Abstract
This study investigated adrenal androgens (AA), gonadotropins, and cortisol in castrated and gonad-intact male rhesus macaques from birth through infancy. Blood samples were collected longitudinally from castrated (n = 6; weekly, 1–40 wk) and intact (n = 4; every other week, 1–17 wk) males. Plasma concentrations of AA were determined by liquid chromatography-tandem mass spectrometry, and plasma concentrations of cortisol and gonadotropins were determined by RIA. Dehydroepiandrosterone sulfate (DHEAS) concentrations increased almost threefold (to 8 wk), dehydroepiandrosterone (DHEA) increased more than eightfold (to 11 wk), and androstenedione doubled (to 15 wk) in five castrated infant males and declined continuously thereafter. A sixth castrated male had markedly different temporal patterns and concentrations (many times more than 2 SDs from the cohort mean) of AA and gonadotropins from first sampling (3 wk) and was excluded from analysis. Cortisol increased over 16 wk but correlated poorly with DHEAS. Luteinizing and follicle-stimulating hormones increased to peaks at 3 and 7 wk, respectively. Testis-intact males exhibited similar profiles, but with earlier peaks of DHEAS (5 wk) and DHEA and androstenedione (7 wk). Peak concentrations of DHEAS were lower and those of DHEA and androstenedione were higher in intact than castrated infants. Testosterone was undetectable in castrated males and >0.5 ng/ml in intact males but was not correlated with DHEA or DHEAS. These are the first data documenting a transient increase in AA secretion during infancy in an Old World primate and are consistent with the previously documented time course of zona reticularis development that accompanies increases in androgen synthetic capacity of the adrenal. The rhesus is a promising model for androgen secretion from the human adrenal cortex.
Keywords: adrenarche, human, nonhuman primate, adrenal cortex, zona reticularis, dehydroepiandrosterone, dehydroepiandrosterone sulfate, castrate, prepubertal, neonate, infant, longitudinal sampling, cortisol
the adrenal cortex synthesizes the most abundant circulating steroid in the human body, dehydroepiandrosterone sulfate (DHEAS), as well as its unconjugated form (DHEA), which are collectively referred to as adrenal androgens. Although they exhibit little or no androgenic activity themselves, DHEAS and DHEA can be used as substrates for bioactive sex steroid synthesis in tissues and cells with the appropriate complement of enzymes (27). Consequently, adrenal androgen secretion remains an important source of precursors for local estrogen production when gonadal sources are absent or suppressed (28). Diseases promoted by sex steroids, such as prostate and breast cancers, are therefore more difficult to control because of adrenal DHEAS and DHEA production (37, 74, 78). Adrenal androgens contribute significantly to hyperandrogenism associated with polycystic ovary syndrome in women (77) and in a nonhuman primate model for polycystic ovary syndrome (18, 79). There are two well-recognized peaks in circulating concentrations of DHEA and DHEAS in men and women (57): the first occurs during fetal development (35), and the second is initiated prepubertally (16, 25, 62, 63) in childhood, an event referred to as adrenarche (2). The adrenarchial rise peaks in the second or third decade of life (23, 36), and there is a slow and continual decline in adrenal androgens thereafter, at least in men (47). More recent studies have identified a third, transient increase in women coincident with the perimenopause (14, 29). Animal models would be valuable in elucidating how adrenal androgen secretion is regulated and affects well-being.
Adrenal androgen secretion is not a common characteristic among mammals. Although concentrations comparable to those in humans are found among the great apes and Old World monkeys (12, 41), not all primates share this characteristic (50). Examples of increased adrenal androgen secretion among other mammals suggest that it can be exceedingly brief in some (52) but still very clearly adaptive in others (6). Adrenal androgen secretion is attributed to specialized cortical zones, the fetal zone during fetal development and the zona reticularis (ZR) postnatally (41). The fetal zone regresses after birth in humans (4, 69), rhesus (34), baboons (17), and marmosets (31, 51). In humans, the subsequent development of the ZR and associated increase in adrenal androgen concentrations are known as adrenarche (40, 72), an event that occurs in early childhood (46, 63). The rhesus macaque develops a ZR with the same steroidogenic phenotype (32), and differentiation takes place in infancy at 2 and 3 mo of age (43). During this same period in the rhesus monkey, there is a corresponding increase in androgen synthetic capacity of adrenal tissues (42), but whether this is reflected in circulating concentrations of androgens is unclear. Longitudinal sampling has provided evidence of a transient rise in circulating immunoreactive (otherwise unidentified) androgen between 2 and 5 mo of age in neonatally castrated male rhesus (54) that was not detectable after adrenalectomy (56). Others who have measured adrenal androgens during the first few months of rhesus development, using cross-sectional and relatively infrequent sampling, reported only a decline in concentrations from birth (26, 64, 65).
The evidence of ZR maturation in infancy (42, 43), together with the prior observation of a transient increase in immunoreactive androgens in castrated male rhesus at this stage of development (54, 56), prompted us to reinvestigate circulating adrenal androgens in castrated and testis-intact male rhesus infants, utilizing an opportunistic study design that capitalized on samples from ongoing experiments. Concentrations of DHEAS, DHEA, androstenedione, and cortisol were examined in monkeys sampled longitudinally from soon after birth through infancy, the interval during which ZR development and differentiation are completed (43). Gonadotropins were also measured, because luteinizing hormone (LH), in particular, may influence adrenal growth and steroidogenesis (5). The study utilized samples taken weekly from males castrated at 1 wk of age (study 1) and every other week from testis-intact males (study 2). DHEAS data from two of the castrated and two of the testis-intact males were reported preliminarily in a recent review (11).
