Abstract
The title compound, C19H19N3O7S·CH3OH, was synthesized from syringic acid and sulfamethoxazole. The benzene rings make a dihedral angle of 41.8 (1)° and the isoxazole ring is twisted by 74.3 (1)° from the central benzene ring. The crystal packing features O—H⋯O and O—H⋯N hydrogen bonds in which the hydroxy groups from the main molecule and methanol solvent molecules serve as donor groups.
Related literature
For the biological activity of syringic acid and sulfamethoxazole, see: Wu et al. (2009 ▶); Itoh et al. (2009 ▶, 2010 ▶); Ramachandran & Raja (2010 ▶); Ma et al. (2007 ▶); Hida et al. (2005 ▶); Liu et al. (2003 ▶). For related structures, see: Camerman et al. (1979 ▶); Yan et al. (2009 ▶); Yasmeen et al. (2010 ▶).
Experimental
Crystal data
C19H19N3O7S·CH4O
M r = 465.47
Monoclinic,
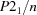
a = 12.133 (11) Å
b = 8.684 (8) Å
c = 20.983 (19) Å
β = 102.043 (13)°
V = 2162 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.20 mm−1
T = 296 K
0.35 × 0.24 × 0.20 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.933, T max = 0.961
11585 measured reflections
3807 independent reflections
2900 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.140
S = 1.05
3807 reflections
298 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.34 e Å−3
Δρmin = −0.48 e Å−3
Data collection: SMART (Bruker, 2004 ▶); cell refinement: SMART (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811055991/bh2401sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055991/bh2401Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811055991/bh2401Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯N3i | 0.85 (3) | 2.05 (3) | 2.852 (4) | 157 (3) |
| O8—H8⋯O2ii | 0.94 (5) | 2.12 (6) | 3.004 (4) | 156 (5) |
| O8—H8⋯O1ii | 0.94 (5) | 2.44 (5) | 3.133 (4) | 131 (4) |
Symmetry codes: (i) 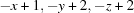 ; (ii)
; (ii)  .
.
Acknowledgments
This work was supported by grants from the China Postdoctoral Science Foundation (grant No. 2011M500130), the Youth Foundation of Guangxi Botanical Garden of Medicinal Plants project (grant No. guiyaoji 201108) and the National Natural Science Foundation of China (grant nos. 20962002, 20662001).
supplementary crystallographic information
Comment
Syringic acid, a natural compound occurring in many kinds of plant species, was synthesized and used widely in medicine, perfume, pesticide chemistry and organic synthetic industry. Syringic acid showed antifungal activity at high concentration (Wu et al., 2009), anti-endotoxic effects (Liu et al., 2003), hepatoprotective effect (Itoh et al., 2009, 2010; Ramachandran & Raja, 2010). Sulfamethoxazole was usually used as anti-infective (anti-bacterial or anti-fungal) drug (Ma et al., 2007; Hida et al., 2005). Whether the title product (Fig. 1, 2 and 3) shows combined-effects (combining the activities of syringic acid with those of sulfamethoxazole) or has some novel properties should be investigated in future. Some structures closely related to the title compound were previously published, which include the syringic or sulfamethoxazole fragment (Camerman et al., 1979; Yan et al., 2009; Yasmeen et al., 2010).
Experimental
4000 mg (20 mmol) of syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid) in 30 ml of acetic anhydride was stirred and refluxed at 120 °C for 2 h. 600 ml of water was added to the above solution to get precipitates (3610 mg) by method of pumping filtration. 3000 mg (12 mmol) of above dry precipitates in 20 ml of thionyl chloride was stirred and refluxed at 80 °C for 6 h under anhydrous conditions. The solution was removed under reduced pressure to get residue (3415 mg). 3100 mg (12 mmol) of the residue, 3040 mg (12 mmol) of sulfamethoxazole [4-amino-N-(5-methyl-3-isoxazolyl)benzenesulfonamide] and 6 ml of pyridine in 200 ml of THF were stirred at 0 °C for 2 h. and then at room temperature for 24 h. 4000 ml of water was added to the above reaction solution to get 4165 mg of precipitates after pumping filtration. 4000 mg (8.4 mmol) of these precipitates and 20 ml of 10 mmol.mL-1 hydrochloric acid in 150 ml of THF were stirred and refluxed at 60 °C for 1 h. 3000 ml of water was added to the hydrolytic solution to get product (2720 mg) after pumping filtration. This final synthetic product was detected by electrospray ionization mass spectroscopy (ESI) to give a molecular ion at m/z value of 432.0 ([M—H]-). This product was redissolved in mixed solution of THF and methanol and then left for evaporating at room temperature. After crystallization and recrystallization from the mixed solution, colorless crystals suitable for X-ray analysis were obtained (mp. 494–495 K).
