Abstract
In the crystal structure of the title solvated salt, C16H19N2
+·C8H5O7S−·CH3OH, the anions and the methanol solvent molecules are linked by O—H⋯O hydrogen bonds. The cations and anions are packed as alternate layers parallel to (11 ). The crystal structure is further stabilized by a π–π interaction between the pyridinium and benzene rings of the cations, with a centroid–centroid distance of 3.5492 (4) Å.
). The crystal structure is further stabilized by a π–π interaction between the pyridinium and benzene rings of the cations, with a centroid–centroid distance of 3.5492 (4) Å.
Related literature
The title compound was synthesized as part of our continuing research on the nonlinear optical properties of DAS (4-N,N-dimethylamino-4′-N′-methylstilbazolium) derivatives. For the synthesis, see: Okada et al. (1990 ▶). For background to non-linear optical materials, see: Bosshard et al. (1995 ▶); Nalwa & Miyata (1997 ▶); Yang, Mutter et al. (2007 ▶); Ruiz et al. (2006 ▶). For the effects of different substituents of benzene sulfonate on its non-linear optical properties, see: Okada et al. (2003 ▶); Yang, Wörle et al. (2007 ▶); Ogawa et al. (2008 ▶), Yang et al. (2005 ▶). For standard bond-lengths, see: Allen et al. (1987 ▶).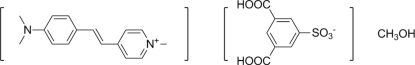
Experimental
Crystal data
C16H19N2 +·C8H5O7S−·CH4O
M r = 516.55
Triclinic,
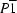
a = 7.5277 (12) Å
b = 10.5517 (17) Å
c = 16.467 (3) Å
α = 106.750 (7)°
β = 97.504 (8)°
γ = 100.273 (8)°
V = 1209.2 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.19 mm−1
T = 173 K
0.45 × 0.31 × 0.22 mm
Data collection
Rigaku Saturn724+ CCD diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2008 ▶) T min = 0.629, T max = 1.000
15622 measured reflections
5523 independent reflections
5192 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.116
S = 1.13
5523 reflections
332 parameters
H-atom parameters constrained
Δρmax = 0.33 e Å−3
Δρmin = −0.33 e Å−3
Data collection: CrystalClear (Rigaku, 2008 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811054419/ez2270sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811054419/ez2270Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811054419/ez2270Isup3.cdx
Supplementary material file. DOI: 10.1107/S1600536811054419/ez2270Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O6i | 0.84 | 1.89 | 2.6796 (18) | 156 |
| O4—H4A⋯O8ii | 0.84 | 1.79 | 2.6156 (18) | 168 |
| O8—H8⋯O7iii | 0.84 | 1.86 | 2.6897 (18) | 169 |
Symmetry codes: (i) 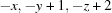 ; (ii)
; (ii) 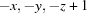 ; (iii)
; (iii) 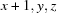 .
.
Acknowledgments
The authors thank the Natural Science Foundation (grant No. 50803005), the Fundamental Research Funds for the Central Universities, the Scientific Research Foundation for Returned Overseas Chinese Scholars and the National Natural Science Fund for Distinguished Young Scholars (grant No. 51025313).
supplementary crystallographic information
Comment
During the last three decades, nonlinear optical materials have been of considerable interest for their potential applications such as frequency conversion, electro-optic modulation and THz generation (Bosshard et al., 1995; Nalwa & Miyata, 1997; Yang, Mutter et al., 2007). In order to create efficient NLO materials, both the molecular and bulk properties must be optimized (Yang et al., 2005; Ruiz et al., 2006; Yang, Wörle et al., 2007). The title compound was synthesized as part of our continuing research on the nonlinear optical properties of DAS (4-N, N-dimethylamino-4'-N'-methyl-stilbazolium) derivatives.