MATERIALS AND METHODS
Animals and blood sampling.
Neonatal rhesus monkeys (Macaca mulatta; 0.51–0.66 kg body wt) used in the study were born at the Primate Core of the Specialized Centers Program for Research in Infertility and Reproduction, University of Pittsburgh School of Medicine (n = 6) or at the Wisconsin National Primate Research Center, University of Wisconsin, Madison (n = 4). Neonates and infants were housed with their mothers in individual cages under controlled conditions (lights on between 0700 and 1900, ambient temperature 20°C) and in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. The experiments were approved by the University of Pittsburgh or University of Wisconsin Institutional Animal Care and Use Committee. The males from the Pittsburgh facility were castrated bilaterally at 1–6 days of age, as described previously (71). Briefly, mothers were sedated with ketamine hydrochloride (20–25 mg/kg body wt im; Ketaject, Phoenix Scientific, St. Joseph, MO), and infants were castrated under anesthesia (ketamine, 20–25 mg im, 0.5-mg supplements as needed) with local anesthetic irrigation (0.5% bupivacaine HCl; Marcaine Hospira, Lake Forest, IL) as needed. Analgesic (flunixin meglumine, 2.5 mg im; Banamine, Schering-Plough Animal Health, Union, NJ) and prophylactic antibiotics were given postoperatively before infants were returned to their mothers. Blood samples (1–2 ml) were drawn weekly in the morning (∼0900–1000) via femoral venipuncture while mothers were sedated (ketamine, 20 mg/kg body wt im), and after 4–6 mo of age, samples were collected after sedation of the infants with ketamine (5 mg im). Plasma was separated and stored at −20°C until assayed. Males utilized at the Wisconsin facility remained intact and did not undergo surgery, and blood samples were taken similarly in the morning (∼0900–1000) via femoral venipuncture in alternate weeks from 1 to 17 wk of age.
Liquid chromatography-tandem mass spectrometry.
Androgen quantitation was accomplished using online sample extraction by two-dimensional liquid chromatography-tandem mass spectrometry (2D-LC-MS/MS). Authentic steroid standards were obtained from Steraloids (Newport, RI). The standard for d3-testosterone and d7-androstenedione were obtained from Cerilliant (Round Rock, TX); acetonitrile, methanol, and water were HPLC grade (Burdick and Jackson, Muskegon, MI); acetone, isopropanol, and ammonium hydroxide were Optima grade (Fisher, St. Louis, MO); and formic acid was American Chemical Society grade (EMD, Gibbstown, NJ). Reference solutions (1 mg/ml) were prepared with 5 ml of acetonitrile or methanol. Dilutions were made at 10, 1, 0.1, and 0.01 ng/μl in methanol. Three levels of standards were used in generating calibration curves for the analytes monitored depending on the ionization efficiency and physiological range of the steroids measured. A lower calibration curve (0.125–25 ng/ml) was used for testosterone and androstenedione determinations, a middle curve (2.5–250 ng/ml) for DHEA, and an upper curve (100–15,000 ng/ml) for DHEAS. All curves incorporated between six and nine calibrators, along with a zero calibrator (no analyte). Quality control (QC) samples were used to monitor accuracy and precision at the following concentrations; 0.4, 0.8, 4, and 30 ng/ml for testosterone and androstenedione, 4, 8, 40, and 300 ng/ml for DHEA, and 750 and 7,500 ng/ml for DHEAS. Internal standard (IS) solutions containing d3-testosterone and d7-androstenedione, both at 4 ng/ml, and d3-testosterone sulfate at 40 ng/ml in HPLC grade water were used in all test samples.
First, 300 μl of plasma and then 150 μl of the IS solution in water were added to autosampler vials. Samples were capped, vortexed for 30 s, centrifuged (2,000 g for 3 min), and stored at 7°C in a temperature-controlled sample compartment until a 75-μl injection introduced the diluted plasma to the 2D-LC-MS/MS system. Calibrators and QC samples were prepared at the same time as the test samples by drying standard solutions in autosampler vials in a concentrator (Turbovap; Zymark, Hopkinton, MA) at 40°C with N2 and then redissolving them in charcoal-stripped plasma. Calibration samples were run at the beginning and the end of each sample set, while QC samples were interspersed throughout each run sequence.
Online sample extraction and separation by 2D-LC was accomplished using a multiplexed turbulent-flow chromatography system (Aria TLX-2, Thermo Fisher Scientific, Franklin, MA) composed of two online degassers, four HPLC pumps (2 quaternary pumps and 2 binary pumps; LC-10AD, Shimadzu, Columbia, MD), a valve interface module, and a temperature-controlled CTC/Leap autosampler. The instrument was controlled using Aria software (version 1.6.1). An extraction column (0.5 × 50 mm, 60-μm particle size, Cyclone P, Thermo Fisher Scientific) was utilized for online sample extraction of diluted serum, and an ACE C18 analytical column (2.1 × 100 mm, 3-μm particle size; Chadds, Ford, PA) with an ACE C18 guard column (2.1 × 10 mm, 3-μm particle size) maintained at 30°C was used for reverse-phase gradient separation prior to MS introduction. Mobile phases A, B, C, and D were as water with 0.2% formic acid (A), methanol (B), acetonitrile-isopropyl alcohol-acetone [60:30:10 (vol/vol); C], and water-acetonitrile [98:2 (vol/vol); D] with 0.1% ammonium hydroxide. The quaternary pumps were used for online sample extraction, while the binary pumps were used for reverse-phase gradient separation of analytes over the total 2D-LC duration of 24.15 min. Thereby, target analytes were extracted from plasma, transferred to the second dimension, separated by column retention, and introduced to the mass spectrometer detector.