Refinement
H atoms bonded to C and N atoms were positioned geometrically with d(N—H) = 0.86 Å, d(C—H) = 0.93 (aromatic CH) or 0.96 Å (methyl CH3), and treated as riding atoms. Hydroxyl H atoms H2 and H8 were refined freely. For all H atoms, isotropic displacement parameters were calculated as Uiso(H) = xUeq(N,C,O) with x = 1.2 or 1.5.
Figures
Fig. 1.
Synthesis of the title molecule.
Fig. 2.
A view of the title compound, with 30% probability displacement ellipsoids.
Fig. 3.
Crystal packing, viewed along the b axis, of the title complex. The O—H···O, O—H···N and N—H···O interactions are shown as dashed lines.
Crystal data
| C19H19N3O7S·CH4O | F(000) = 976 |
| Mr = 465.47 | Dx = 1.430 Mg m−3 |
| Monoclinic, P21/n | Melting point: 494 K |
| Hall symbol: -P 2yn | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.133 (11) Å | Cell parameters from 3807 reflections |
| b = 8.684 (8) Å | θ = 1.8–25.0° |
| c = 20.983 (19) Å | µ = 0.20 mm−1 |
| β = 102.043 (13)° | T = 296 K |
| V = 2162 (3) Å3 | Block, colourless |
| Z = 4 | 0.35 × 0.24 × 0.20 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3807 independent reflections |
| Radiation source: fine-focus sealed tube | 2900 reflections with I > 2σ(I) |
| graphite | Rint = 0.042 |
| φ and ω scans | θmax = 25.0°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | h = −13→14 |
| Tmin = 0.933, Tmax = 0.961 | k = −10→10 |
| 11585 measured reflections | l = −23→24 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.140 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0122P)2 + 1.2353P] where P = (Fo2 + 2Fc2)/3 |
| 3807 reflections | (Δ/σ)max = 0.001 |
| 298 parameters | Δρmax = 0.34 e Å−3 |
| 0 restraints | Δρmin = −0.48 e Å−3 |
| 0 constraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.18631 (5) | 0.23253 (8) | 1.00904 (3) | 0.0372 (2) | |
| O1 | 0.29542 (15) | 1.3250 (2) | 0.77380 (10) | 0.0480 (5) | |
| O2 | 0.49358 (17) | 1.4549 (2) | 0.80363 (10) | 0.0463 (5) | |
| O3 | 0.66996 (15) | 1.3222 (2) | 0.88701 (11) | 0.0533 (6) | |
| O4 | 0.51958 (15) | 0.7933 (2) | 0.92734 (11) | 0.0539 (6) | |
| O5 | 0.06867 (14) | 0.2401 (2) | 1.00843 (10) | 0.0463 (5) | |
| O6 | 0.23161 (17) | 0.1065 (2) | 0.97917 (10) | 0.0501 (5) | |
| O7 | 0.24567 (19) | 0.4522 (3) | 1.22145 (11) | 0.0599 (6) | |
| O8 | 0.40341 (19) | 0.4397 (3) | 0.65913 (12) | 0.0639 (7) | |
| N1 | 0.33231 (17) | 0.8292 (2) | 0.91667 (11) | 0.0384 (5) | |
| N2 | 0.25363 (17) | 0.2263 (3) | 1.08544 (11) | 0.0398 (6) | |
| N3 | 0.3029 (2) | 0.3712 (3) | 1.17963 (13) | 0.0522 (7) | |
| C1 | 0.4473 (2) | 1.0346 (3) | 0.88707 (12) | 0.0331 (6) | |
| C2 | 0.5519 (2) | 1.1046 (3) | 0.90221 (13) | 0.0373 (6) | |
| C3 | 0.5688 (2) | 1.2450 (3) | 0.87506 (13) | 0.0375 (6) | |