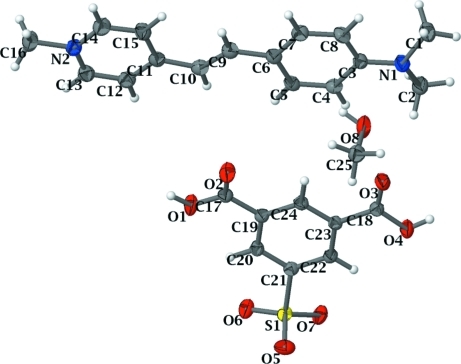
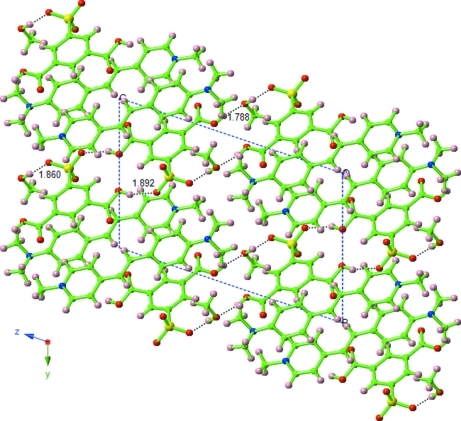
Fig. 1 illustrates the molecular structure of the title compound together with the atomic numbering scheme. The unit cell of the title compound contains two asymmetric units, each consisting of one C16H19N2+ cation, one C8H5O7S- anion and one methanol molecule. The bond distances and angles in both the cation and anion are in normal ranges (Allen et al., 1987). In the crystal structure, atoms O4, O6, O7 and O8 of the anion are involved in O—H···O interactions. The cations and anions are stacked in a parallel manner and form alternating layers parallel to the (112) plane. The crystal structure is further stabilized by a π···π interaction between the pyridinium and C3–C8 benzene rings with a centroid–centroid distance of 3.5492 (4) Å, which combine with the intermolecular O—H···O interactions to form a three-dimensional network.
Experimental
4-{2-[4-(Dimethylamino)phenyl]ethenyl}-1-methylpyridinium 3,5-dicarboxybenzenesulfonate was prepared by the metathesization of 4-N,N-dimethylamino-4'-N'-methyl-stilbazolium iodide (Okada et al., 1990) with the sodium salt of the 3,5-dicarboxybenzenesulfonic acid. The title compound was then recrystallized from methanol to get high purity material for crystal growth. 4-{2-[4-(Dimethylamino)phenyl]ethenyl}-1-methylpyridinium 3,5-dicarboxybenzenesulfonate: yield 72%; 1H-NMR (400 MHz, DMSO-d6): 8.67 (d, 2H, J= 6.8Hz, C5H4N), 8.39 (s, 1H, C6H3SO3-), 8.34 (d, 2H, C6H3SO3-), 8.03(d, 2H, J= 6.8Hz, C5H4N), 7.91 (d, 1H, J= 16.0Hz, CH), 7.59(d, 2H, J= 8.4Hz, C6H3SO3-), 7.17 (d, 1H, J= 16.0Hz, CH), 6.79 (d, 2H, J= 8.8Hz, C6H4), 4.16 (s, 3H, NMe), 3.01 (s, 6H, NMe2). C, H, N analysis calcd. for C24H24N2O7S: C 59.49, H 4.99, N 5.78; found: C 59.39, H 5.02, N 5.79. Crystals were obtained by slow cooling method from 45°C to room temperature in methanol.
Refinement
All H atoms were located geometrically (methyl C-H = 0.98 Å, aromatic C-H = 0.95Å and O-H = 0.84 Å) and refined using a riding model, with Uiso(H) = 1.2 or 1.5Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound showing the atom numbering scheme and 50% probability displacement ellipsoids.
Fig. 2.
A packing diagram of the title compound showing the hydrogen bonds as dashed lines.