Mass spectral detection was accomplished using a triple-quadrupole mass spectrometer (TSQ Vantage, Thermo Scientific, San Jose, CA) with heated electrospray (HESI) source operating at room temperature in the positive mode for testosterone, androstenedione, and DHEA and in the negative mode for DHEAS. The mass spectrometer was controlled using Xcalibur (version 2.0.7), and data were processed using LCquan (version 2.5.6) software. HESI source conditions for sheath gas, ion sweep, and auxiliary gases were held at 45, 0.5, and 30 arbitrary units of dry nitrogen, respectively. Spray voltage was set to 4,000 V in the positive mode and 5,000 V in the negative mode. The ion transfer tube temperature was set to 350°C. The skimmer offset was set at 0 V. Argon was used as a collision gas and set to 1.5 arbitrary units. Resolution parameters were set with Q1 and Q3 at 0.1 and 0.7 mass-to-charge (m/z) ratio, respectively. Detection and quantitation of all analytes were accomplished using highly selective reaction monitoring, with a minimum of three transitions monitored per analyte. The method was split into six segments over a 12.25-min period, with polarity switching in the first and second segments to allow for multiplexing of the 2D-LC system. Chromatographic peaks at the expected retention times were integrated and quantified by LCquan using a single transition of each androgen corresponding to 271.2–213.2, 367.1–96.9, 289.2–97.1, and 287.2–97.1 m/z for DHEA, DHEAS, testosterone, and androstenedione, respectively.
The concentration of the monitored androgens in each sample (e.g., calibrators, QC, and unknowns) was determined by an IS method using the peak area ratio and linear regression analysis. Recovery of DHEA, DHEAS, testosterone, and androstenedione at the monitored QC concentrations averaged 86, 38, 86, and 88%, respectively. Instrumental response was linear over the previously noted calibration ranges, with a correlation coefficient of >0.99. Average percent accuracy was determined as 101, 85, 117, and 98% of expected concentration and average percent relative standard deviation was 10, 8, 9, and 8% for DHEA, DHEAS, testosterone, and androstenedione, respectively. The technique was optimized to provide limits of quantitation at 2.5, 100, 0.125, and 0.125 ng/ml for DHEA, DHEAS, androstenedione, and testosterone, respectively.
RIAs.
Circulating cortisol concentrations were measured using a commercial kit (Coat-A-Count, Diagnostic Products, Los Angeles, CA) with a mean sensitivity of 0.9 ng/ml. The mean intra- and interassay coefficients of variation were less than 5% and 7%, respectively, per kit specification. Plasma follicle-stimulating hormone (FSH) and LH levels were measured using respective homologous RIA, as described previously (19, 58). The mean sensitivities of the FSH and LH assays were 0.06 and 0.10 ng/ml, respectively, and the mean intra- and interassay coefficients of variation were less than 5% and 7% for FSH and 5% and 9% for LH. Hormone concentrations below detectable limit were assigned a value equivalent to the minimum detectable concentration of the respective assay.
Statistical analysis.
Normality of the data was determined using a Shapiro-Wilk test and other tests, and, if not normally distributed, data were logarithmically transformed and then analyzed by analysis of variance using the general linear and mixed-model functions of SAS (version 9.2, SAS Institute, Cary, NC). The repeated-measures model included linear and quadratic effects of age, paired t-tests were used to verify the significance of differences in hormone concentrations between means at different ages, and Pearson's correlation coefficients were calculated among hormones. One castrated male exhibited an exceptional pattern of DHEAS secretion, with markedly elevated androgen concentrations at the very first sampling (3 wk) that declined with age. In fact, concentrations of DHEAS, DHEA, androstenedione, LH, and FSH were >2 SDs from the mean of the cohort at many time points in the first 10 wk of sampling. Exclusion of data from this single animal halved the skewness and vastly improved normality by several tests (Shapiro-Wilk, Kolmogrov-Smirnov, Cramer-von Mises, and Anderson-Darling tests). Data from this animal were therefore excluded from further analysis.
RESULTS
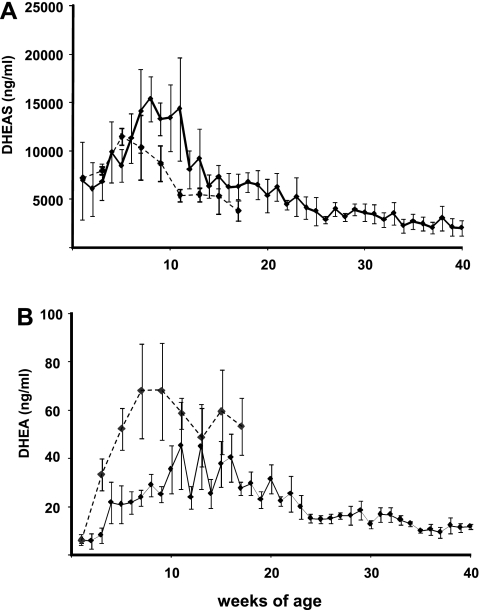
In castrated male rhesus macaques, DHEAS and DHEA concentrations (Fig. 1) varied significantly with age, reaching peaks (by 8 and 11 wk of age for DHEAS and DHEA, respectively) before declining, based on a highly significant quadratic effect of time (P < 0.0001, n = 5). Concentrations of DHEAS (Fig. 1A) more than doubled, from 6,761 ± 1,863 ng/ml at 3 wk of age to a peak of 15,367 ± 2,330 ng/ml at 8 wk of age (P < 0.025), and thereafter declined steadily and continuously to 40 wk of age (P < 0.001). Unconjugated DHEA concentrations (Fig. 1B) at 2 wk of age were ∼5 ng/ml, three orders of magnitude lower than DHEAS, and by 8 wk of age had increased fivefold (P < 0.01). DHEA peaked at >40 ng/ml between 11 and 13 wk of age (P = 0.001) before declining steadily for the remainder of the study period.
