechanisms have been identified in the neural processes that occur in the context of benzodiazepine withdrawal (Das, et al., 2008, Das, et al., 2010), nicotine dependence and reward (Berrendero, et al., 2010), and cannabinoid exposure (Good and Lupica, 2010). Glutamatergic-opioid interactions have already been demonstrated in studies of the delta-opioid receptor function in the amygdala following ethanol exposure (Bie, et al., 2009, Bie, et al., 2009).
Future studies will be necessary to provide continued assessment of the potential ways in which opioid signaling may impact glutamatergic involvement in drug seeking, reinstatement, reward and reinforcement. Given what is known about the role of opioids in neural processes such as reward, motivation and nociception, it is quite possible that the neuroanatomical evidence for glutamatergic-opioid interactions reviewed here may lead to functional discoveries that expand our understanding of the synaptic dynamics of these important receptor systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aicher SA, Goldberg A, Sharma S, Pickel VM. mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol. 2000;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 3.Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- 5.Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckerman MA, Glass MJ. Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrendero F, Robledo P, Trigo JM, Martin-Garcia E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bie B, Peng Y, Zhang Y, Pan ZZ. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci. 2005;25:3824–3832. doi: 10.1523/JNEUROSCI.5010-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bie B, Zhu W, Pan ZZ. Ethanol-induced delta-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience. 2009;160:348–358. doi: 10.1016/j.neuroscience.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bie B, Zhu W, Pan ZZ. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther. 2009;329:290–296. doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, Moron JA. Increased insertion of glutamate receptor 2-lacking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown TH, Chapman PF, Kairiss EW, Keenan CL. Long-term synaptic potentiation. Science. 1988;242:724–728. doi: 10.1126/science.2903551. [DOI] [PubMed] [Google Scholar]
- 14.Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol. 1989;281:320–333. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Huang LY. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 16.Cheng PY, Liu-Chen LY, Chen C, Pickel VM. Immunolabeling of Mu opioid receptors in the rat nucleus of the solitary tract: extrasynaptic plasmalemmal localization and association with Leu5-enkephalin. J Comp Neurol. 1996;371:522–536. doi: 10.1002/(SICI)1096-9861(19960805)371:4<522::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Commons KG, van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408:549–559. [PubMed] [Google Scholar]
- 18.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 19.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Das P, Lilly SM, Zerda R, Gunning WT, 3rd, Alvarez FJ, Tietz EI. Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol. 2008;511:832–846. doi: 10.1002/cne.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das P, Zerda R, Alvarez FJ, Tietz EI. Immunogold electron microscopic evidence of differential regulation of GluN1, GluN2A, and GluN2B, NMDA-type glutamate receptor subunits in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol. 2010;518:4311–4328. doi: 10.1002/cne.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliava M, Yilmazer-Hanke D, Asan E. Interrelations between monoaminergic afferents and corticotropin-releasing factor-immunoreactive neurons in the rat central amygdaloid nucleus: ultrastructural evidence for dopaminergic control of amygdaloid stress systems. Histochem Cell Biol. 2003;120:183–197. doi: 10.1007/s00418-003-0557-9. [DOI] [PubMed] [Google Scholar]
- 24.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 25.Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Famous KR, Schmidt HD, Pierce RC. When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett. 2007;420:169–173. doi: 10.1016/j.neulet.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farb CR, Aoki C, Ledoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J Comp Neurol. 1995;362:86–108. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Giacchino JL, Henriksen SJ. Opioid effects on activation of neurons in the medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1157–1178. doi: 10.1016/s0278-5846(98)00053-0. [DOI] [PubMed] [Google Scholar]
- 32.Glass MJ. The role of functional postsynaptic NMDA receptors in the central nucleus of the amygdala in opioid dependence. Vitam Horm. 2010;82:145–166. doi: 10.1016/S0083-6729(10)82008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58:1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- 34.Glass MJ, Kruzich PJ, Kreek MJ, Pickel VM. Decreased plasma membrane targeting of NMDA-NR1 receptor subunit in dendrites of medial nucleus tractus solitarius neurons in rats self-administering morphine. Synapse. 2004;53:191–201. doi: 10.1002/syn.20049. [DOI] [PubMed] [Google Scholar]
- 35.Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210:750–761. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass MJ, Vanyo L, Quimson L, Pickel VM. Ultrastructural relationship between N-methyl-D-aspartate-NR1 receptor subunit and mu-opioid receptor in the mouse central nucleus of the amygdala. Neuroscience. 2009;163:857–867. doi: 10.1016/j.neuroscience.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci. 2010;30:7900–7909. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gracy KN, Svingos AL, Pickel VM. Dual ultrastructural localization of mu-opioid receptors and NMDA-type glutamate receptors in the shell of the rat nucleus accumbens. J Neurosci. 1997;17:4839–4848. doi: 10.1523/JNEUROSCI.17-12-04839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- 40.Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 41.Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2010:1–12. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Wang H, Pickel VM. Rostrocaudal variation in targeting of N-methyl-D-aspartate and mu-opioid receptors in the rat medial nucleus of the solitary tract. J Comp Neurol. 2000;421:400–411. [PubMed] [Google Scholar]