Crystal data
| C16H19N2+·C8H5O7S−·CH4O | Z = 2 |
| Mr = 516.55 | F(000) = 544 |
| Triclinic, P1 | Dx = 1.419 Mg m−3 |
| a = 7.5277 (12) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.5517 (17) Å | Cell parameters from 4222 reflections |
| c = 16.467 (3) Å | θ = 1.3–27.5° |
| α = 106.750 (7)° | µ = 0.19 mm−1 |
| β = 97.504 (8)° | T = 173 K |
| γ = 100.273 (8)° | Block, red |
| V = 1209.2 (3) Å3 | 0.45 × 0.31 × 0.22 mm |
Data collection
| Rigaku Saturn724+ CCD diffractometer | 5523 independent reflections |
| Radiation source: fine-focus sealed tube | 5192 reflections with I > 2σ(I) |
| graphite | Rint = 0.037 |
| Detector resolution: 28.5714 pixels mm-1 | θmax = 27.5°, θmin = 2.8° |
| ω scans at fixed χ = 45° | h = −9→9 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2008) | k = −13→13 |
| Tmin = 0.629, Tmax = 1.000 | l = −21→21 |
| 15622 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.116 | H-atom parameters constrained |
| S = 1.13 | w = 1/[σ2(Fo2) + (0.0477P)2 + 0.4666P] where P = (Fo2 + 2Fc2)/3 |
| 5523 reflections | (Δ/σ)max = 0.001 |
| 332 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | −0.38860 (5) | 0.41490 (4) | 0.77513 (3) | 0.02588 (12) | |
| O1 | 0.1455 (2) | 0.37615 (12) | 0.99887 (8) | 0.0390 (3) | |
| H1 | 0.2036 | 0.3798 | 1.0470 | 0.059* | |
| O2 | 0.1780 (2) | 0.16194 (13) | 0.96041 (8) | 0.0420 (3) | |
| O3 | −0.09373 (18) | −0.12371 (12) | 0.64695 (8) | 0.0350 (3) | |
| O4 | −0.28863 (18) | −0.04200 (13) | 0.57227 (8) | 0.0346 (3) | |
| H4A | −0.2926 | −0.1141 | 0.5327 | 0.052* | |
| O5 | −0.55084 (17) | 0.36035 (13) | 0.80299 (9) | 0.0374 (3) | |
| O6 | −0.28648 (18) | 0.54879 (12) | 0.83184 (8) | 0.0347 (3) | |
| O7 | −0.42469 (17) | 0.41121 (12) | 0.68482 (8) | 0.0332 (3) | |
| O8 | 0.2880 (2) | 0.24475 (14) | 0.56382 (8) | 0.0436 (4) | |
| H8 | 0.3854 | 0.2890 | 0.5993 | 0.065* | |
| N1 | 0.3505 (2) | −0.25772 (15) | 0.60065 (10) | 0.0338 (3) | |
| N2 | 0.9237 (2) | 0.38403 (14) | 1.25311 (10) | 0.0313 (3) | |
| C1 | 0.4220 (3) | −0.37550 (19) | 0.56160 (13) | 0.0406 (4) | |
| H1C | 0.3964 | −0.4424 | 0.5918 | 0.061* | |
| H1A | 0.5552 | −0.3472 | 0.5662 | 0.061* | |
| H1B | 0.3626 | −0.4162 | 0.5006 | 0.061* | |
| C2 | 0.2606 (3) | −0.1979 (2) | 0.54209 (12) | 0.0401 (4) | |
| H2B | 0.3443 | −0.1152 | 0.5424 | 0.060* | |
| H2A | 0.1488 | −0.1752 | 0.5610 | 0.060* | |
| H2C | 0.2280 | −0.2631 | 0.4835 | 0.060* | |
| C3 | 0.4215 (2) | −0.18111 (16) | 0.68546 (11) | 0.0261 (3) | |
| C4 | 0.3830 (2) | −0.05246 (17) | 0.72159 (11) | 0.0281 (3) | |
| H4 | 0.3044 | −0.0190 | 0.6870 | 0.034* | |