Fig. 1.
Adrenal androgen [dehydroepiandrosterone sulfate (DHEAS, A) and unconjugated dehydroepiandrosterone (DHEA, B)] concentrations in peripheral blood of castrated (n = 5, solid line) and intact (n = 4, dashed line) male rhesus macaques sampled longitudinally from 1 wk of age. Values are means ± SE.
In testis-intact males, DHEAS (quadratic only, P < 0.02) and DHEA (linear and quadratic, P < 0.001) were significantly affected by age (Fig. 1). DHEAS concentrations averaged ∼6,000 ng/ml at 1 wk and ∼11,000 ng/ml by 7 wk of age. Despite the significant quadratic effect of time, a pair-wise comparison of peak DHEAS concentration at 5 wk of age was not different from that at 1 wk (P > 0.1). Concentrations of DHEAS declined steadily from 7 wk (P < 0.05) to <5,000 ng/ml at 17 wk, when the last samples from testis-intact males were drawn. As in the castrated males, DHEA concentrations in the testis-intact males were orders of magnitude lower than DHEAS concentrations, but a far greater increase in unconjugated DHEA, from <6 to >60 ng/ml, occurred from 1 to 7 wk of age (P < 0.05), and concentrations decreased thereafter (P < 0.005, quadratic effect of time). Peak DHEAS concentrations were lower (P < 0.05) and DHEA concentrations were higher (P < 0.05) in testis-intact than castrated males.
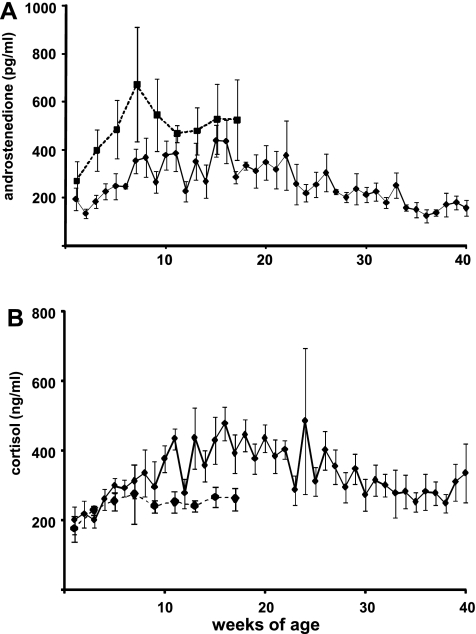
In castrated males, concentrations of androstenedione (Fig. 2A) were consistently <1 ng/ml throughout sampling, increasing from ∼200 pg/ml at 1 wk of age to >400 pg/ml between 15 and 16 wk (P < 0.001) and then decreasing progressively to 40 wk of age. Androstenedione concentrations in testis-intact males (Fig. 2A) were more variable than DHEAS and DHEA concentrations (no difference between 1 and 7 wk, P > 0.1), remaining <1 ng/ml throughout the sampling period. Testosterone was not detected in the castrated males, and concentrations of this steroid were extremely variable in testis-intact males, especially through the first 2 mo, during which they averaged between 0.5 and 2 ng/ml, stabilizing at slightly less than 0.5 ng/ml thereafter (data not shown). In castrated males, DHEAS, DHEA, and androstenedione were positively correlated (DHEAS vs. DHEA, r = 0.54; DHEA vs. androstenedione, r = 0.69; androstenedione vs. DHEAS, r = 0.43; P < 0.0001). There was a lower, although still significant, correlation between DHEAS and DHEA in testis-intact (r = 0.35, P < 0.05) than castrated males, and correlations between androstenedione and DHEAS (r = 0.58, P < 0.001) and between androstenedione and DHEA (r = 0.77, P < 0.0001) were much higher than correlations between DHEAS and DHEA. There were no significant correlations between testosterone and any other androgen (P > 0.3).
Fig. 2.
Androstenedione (A) and cortisol (B) concentrations in peripheral blood of castrated (n = 5, solid line) and intact (n = 4, dashed line) male rhesus macaques sampled longitudinally from 1 wk of age. Values are means ± SE.
In castrated males, cortisol concentrations increased from 199.2.1 ± 21.3 at 3 wk to a peak of 477.1 ± 47.9 ng/ml at 16 wk of age (Fig. 2B; P < 0.05), but this was gradual, and concentrations did not increase significantly between 3 and 8 wk of age (334.7 ± 67.1 ng/ml), when DHEAS reached its peak. There was no significant effect of time on cortisol concentrations in testis-intact males (Fig. 2B). Cortisol was more highly correlated with androstenedione (r = 0.48, P < 0.0001) than with DHEA (0.43, P < 0.0001) or DHEAS (r = 0.17, P < 0.05) in castrated males. Cortisol was correlated with DHEA (r = 0.62, P < 0.0001), but not with either DHEAS (P > 0.5) or androstenedione (P > 0.05), in testis-intact males.