- 43.Ishida S, Shimosaka R, Kawasaki Y, Jin C, Kitamura Y, Araki H, Sendo T, Gomita Y. Involvement of the amygdala on place aversion induced by naloxone in single-dose morphine-treated rats. Yakugaku Zasshi. 2008;128:395–403. doi: 10.1248/yakushi.128.395. [DOI] [PubMed] [Google Scholar]
- 44.Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159:526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;1(56 Suppl):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- 48.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Koob GF. Hedonic Homeostatic Dysregulation as a Driver of Drug-Seeking Behavior. Drug Discov Today Dis Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, Kreek MJ, Pickel VM. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28:9670–9681. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 56.Leranth C, Pickel VM. Electron microscopic preembedding double-immunostaining methods. In: Heimer L, Záborszky L, editors. Neuroanatomical tract tracing methods 2: recent progress. Plenum Press; New York: 1989. pp. 129–172. [Google Scholar]
- 57.Li Y, He L, Chen Q, Zhou Y. Changes of micro-opioid receptors and GABA in visual cortex of chronic morphine treated rats. Neurosci Lett. 2007;428:11–16. doi: 10.1016/j.neulet.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 58.Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 60.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 61.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 62.Mansour A, Lewis ME, Khachaturian H, Akil H, Watson SJ. Pharmacological and anatomical evidence of selective mu, delta, and kappa opioid receptor binding in rat brain. Brain Res. 1986;399:69–79. doi: 10.1016/0006-8993(86)90601-3. [DOI] [PubMed] [Google Scholar]
- 63.Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 64.McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- 65.Mickiewicz AL, Napier TC. Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur J Neurosci. 2011;33:259–265. doi: 10.1111/j.1460-9568.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- 66.Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology. 2010;35:955–966. doi: 10.1038/npp.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narita M, Aoki T, Suzuki T. Molecular evidence for the involvement of NR2B subunit containing N-methyl-D-aspartate receptors in the development of morphine-induced place preference. Neuroscience. 2000;101:601–606. doi: 10.1016/s0306-4522(00)00405-x. [DOI] [PubMed] [Google Scholar]
- 68.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ostermeier AM, Schlosser B, Schwender D, Sutor B. Activation of mu- and delta-opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology. 2000;93:1053–1063. doi: 10.1097/00000542-200010000-00029. [DOI] [PubMed] [Google Scholar]
- 70.Pacchioni AM, Kalivas PW. The Role of AMPAR Trafficking Mediated by Neuronal Pentraxins in Cocaine-induced Neuroadaptations. Mol Cell Pharmacol. 2009;1:183–192. doi: 10.4255/mcpharmacol.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickel VM, Colago EE. Presence of mu-opioid receptors in targets of efferent projections from the central nucleus of the amygdala to the nucleus of the solitary tract. Synapse. 1999;33:141–152. doi: 10.1002/(SICI)1098-2396(199908)33:2<141::AID-SYN4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 72.Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 73.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 74.Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 75.Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496:859–876. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- 76.Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. J Physiol. 2010;588:93–99. doi: 10.1113/jphysiol.2009.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reyes BA, Chavkin C, Van Bockstaele EJ. Agonist-induced internalization of kappa-opioid receptors in noradrenergic neurons of the rat locus coeruleus. J Chem Neuroanat. 2010;40:301–309. doi: 10.1016/j.jchemneu.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 80.Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rezayof A, Golhasani-Keshtan F, Haeri-Rohani A, Zarrindast MR. Morphine-induced place preference: involvement of the central amygdala NMDA receptors. Brain Res. 2007;1133:34–41. doi: 10.1016/j.brainres.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 82.Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin Neuropharmacol. 2009;32:277–289. doi: 10.1097/wnf.0b013e3181a91655. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 84.Sepehrizadeh Z, Bahrololoumi Shapourabadi M, Ahmadi S, Hashemi Bozchlou S, Zarrindast MR, Sahebgharani M. Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol. 2008;35:1321–1330. doi: 10.1111/j.1440-1681.2008.05004.x. [DOI] [PubMed] [Google Scholar]
- 85.Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou SH, Zarrindast MR. Morphine-induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacology. 2008;81:333–343. doi: 10.1159/000122959. [DOI] [PubMed] [Google Scholar]
- 86.Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka H, Grooms SY, Bennett MV, Zukin RS. The AMPAR subunit GluR2: still front and center-stage. Brain Res. 2000;886:190–207. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 89.Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- 91.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Gracy KN, Pickel VM. Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J Comp Neurol. 1999;412:132–146. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- 94.Yang TT, Hung CF, Lee YJ, Su MJ, Wang SJ. Morphine inhibits glutamate exocytosis from rat cerebral cortex nerve terminals (synaptosomes) by reducing Ca2+ influx. Synapse. 2004;51:83–90. doi: 10.1002/syn.10290. [DOI] [PubMed] [Google Scholar]
- 95.Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127:871–879. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 97.Zhu W, Pan ZZ. Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience. 2005;133:97–103. doi: 10.1016/j.neuroscience.2005.02.004. [DOI] [PubMed] [Google Scholar]