| C5 | 0.4577 (2) | 0.02533 (16) | 0.80627 (11) | 0.0271 (3) | |
| H5 | 0.4301 | 0.1116 | 0.8288 | 0.033* | |
| C6 | 0.5736 (2) | −0.02072 (16) | 0.85963 (11) | 0.0271 (3) | |
| C7 | 0.6059 (2) | −0.15028 (17) | 0.82433 (11) | 0.0297 (4) | |
| H7 | 0.6799 | −0.1853 | 0.8599 | 0.036* | |
| C8 | 0.5340 (2) | −0.22835 (16) | 0.73985 (11) | 0.0297 (4) | |
| H8A | 0.5606 | −0.3151 | 0.7180 | 0.036* | |
| C9 | 0.6622 (2) | 0.05654 (17) | 0.94810 (11) | 0.0290 (4) | |
| H9 | 0.7296 | 0.0107 | 0.9785 | 0.035* | |
| C10 | 0.6608 (2) | 0.18460 (17) | 0.99228 (11) | 0.0296 (4) | |
| H10 | 0.5937 | 0.2334 | 0.9642 | 0.036* | |
| C11 | 0.7569 (2) | 0.25224 (17) | 1.08083 (11) | 0.0282 (3) | |
| C12 | 0.7411 (2) | 0.38370 (18) | 1.12496 (12) | 0.0325 (4) | |
| H12 | 0.6721 | 0.4299 | 1.0956 | 0.039* | |
| C13 | 0.8239 (2) | 0.44591 (18) | 1.20961 (12) | 0.0333 (4) | |
| H13 | 0.8107 | 0.5347 | 1.2384 | 0.040* | |
| C14 | 0.9459 (3) | 0.25904 (18) | 1.21244 (12) | 0.0351 (4) | |
| H14 | 1.0186 | 0.2166 | 1.2432 | 0.042* | |
| C15 | 0.8659 (2) | 0.19230 (17) | 1.12795 (12) | 0.0333 (4) | |
| H15 | 0.8840 | 0.1044 | 1.1006 | 0.040* | |
| C16 | 1.0116 (3) | 0.4516 (2) | 1.34462 (12) | 0.0421 (5) | |
| H16B | 0.9368 | 0.5113 | 1.3731 | 0.063* | |
| H16C | 1.1346 | 0.5056 | 1.3485 | 0.063* | |
| H16A | 1.0221 | 0.3828 | 1.3732 | 0.063* | |
| C17 | 0.1147 (2) | 0.25092 (16) | 0.94444 (11) | 0.0280 (3) | |
| C18 | −0.1751 (2) | −0.03581 (16) | 0.64270 (10) | 0.0256 (3) | |
| C19 | −0.0110 (2) | 0.23178 (16) | 0.86173 (10) | 0.0242 (3) | |
| C20 | −0.1150 (2) | 0.32637 (15) | 0.85514 (10) | 0.0242 (3) | |
| H20 | −0.1007 | 0.4069 | 0.9023 | 0.029* | |
| C21 | −0.2395 (2) | 0.30264 (15) | 0.77944 (10) | 0.0231 (3) | |
| C22 | −0.2594 (2) | 0.18634 (15) | 0.70939 (10) | 0.0241 (3) | |
| H22 | −0.3434 | 0.1714 | 0.6574 | 0.029* | |
| C23 | −0.1550 (2) | 0.09165 (15) | 0.71595 (10) | 0.0236 (3) | |
| C24 | −0.0322 (2) | 0.11394 (16) | 0.79225 (10) | 0.0248 (3) | |
| H24 | 0.0373 | 0.0487 | 0.7970 | 0.030* | |
| C25 | 0.1596 (3) | 0.19091 (19) | 0.60747 (12) | 0.0368 (4) | |
| H25A | 0.1064 | 0.0948 | 0.5753 | 0.055* | |
| H25C | 0.0616 | 0.2410 | 0.6118 | 0.055* | |
| H25B | 0.2218 | 0.1996 | 0.6656 | 0.055* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0280 (2) | 0.02105 (19) | 0.0269 (2) | 0.00778 (15) | −0.00144 (16) | 0.00678 (15) |
| O1 | 0.0533 (8) | 0.0281 (6) | 0.0274 (6) | 0.0160 (6) | −0.0121 (6) | 0.0009 (5) |
| O2 | 0.0555 (8) | 0.0343 (7) | 0.0324 (7) | 0.0236 (6) | −0.0088 (6) | 0.0042 (5) |
| O3 | 0.0403 (7) | 0.0317 (6) | 0.0295 (6) | 0.0159 (5) | 0.0021 (5) | 0.0017 (5) |