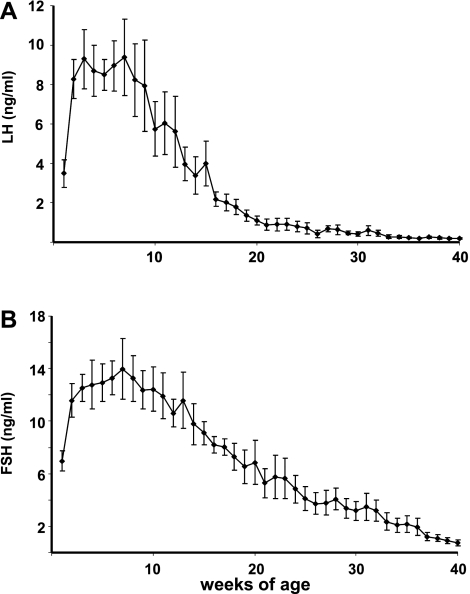
Concentrations of gonadotropins in samples taken from the castrated males also changed with age (P < 0.001). LH (Fig. 3A) and FSH (Fig. 3B) increased in parallel from ∼3 to >9 ng/ml (LH) and from 6 to >14 ng/ml (FSH), between 1 and 7 wk of age. LH and FSH concentrations declined continuously thereafter in the castrated males. In testis-intact males, LH was below assay sensitivity in almost half of the samples and was >1 ng/ml in only three of the samples (data not shown). In castrated males, the highest correlations between gonadotropins and steroids (P < 0.0001) were those for DHEAS with LH (r = 0.68), DHEAS with FSH (r = 0.67), DHEA with FSH (r = 0.45), and androstenedione with FSH (r = 0.37). LH and FSH were also highly correlated (r = 0.83, P < 0.0001).
Fig. 3.
Gonadotropin [luteinizing hormone (LH, A) and follicle-stimulating hormone (FSH, B)] concentrations in peripheral blood of castrated male rhesus macaques (n = 5) sampled longitudinally from 1 wk of age. Values are means ± SE.
DISCUSSION
These are the first data to document a transient, prepubertal rise in adrenal androgen output in an Old World monkey, reflecting development and maturation of the ZR that have been defined previously histologically (43) and biochemically (42). Addition of endocrine to histological and biochemical data provides strong evidence that this event in the infant rhesus is akin to adrenarche in humans, which has not previously been considered to occur in any nonhuman primate, except some of the great apes. The only prior study to employ longitudinal sampling of a neonatal/infant nonhuman primate was conducted in intact baboons (17). DHEAS was measured by immunoassay in samples taken daily for 3 mo, but no increase was observed in this Old World monkey. Moreover, it appears from the data presented here that adrenal androgen secretion during infancy is affected by castration, shifting the balance from sulfated to more unconjugated DHEA. The cumulative evidence of the occurrence of a phenomenon in an Old World primate that resembles human adrenarche in fundamental ways represents a paradigm shift. Clear differences also exist, namely, the timing of the peak of adrenal androgen secretion (prepubertal in rhesus monkeys but postpubertal in humans) and the progression (much more dynamic in time course and magnitude in rhesus monkeys than humans). However, these differences suggest that the rhesus monkey offers a promising opportunity to gain a much broader understanding of the physiological and evolutionary (7) significance of prepubertal increases in adrenal androgen output.
This also constitutes the first longitudinal study to report concentrations of specific adrenal androgens in serum of rhesus macaques from birth through infancy. Importantly, the transient increase in adrenal androgen secretion in castrated and intact males correlates very well with the observed morphological maturation of the ZR (43) and increasing 17,20-lyase enzyme activity in adrenal microsomes (42) occurring concurrently during the same developmental time period. Adrenal gland weight decreases in the first 2 wk of life but remains constant thereafter to 6 mo of age (34), suggesting that changes in adrenal steroid output result more from the zone-specific maturation (43) than organ growth. DHEAS more than doubled between 3 and 8 wk of age in castrated males, and there was an even more dramatic increase in DHEA during the same interval in castrated as well as testis-intact males. Previous failure to detect an increase in adrenal androgen secretion in prepubertal rhesus monkeys is probably due to relatively infrequent cross-sectional sampling and to variability in the timing of peaks among individuals that obscures detection of common secretion patterns. Castration also appears to alter the ratio of DHEAS to DHEA in this early period of development, favoring DHEA over DHEAS. This may indicate an effect of castration on expression of SULT2A1, which catalyzes sulfation of DHEA and is expressed in the ZR of the rhesus (48), as it is in the human (72), adrenal. SULT2A1 expression in mouse liver is suppressed, at least in part, by androgens (3).
Morphological, biochemical, and endocrine evidence establishes a much narrower period of development during which adrenal ZR maturation occurs in this monkey than in humans and perhaps other nonhuman primates. The increases in concentrations of adrenal androgens in these rhesus macaques were also more dramatic in their range, starting from lower prenatal concentrations (49) and reaching higher postnatal peaks (44, 70) than occur during human development. This suggests that rhesus macaques may provide a particularly useful model with which to investigate the mechanisms regulating the increase in adrenal DHEAS and DHEA secretion, because the changes are so dynamic. Limited data indicate that great apes, principally chimpanzees, experience a prepubertal increase in adrenal androgen secretion that takes place over years (10, 13, 15, 68, 76), but there are no corresponding morphological or biochemical data documenting ZR maturation, as exists for the rhesus monkey. Indeed, not all studies have observed an increase in DHEAS concentrations in the developing chimpanzee (39). Androgen secretion by the rhesus adrenal cortex in male neonates and infants may differ from that of other Old World primates. Results of careful longitudinal studies in neonatal and infant baboons (17), consistent with cross-sectional studies (8), indicate that DHEAS concentrations only decline with age from birth. Morphological studies of the baboon adrenal suggest that postnatal regression of the fetal zone occurred concurrent with establishment of the ZR (17), potentially precluding distinction of one event from the other solely on the basis of circulating adrenal androgens (8). The data presented here, however, indicate that the decline in androgen secretion from the regressing fetal zone in neonates and the increase from the differentiating ZR in infant rhesus monkeys can be seen in circulating concentrations of DHEAS and DHEA when frequent longitudinal sampling is employed.
Gonadotropin concentrations, in addition to circulating steroids, were examined in the current study. Castration eliminates the potential complication of contributions from the testes to circulating androgen concentrations but also stimulates gonadotropin secretion (53, 54). Gonadotropins, and LH in particular, are thought to be capable of influencing adrenal growth and steroidogenesis (5) and, therefore, could have influenced adrenal androgen secretion in the castrated animals in this study. The human adrenal cortex expresses LH receptors prenatally (1) and, importantly, postnatally in the ZR (45). Androgen secretion in adrenal hyperplasia (22) and adrenal adenomas (21, 30, 75) is believed to be stimulated by LH, and LH receptors are expressed in hyperplastic adrenal cortex (20, 22, 60), as well as adrenal adenomas (30). Patients respond to gonadotropin-releasing hormone, LH, and human chorionic gonadotropin by increased androgen secretion, suggesting a trophic effect on the ZR (20, 22, 30), and a similar increase in androgen secretion has been observed in adrenal carcinoma cell lines (59). No comparable, published data are available from postnatal studies of rhesus monkeys. Nevertheless, there were positive correlations between gonadotropin and adrenal androgen concentrations, and castration apparently changed adrenal androgen secretion, increasing DHEAS at the expense of DHEA. Few comparable human data exist on the effect of gonadal function on adrenal androgen secretion. It is interesting to note, however, that while girls with Turner syndrome experience adrenarche, those with premature ovarian failure have an earlier adrenarche and higher adrenal androgen concentrations than those that reach puberty (33). Furthermore, recent studies have identified a transient increase in DHEAS concentrations in women transitioning into menopause with its associated increase in gonadotropins (14, 29). A similar transient rise may be present in perimenopausal female rhesus monkeys (67). As stated in a recent review, “while adrenarche and gonadarche occur independently, are controlled and initiated by separate mechanisms, and have separate pathways, there appears to be a temporal link between adrenarche and gonadarche: early adrenarche is associated with earlier gonadarche and conversely delayed or inadequate adrenarche with later gonadarche” (38). Further studies are required to determine the role of LH in regulating the postnatal pattern of adrenal androgen secretion.
Patterns in circulating concentrations of cortisol during infancy in the rhesus monkey reported previously have been variable (9, 56, 66). This is perhaps not surprising, because levels of this stress hormone are likely to be highly dependent on environmental conditions and sampling procedures. In the present study, a modest progressive rise in cortisol was observed from birth to 8 wk of age in testis-intact and castrated males, with the increase in castrated animals continuing until 16 wk of age. Correlations with adrenal androgen levels were generally weak and did not reveal any consistency between intact and castrated monkeys, likely reflecting distinct patterns of development for the ZR and zona fascicularis, the site of cortisol synthesis.
The observed transient increase in adrenal androgen secretion based on frequent longitudinal sampling provides endocrine support for what has previously been described as morphological and biochemical “adrenarche” in rhesus macaques (42, 43). Whether the rhesus is now to be recognized to exhibit adrenarche, however, relies very much on the definition of the phenomenon. The most common definition, namely, an increase in adrenal androgen secretion in infancy or early childhood, appears to occur in humans (63) and rhesus monkeys (current data), although there are differences. The most obvious include the relatively rapid onset after birth (months, rather than years) and the short duration (months, rather than decades), such that the peak of adrenal androgen concentrations is prepubertal in the male rhesus monkey and occurs many years after puberty in humans. Still, the defining aspect of adrenarche is the timing of the initial rise, not the peak. Adrenal androgens peak earlier in women than men (61, 70), but adrenarche is still considered to occur at the same age in each sex (23). Precocious and delayed adrenarche are also considered to reflect abnormally early- or late-childhood onset of adrenal androgen secretion (24, 73), not differences in age at the peak as in adults. However adrenarche is defined, understanding ZR development and function in the rhesus monkey will provide valuable insights into the regulation of adrenal androgen secretion in humans.
GRANTS
Partial support for this work was provided by National Institute of Child Health and Human Development Grants HD-13254 and HD-08160 (T. M. Plant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.C. and T.M.P. are responsible for conception and design of the research; A.J.C., T.M.P., B.C.M., and S.D.S. analyzed the data; A.J.C., T.M.P., and D.H.A. interpreted the results of the experiments; A.J.C. prepared the figures; A.J.C. drafted the manuscript; A.J.C., T.M.P., D.H.A., B.C.M., and S.D.S. edited and revised the manuscript; A.J.C., T.M.P., D.H.A., B.C.M., and S.D.S. approved the final version of the manuscript; T.M.P. and D.H.A. performed the experiments.