| O4 | 0.0414 (7) | 0.0339 (7) | 0.0227 (6) | 0.0112 (6) | −0.0017 (5) | 0.0020 (5) |
| O5 | 0.0325 (7) | 0.0365 (7) | 0.0499 (8) | 0.0159 (5) | 0.0121 (6) | 0.0171 (6) |
| O6 | 0.0427 (7) | 0.0219 (6) | 0.0335 (7) | 0.0094 (5) | −0.0085 (6) | 0.0055 (5) |
| O7 | 0.0386 (7) | 0.0299 (6) | 0.0284 (6) | 0.0083 (5) | −0.0059 (5) | 0.0101 (5) |
| O8 | 0.0445 (8) | 0.0437 (8) | 0.0262 (6) | −0.0102 (6) | 0.0031 (6) | 0.0001 (6) |
| N1 | 0.0385 (8) | 0.0296 (7) | 0.0305 (8) | 0.0129 (6) | 0.0022 (6) | 0.0042 (6) |
| N2 | 0.0292 (7) | 0.0294 (7) | 0.0306 (8) | 0.0019 (6) | 0.0038 (6) | 0.0064 (6) |
| C1 | 0.0514 (12) | 0.0325 (9) | 0.0338 (10) | 0.0152 (8) | 0.0048 (9) | 0.0027 (8) |
| C2 | 0.0436 (11) | 0.0466 (11) | 0.0281 (9) | 0.0186 (9) | 0.0003 (8) | 0.0065 (8) |
| C3 | 0.0260 (8) | 0.0239 (8) | 0.0274 (8) | 0.0048 (6) | 0.0058 (7) | 0.0069 (6) |
| C4 | 0.0286 (8) | 0.0272 (8) | 0.0318 (9) | 0.0102 (7) | 0.0061 (7) | 0.0123 (7) |
| C5 | 0.0296 (8) | 0.0230 (7) | 0.0304 (8) | 0.0079 (6) | 0.0096 (7) | 0.0081 (6) |
| C6 | 0.0276 (8) | 0.0269 (8) | 0.0280 (8) | 0.0048 (6) | 0.0079 (7) | 0.0106 (6) |
| C7 | 0.0327 (9) | 0.0301 (8) | 0.0303 (9) | 0.0107 (7) | 0.0057 (7) | 0.0135 (7) |
| C8 | 0.0356 (9) | 0.0229 (8) | 0.0337 (9) | 0.0106 (7) | 0.0096 (7) | 0.0101 (7) |
| C9 | 0.0304 (9) | 0.0297 (8) | 0.0295 (9) | 0.0072 (7) | 0.0074 (7) | 0.0126 (7) |
| C10 | 0.0294 (9) | 0.0298 (8) | 0.0323 (9) | 0.0080 (7) | 0.0067 (7) | 0.0130 (7) |
| C11 | 0.0261 (8) | 0.0284 (8) | 0.0300 (9) | 0.0032 (6) | 0.0085 (7) | 0.0095 (7) |
| C12 | 0.0320 (9) | 0.0296 (8) | 0.0368 (9) | 0.0092 (7) | 0.0046 (7) | 0.0118 (7) |
| C13 | 0.0320 (9) | 0.0267 (8) | 0.0389 (10) | 0.0072 (7) | 0.0067 (8) | 0.0066 (7) |
| C14 | 0.0360 (10) | 0.0310 (9) | 0.0374 (10) | 0.0089 (7) | 0.0017 (8) | 0.0114 (7) |
| C15 | 0.0385 (10) | 0.0257 (8) | 0.0345 (9) | 0.0098 (7) | 0.0058 (8) | 0.0071 (7) |
| C16 | 0.0416 (11) | 0.0423 (11) | 0.0315 (10) | 0.0009 (9) | 0.0004 (8) | 0.0034 (8) |
| C17 | 0.0295 (8) | 0.0271 (8) | 0.0267 (8) | 0.0116 (7) | 0.0024 (7) | 0.0057 (6) |
| C18 | 0.0243 (8) | 0.0284 (8) | 0.0229 (8) | 0.0049 (6) | 0.0049 (6) | 0.0070 (6) |
| C19 | 0.0246 (8) | 0.0247 (7) | 0.0233 (8) | 0.0066 (6) | 0.0040 (6) | 0.0075 (6) |
| C20 | 0.0266 (8) | 0.0209 (7) | 0.0240 (8) | 0.0051 (6) | 0.0039 (6) | 0.0062 (6) |
| C21 | 0.0229 (7) | 0.0216 (7) | 0.0261 (8) | 0.0057 (6) | 0.0044 (6) | 0.0094 (6) |
| C22 | 0.0238 (8) | 0.0256 (8) | 0.0226 (7) | 0.0050 (6) | 0.0030 (6) | 0.0082 (6) |
| C23 | 0.0237 (7) | 0.0238 (7) | 0.0230 (8) | 0.0051 (6) | 0.0061 (6) | 0.0067 (6) |