ACKNOWLEDGMENTS
The authors thank Jo Corbin for technical assistance. The authors acknowledge the invaluable contributions of Dr. Suresh Ramaswamy, the staff of the Primate Core of the Center for Research in Reproductive Physiology (Michael Cicco and Rachel Rosland), and Carolyn Phalin in the Assay Core of the Pittsburgh Center, who performed surgical procedures, collected sequential plasma samples from the agonadal male monkeys, and conducted immunoassays. The authors are equally grateful for the technical assistance of Amber Edwards and Tammie Frost (Wisconsin National Primate Research Center), who sampled intact males.
REFERENCES
- 1. Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima ST, Jauniaux E, Rao C. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J Clin Endocrinol Metab 89: 952–956, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Albright F. Osteoporosis. Ann Intern Med 27: 861–882, 1947 [DOI] [PubMed] [Google Scholar]
- 3. Alnouti Y, Klaassen CD. Mechanisms of gender-specific regulation of mouse sulfotransferases (Sults). Xenobiotica 41: 187–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benner MC. Studies on the involution of the fetal cortex of the adrenal glands. Am J Pathol 16: 787–798, 1940 [PMC free article] [PubMed] [Google Scholar]
- 5. Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol Metab 19: 231–238, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Boonstra R, Bradley AJ, Delehanty B. Preparing for hibernation in ground squirrels: adrenal androgen production in summer linked to environmental severity in winter. Funct Ecol. In press [Google Scholar]
- 7. Campbell B. Adrenarche in comparative perspective. Am J Hum Biol 23: 44–52, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Castracane VD, Cutler GB, Jr, Loriaux DL. Pubertal endocrinology of the baboon: adrenarche. Am J Physiol Endocrinol Metab 241: E305–E309, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol 26: 433–446, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Collins DC, Nadler RD, Preedy JRK. Adrenarche in the Great Apes (Abstract). Am J Primatol 1: 344, 1981 [Google Scholar]
- 11. Conley AJ, Moeller BC, Nguyen AD, Stanley SD, Plant TM, Abbott DH. Defining adrenarche in the rhesus macaque (Macaca mulatta), a non-human primate model for adrenal androgen secretion. Mol Cell Endocrinol 336: 110–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med 22: 311–326, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Copeland KC, Eichberg JW, Parker CR, Jr, Bartke A. Puberty in the chimpanzee: somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. J Clin Endocrinol Metab 60: 1154–1160, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab 94: 2945–2951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cutler GJ, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology 103: 2112–2118, 1978 [DOI] [PubMed] [Google Scholar]
- 16. de Peretti E, Forest MG. Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab 47: 572–577, 1978 [DOI] [PubMed] [Google Scholar]
- 17. Ducsay CA, Hess DL, McClellan MC, Novy MJ. Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. J Clin Endocrinol Metab 73: 385–395, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril 77: 167–172, 2002 [DOI] [PubMed] [Google Scholar]
- 19. El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol 12: 167–176, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Feelders RA, Lamberts SW, Hofland LJ, van Koetsveld PM, Verhoef-Post M, Themmen AP, de Jong FH, Bonjer HJ, Clark AJ, van der Lely AJ, de Herder WW. Luteinizing hormone (LH)-responsive Cushing's syndrome: the demonstration of LH receptor messenger ribonucleic acid in hyperplastic adrenal cells, which respond to chorionic gonadotropin and serotonin agonists in vitro. J Clin Endocrinol Metab 88: 230–237, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Givens JR, Andersen RN, Wiser WL, Coleman SA, Fish SA. A gonadotropin-responsive adrenocortical adenoma. J Clin Endocrinol Metab 38: 126–133, 1974 [DOI] [PubMed] [Google Scholar]
- 22. Goodarzi MO, Dawson DW, Li X, Lei Z, Shintaku P, Rao CV, Van Herle AJ. Virilization in bilateral macronodular adrenal hyperplasia controlled by luteinizing hormone. J Clin Endocrinol Metab 88: 73–77, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med 22: 337–347, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Ibanez L, DiMartino-Nardi J, Potau N, Saenger P. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev 21: 671–696, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Kelnar CJ, Brook CG. A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clin Endocrinol (Oxf) 19: 117–129, 1983 [DOI] [PubMed] [Google Scholar]
- 26. Koritnik DR, Laherty RF, Rotten D, Jaffe RB. A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids 42: 653–667, 1983 [DOI] [PubMed] [Google Scholar]
- 27. Labrie F. Intracrinology. Mol Cell Endocrinol 78: C113–C118, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24: 152–182, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab 87: 3760–3767, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Leinonen P, Ranta T, Siegberg R, Pelkonen R, Heikkila P, Kahri A. Testosterone-secreting virilizing adrenal adenoma with human chorionic gonadotrophin receptors and 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 34: 31–35, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Levine J, Wolfe LG, Schiebinger RJ, Loriaux DL, Cutler GB., Jr Rapid regression of fetal adrenal zone and absence of adrenal reticular zone in the marmoset. Endocrinology 111: 1797–1802, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Mapes S, Corbin CJ, Tarantal A, Conley A. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD). J Clin Endocrinol Metab 84: 3382–3385, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Martin DD, Schweizer R, Schwarze CP, Elmlinger MW, Ranke MB, Binder G. The early dehydroepiandrosterone sulfate rise of adrenarche and the delay of pubarche indicate primary ovarian failure in Turner syndrome. J Clin Endocrinol Metab 89: 1164–1168, 2004 [DOI] [PubMed] [Google Scholar]
- 34. McNulty WP, Novy MJ, Walsh SW. Fetal and postnatal development of the adrenal glands in Macaca mulatta. Biol Reprod 25: 1079–1089, 1981 [DOI] [PubMed] [Google Scholar]