| C24 | 0.0261 (8) | 0.0250 (7) | 0.0251 (8) | 0.0096 (6) | 0.0056 (6) | 0.0080 (6) |
| C25 | 0.0352 (10) | 0.0354 (9) | 0.0347 (10) | 0.0039 (8) | 0.0056 (8) | 0.0062 (8) |
Geometric parameters (Å, °)
| S1—O5 | 1.4427 (13) | C7—C8 | 1.375 (2) |
| S1—O6 | 1.4572 (12) | C7—H7 | 0.9500 |
| S1—O7 | 1.4643 (13) | C8—H8A | 0.9500 |
| S1—C21 | 1.7801 (16) | C9—C10 | 1.340 (2) |
| O1—C17 | 1.325 (2) | C9—H9 | 0.9500 |
| O1—H1 | 0.8400 | C10—C11 | 1.449 (2) |
| O2—C17 | 1.203 (2) | C10—H10 | 0.9500 |
| O3—C18 | 1.212 (2) | C11—C12 | 1.402 (2) |
| O4—C18 | 1.326 (2) | C11—C15 | 1.406 (2) |
| O4—H4A | 0.8400 | C12—C13 | 1.364 (3) |
| O8—C25 | 1.412 (2) | C12—H12 | 0.9500 |
| O8—H8 | 0.8400 | C13—H13 | 0.9500 |
| N1—C3 | 1.375 (2) | C14—C15 | 1.364 (3) |
| N1—C1 | 1.455 (2) | C14—H14 | 0.9500 |
| N1—C2 | 1.456 (2) | C15—H15 | 0.9500 |
| N2—C13 | 1.345 (2) | C16—H16B | 0.9800 |
| N2—C14 | 1.347 (2) | C16—H16C | 0.9800 |
| N2—C16 | 1.473 (2) | C16—H16A | 0.9800 |
| C1—H1C | 0.9800 | C17—C19 | 1.493 (2) |
| C1—H1A | 0.9800 | C18—C23 | 1.494 (2) |
| C1—H1B | 0.9800 | C19—C20 | 1.393 (2) |
| C2—H2B | 0.9800 | C19—C24 | 1.394 (2) |
| C2—H2A | 0.9800 | C20—C21 | 1.388 (2) |
| C2—H2C | 0.9800 | C20—H20 | 0.9500 |
| C3—C8 | 1.406 (2) | C21—C22 | 1.390 (2) |
| C3—C4 | 1.414 (2) | C22—C23 | 1.396 (2) |
| C4—C5 | 1.380 (2) | C22—H22 | 0.9500 |
| C4—H4 | 0.9500 | C23—C24 | 1.392 (2) |
| C5—C6 | 1.402 (2) | C24—H24 | 0.9500 |
| C5—H5 | 0.9500 | C25—H25A | 0.9800 |
| C6—C7 | 1.401 (2) | C25—H25C | 0.9800 |
| C6—C9 | 1.449 (2) | C25—H25B | 0.9800 |
| O5—S1—O6 | 114.32 (8) | C12—C11—C15 | 116.21 (16) |
| O5—S1—O7 | 113.04 (8) | C12—C11—C10 | 120.18 (16) |
| O6—S1—O7 | 111.46 (7) | C15—C11—C10 | 123.60 (16) |
| O5—S1—C21 | 105.50 (7) | C13—C12—C11 | 120.71 (17) |
| O6—S1—C21 | 106.09 (7) | C13—C12—H12 | 119.6 |
| O7—S1—C21 | 105.58 (7) | C11—C12—H12 | 119.6 |
| C17—O1—H1 | 109.5 | N2—C13—C12 | 121.34 (16) |
| C18—O4—H4A | 109.5 | N2—C13—H13 | 119.3 |
| C25—O8—H8 | 109.5 | C12—C13—H13 | 119.3 |
| C3—N1—C1 | 119.25 (15) | N2—C14—C15 | 121.23 (17) |
| C3—N1—C2 | 119.96 (15) | N2—C14—H14 | 119.4 |
| C1—N1—C2 | 116.88 (15) | C15—C14—H14 | 119.4 |
| C13—N2—C14 | 119.76 (16) | C14—C15—C11 | 120.71 (16) |
| C13—N2—C16 | 120.98 (16) | C14—C15—H15 | 119.6 |
| C14—N2—C16 | 119.25 (16) | C11—C15—H15 | 119.6 |
| N1—C1—H1C | 109.5 | N2—C16—H16B | 109.5 |
| N1—C1—H1A | 109.5 | N2—C16—H16C | 109.5 |
| H1C—C1—H1A | 109.5 | H16B—C16—H16C | 109.5 |
| N1—C1—H1B | 109.5 | N2—C16—H16A | 109.5 |
| H1C—C1—H1B | 109.5 | H16B—C16—H16A | 109.5 |
| H1A—C1—H1B | 109.5 | H16C—C16—H16A | 109.5 |