- 35. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18: 378–403, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10: 3–17, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab 22: 243–258, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nader S. Adrenarche and polycystic ovary syndrome: a tale of two hypotheses. J Pediatr Adolesc Gynecol 20: 353–360, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Nadler RD, Wallis J, Roth-Meyer C, Cooper RW, Baulieu EE. Hormones and behavior of prepubertal and peripubertal chimpanzees. Horm Behav 21: 118–131, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol 174: 111–120, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev 13: 33–54, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology 150: 1748–1756, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol 199: 367–378, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551–555, 1984 [DOI] [PubMed] [Google Scholar]
- 45. Pabon JE, Li X, Lei ZM, Sanfilippo JS, Yussman MA, Rao CV. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab 81: 2397–2400, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler DW, Boepple PA. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab 86: 4536–4542, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Parker CR., Jr Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64: 640–647, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Parker CR, Jr, Jian M, Conley AJ. The localization of DHEA sulfotransferase in steroidogenic and steroid metabolizing tissues of the adult rhesus macaque monkey. Endocr Res 26: 517–522, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Parker CR, Jr, Leveno K, Carr BR, Hauth J, MacDonald PC. Umbilical cord plasma levels of dehydroepiandrosterone sulfate during human gestation. J Clin Endocrinol Metab 54: 1216–1220, 1982 [DOI] [PubMed] [Google Scholar]
- 50. Pattison JC, Abbott DH, Saltzman W, Conley AJ, Bird IM. Plasticity of the zona reticularis in the adult marmoset adrenal cortex: voyages of discovery in the New World. J Endocrinol 203: 313–326, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Pattison JC, Mapes SM, Pryce CR, Conley AJ, Bird IM. Marmosets express a fetal zone at birth but no ZR in adulthood. Endocr Res 28: 675, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol 191: 301–308, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Plant TM. The effects of neonatal orchidectomy on the developmental pattern of gonadotropin-secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 106: 1451–1454, 1980 [DOI] [PubMed] [Google Scholar]
- 54. Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 116: 1341–1350, 1985 [DOI] [PubMed] [Google Scholar]
- 56. Plant TM, Zorub DS. A study of the role of the adrenal glands in the initiation of the hiatus in gonadotropin secretion during prepubertal development in the male rhesus monkey (Macaca mulatta). Endocrinology 114: 560–565, 1984 [DOI] [PubMed] [Google Scholar]
- 57. Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab 13: 234–239, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Ramaswamy S, Pohl CR, McNeilly AS, Winters SJ, Plant TM. The time course of follicle-stimulating hormone suppression by recombinant human inhibin A in the adult male rhesus monkey (Macaca mulatta). Endocrinology 139: 3409–3415, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Rao C, Zhou XL, Lei ZM. Functional luteinizing hormone/chorionic gonadotropin receptors in human adrenal cortical H295R cells. Biol Reprod 71: 579–587, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Rask E, Schvarcz E, Hellman P, Hennings J, Karlsson FA, Rao CV. Adrenocorticotropin-independent Cushing's syndrome in pregnancy related to overexpression of adrenal luteinizing hormone/human chorionic gonadotropin receptors. J Endocrinol Invest 32: 313–316, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Rehman KS, Carr BR. Sex differences in adrenal androgens. Semin Reprod Med 22: 349–360, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr 90: 766–770, 1977 [DOI] [PubMed] [Google Scholar]
- 63. Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90: 2015–2021, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Seron-Ferre M, Hess DL, Lindholm U, Jaffe RB. Persistence of fetal zone function in the infant rhesus monkey adrenal gland. J Clin Endocrinol Metab 62: 460–465, 1986 [DOI] [PubMed] [Google Scholar]
- 65. Seron-Ferre M, Taylor NF, Rotten D, Koritnik DR, Jaffe RB. Changes in fetal rhesus monkey plasma dehydroepiandrosterone sulfate: relationship to gestational age, adrenal weight and preterm delivery. J Clin Endocrinol Metab 57: 1173–1178, 1983 [DOI] [PubMed] [Google Scholar]
- 66. Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol 46: 311–321, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod 65: 1718–1725, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS. Further studies on adrenarche in nonhuman primates. Endocrinology 111: 844–848, 1982 [DOI] [PubMed] [Google Scholar]
- 69. Sucheston ME, Cannon MS. Microscopic comparison of the normal and anencephalic human adrenal gland with emphasis on the transient-zone. Obstet Gynecol 35: 544–553, 1970 [PubMed] [Google Scholar]
- 70. Sulcova J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol 154: 57–62, 1997 [DOI] [PubMed] [Google Scholar]
- 71. Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta). Endocrinology 139: 2774–2783, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53: 739–747, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Taha D, Mullis PE, Ibanez L, de Zegher F. Absent or delayed adrenarche in Pit-1/POU1F1 deficiency. Horm Res 64: 175–179, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, Barbieri RL, Hankinson SE. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev 15: 967–971, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Werk EE, Jr, Sholiton LE, Kalejs L. Testosterone-secreting adrenal adenoma under gonadotropin control. N Engl J Med 289: 767–770, 1973 [DOI] [PubMed] [Google Scholar]
- 76. Winter JS, Faiman C, Hobson WC, Reyes FI. The endocrine basis of sexual development in the chimpanzee. J Reprod Fertil Suppl 28: 131–138, 1980 [PubMed] [Google Scholar]
- 77. Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 8: 331–342, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol 27: 36–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab 90: 6630–6637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]