| N1—C2—H2B | 109.5 | O2—C17—O1 | 123.90 (16) |
| N1—C2—H2A | 109.5 | O2—C17—C19 | 123.72 (15) |
| H2B—C2—H2A | 109.5 | O1—C17—C19 | 112.37 (13) |
| N1—C2—H2C | 109.5 | O3—C18—O4 | 123.48 (15) |
| H2B—C2—H2C | 109.5 | O3—C18—C23 | 123.32 (15) |
| H2A—C2—H2C | 109.5 | O4—C18—C23 | 113.19 (14) |
| N1—C3—C8 | 121.18 (15) | C20—C19—C24 | 119.86 (15) |
| N1—C3—C4 | 121.36 (15) | C20—C19—C17 | 120.65 (14) |
| C8—C3—C4 | 117.45 (15) | C24—C19—C17 | 119.37 (14) |
| C5—C4—C3 | 121.16 (15) | C21—C20—C19 | 119.82 (14) |
| C5—C4—H4 | 119.4 | C21—C20—H20 | 120.1 |
| C3—C4—H4 | 119.4 | C19—C20—H20 | 120.1 |
| C4—C5—C6 | 121.17 (15) | C20—C21—C22 | 120.65 (14) |
| C4—C5—H5 | 119.4 | C20—C21—S1 | 119.61 (12) |
| C6—C5—H5 | 119.4 | C22—C21—S1 | 119.54 (12) |
| C7—C6—C5 | 117.35 (15) | C21—C22—C23 | 119.52 (15) |
| C7—C6—C9 | 118.11 (15) | C21—C22—H22 | 120.2 |
| C5—C6—C9 | 124.54 (15) | C23—C22—H22 | 120.2 |
| C8—C7—C6 | 122.09 (16) | C24—C23—C22 | 120.01 (14) |
| C8—C7—H7 | 119.0 | C24—C23—C18 | 118.89 (14) |
| C6—C7—H7 | 119.0 | C22—C23—C18 | 121.09 (14) |
| C7—C8—C3 | 120.72 (15) | C23—C24—C19 | 120.12 (15) |
| C7—C8—H8A | 119.6 | C23—C24—H24 | 119.9 |
| C3—C8—H8A | 119.6 | C19—C24—H24 | 119.9 |
| C10—C9—C6 | 128.01 (16) | O8—C25—H25A | 109.5 |
| C10—C9—H9 | 116.0 | O8—C25—H25C | 109.5 |
| C6—C9—H9 | 116.0 | H25A—C25—H25C | 109.5 |
| C9—C10—C11 | 123.11 (16) | O8—C25—H25B | 109.5 |
| C9—C10—H10 | 118.4 | H25A—C25—H25B | 109.5 |
| C11—C10—H10 | 118.4 | H25C—C25—H25B | 109.5 |
| C1—N1—C3—C8 | −12.7 (3) | C10—C11—C15—C14 | −176.93 (16) |
| C2—N1—C3—C8 | −169.80 (16) | O2—C17—C19—C20 | 163.92 (17) |
| C1—N1—C3—C4 | 167.82 (16) | O1—C17—C19—C20 | −14.8 (2) |
| C2—N1—C3—C4 | 10.8 (3) | O2—C17—C19—C24 | −12.2 (3) |
| N1—C3—C4—C5 | −178.64 (15) | O1—C17—C19—C24 | 169.03 (15) |
| C8—C3—C4—C5 | 1.9 (2) | C24—C19—C20—C21 | 0.2 (2) |
| C3—C4—C5—C6 | −0.3 (3) | C17—C19—C20—C21 | −175.99 (15) |
| C4—C5—C6—C7 | −1.9 (2) | C19—C20—C21—C22 | −1.2 (2) |
| C4—C5—C6—C9 | 177.82 (16) | C19—C20—C21—S1 | 173.64 (12) |
| C5—C6—C7—C8 | 2.5 (2) | O5—S1—C21—C20 | −89.52 (14) |
| C9—C6—C7—C8 | −177.17 (15) | O6—S1—C21—C20 | 32.14 (15) |
| C6—C7—C8—C3 | −1.0 (3) | O7—S1—C21—C20 | 150.54 (13) |
| N1—C3—C8—C7 | 179.29 (16) | O5—S1—C21—C22 | 85.32 (14) |
| C4—C3—C8—C7 | −1.2 (2) | O6—S1—C21—C22 | −153.02 (13) |
| C7—C6—C9—C10 | 174.95 (17) | O7—S1—C21—C22 | −34.61 (14) |
| C5—C6—C9—C10 | −4.7 (3) | C20—C21—C22—C23 | 1.0 (2) |
| C6—C9—C10—C11 | −179.27 (15) | S1—C21—C22—C23 | −173.75 (12) |
| C9—C10—C11—C12 | −176.31 (16) | C21—C22—C23—C24 | 0.0 (2) |
| C9—C10—C11—C15 | 2.3 (3) | C21—C22—C23—C18 | 178.81 (14) |
| C15—C11—C12—C13 | −1.9 (3) | O3—C18—C23—C24 | 2.3 (2) |
| C10—C11—C12—C13 | 176.88 (16) | O4—C18—C23—C24 | −176.68 (14) |
| C14—N2—C13—C12 | 1.1 (3) | O3—C18—C23—C22 | −176.44 (16) |
| C16—N2—C13—C12 | −179.62 (17) | O4—C18—C23—C22 | 4.5 (2) |
| C11—C12—C13—N2 | 0.5 (3) | C22—C23—C24—C19 | −1.0 (2) |
| C13—N2—C14—C15 | −1.2 (3) | C18—C23—C24—C19 | −179.82 (14) |
| C16—N2—C14—C15 | 179.51 (17) | C20—C19—C24—C23 | 0.9 (2) |
| N2—C14—C15—C11 | −0.3 (3) | C17—C19—C24—C23 | 177.13 (15) |
| C12—C11—C15—C14 | 1.8 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O6i | 0.84 | 1.89 | 2.6796 (18) | 156. |
| O4—H4A···O8ii | 0.84 | 1.79 | 2.6156 (18) | 168. |
| O8—H8···O7iii | 0.84 | 1.86 | 2.6897 (18) | 169. |
Symmetry codes: (i) −x, −y+1, −z+2; (ii) −x, −y, −z+1; (iii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: EZ2270).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bosshard, Ch., Sutter, K., Prêtre, Ph., Hulliger, J., Flörsheimer, M., Kaatz, P. & Günter, P. (1995). Organic Nonlinear Optical Materials. Advances in Nonlinear Optics, Vol. 1. Amsterdam: Gordon & Breach.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Nalwa, H. S. & Miyata, S. (1997). Editors. Nonlinear Optics of Organic Molecules and Polymers Boca Raton: CRC Press.
- Ogawa, J., Okada, S., Glavcheva, Z. & Nakanishi, H. (2008). J. Cryst. Growth, 310, 836–842.
- Okada, S., Masaki, I., Matsuda, H., Nakanishi, H., Kato, M., Muramatsu, R. & Otsuka, M. (1990). Jpn J. Appl. Phys. 29, 1112–1115.
- Okada, S., Nogi, K., Anwar, Tsuji, K., Duan X. M., Oikawa, H., Matsuda, H. & Nakanishi H. (2003). Jpn J. Appl. Phys. 42, 668–671.
- Rigaku (2008). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Ruiz, B., Yang, Z., Gramlich, V., Jazbinsek, M. & Günter, P. (2006). J. Mater. Chem. 16, 2839–2842.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Yang, Z., Aravazhi, S., Schneider, A., Seiler, P., Jazbinsek, M. & Günter, P. (2005). Adv. Funct. Mater. 15, 1072–1075.
- Yang, Z., Mutter, L., Ruiz, B., Aravazhi, S., Stillhart, M., Jazbinsek, M., Gramlich, V. & Günter, P. (2007). Adv. Funct. Mater. 17, 2018–2023.
- Yang, Z., Wörle, M., Mutter, L., Jazbinsek, M. & Günter, P. (2007). Cryst. Growth Des. 7, 83–86.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811054419/ez2270sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811054419/ez2270Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811054419/ez2270Isup3.cdx
Supplementary material file. DOI: 10.1107/S1600536811054419/ez2270Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Enhanced figure: interactive version of Fig. 1