| C4 | 0.4811 (2) | 1.3181 (3) | 0.83263 (13) | 0.0343 (6) | |
| C5 | 0.3754 (2) | 1.2467 (3) | 0.81737 (13) | 0.0358 (6) | |
| C6 | 0.3588 (2) | 1.1071 (3) | 0.84468 (13) | 0.0351 (6) | |
| C7 | 0.4365 (2) | 0.8765 (3) | 0.91227 (13) | 0.0339 (6) | |
| C8 | 0.1907 (2) | 1.2484 (4) | 0.75269 (19) | 0.0664 (10) | |
| C9 | 0.7670 (2) | 1.2299 (4) | 0.88784 (19) | 0.0639 (10) | |
| C10 | 0.3002 (2) | 0.6841 (3) | 0.93632 (13) | 0.0344 (6) | |
| C11 | 0.1948 (2) | 0.6720 (3) | 0.95179 (14) | 0.0420 (7) | |
| C12 | 0.1587 (2) | 0.5344 (3) | 0.97264 (14) | 0.0436 (7) | |
| C13 | 0.2275 (2) | 0.4053 (3) | 0.97739 (13) | 0.0353 (6) | |
| C14 | 0.3311 (2) | 0.4152 (3) | 0.96069 (14) | 0.0395 (6) | |
| C15 | 0.3678 (2) | 0.5532 (3) | 0.94009 (14) | 0.0405 (7) | |
| C16 | 0.2235 (2) | 0.3183 (3) | 1.13335 (13) | 0.0371 (6) | |
| C17 | 0.1158 (2) | 0.3594 (3) | 1.14200 (15) | 0.0462 (7) | |
| C18 | 0.1341 (3) | 0.4427 (4) | 1.19678 (15) | 0.0505 (8) | |
| C19 | 0.0590 (3) | 0.5249 (5) | 1.23277 (19) | 0.0758 (11) | |
| C20 | 0.4962 (3) | 0.5218 (5) | 0.64565 (19) | 0.0711 (10) | |
| H1A | 0.2792 | 0.8958 | 0.9062 | 0.046* | |
| H2 | 0.560 (3) | 1.487 (4) | 0.8163 (16) | 0.060 (10)* | |
| H2B | 0.3095 | 0.1639 | 1.0962 | 0.048* | |
| H2C | 0.6109 | 1.0569 | 0.9308 | 0.045* | |
| H6A | 0.2881 | 1.0608 | 0.8349 | 0.042* | |
| H8 | 0.418 (4) | 0.420 (6) | 0.704 (3) | 0.15 (2)* | |
| H8A | 0.1408 | 1.3128 | 0.7224 | 0.100* | |
| H8B | 0.1578 | 1.2274 | 0.7896 | 0.100* | |
| H8C | 0.2028 | 1.1534 | 0.7318 | 0.100* | |
| H9A | 0.8330 | 1.2939 | 0.8963 | 0.096* | |
| H9B | 0.7607 | 1.1802 | 0.8464 | 0.096* | |
| H9C | 0.7729 | 1.1534 | 0.9214 | 0.096* | |
| H11A | 0.1481 | 0.7579 | 0.9480 | 0.050* | |
| H12A | 0.0884 | 0.5278 | 0.9835 | 0.052* | |
| H14A | 0.3766 | 0.3284 | 0.9633 | 0.047* | |
| H15A | 0.4378 | 0.5590 | 0.9287 | 0.049* | |
| H17A | 0.0468 | 0.3343 | 1.1154 | 0.055* | |
| H19A | 0.1035 | 0.5718 | 1.2711 | 0.114* | |
| H19B | 0.0072 | 0.4531 | 1.2452 | 0.114* | |
| H19C | 0.0178 | 0.6030 | 1.2053 | 0.114* | |
| H20A | 0.4828 | 0.6303 | 0.6482 | 0.107* | |
| H20B | 0.5060 | 0.4965 | 0.6026 | 0.107* | |
| H20C | 0.5630 | 0.4944 | 0.6769 | 0.107* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0342 (4) | 0.0333 (4) | 0.0451 (4) | −0.0035 (3) | 0.0105 (3) | 0.0010 (3) |
| O1 | 0.0361 (10) | 0.0457 (12) | 0.0569 (13) | −0.0021 (8) | −0.0024 (9) | 0.0198 (10) |
| O2 | 0.0402 (11) | 0.0386 (12) | 0.0569 (13) | −0.0080 (9) | 0.0026 (9) | 0.0146 (10) |
| O3 | 0.0371 (10) | 0.0407 (12) | 0.0760 (15) | −0.0075 (9) | −0.0018 (10) | 0.0086 (11) |
| O4 | 0.0326 (10) | 0.0447 (12) | 0.0844 (16) | 0.0043 (9) | 0.0120 (10) | 0.0239 (11) |
| O5 | 0.0321 (10) | 0.0499 (13) | 0.0566 (13) | −0.0079 (8) | 0.0088 (9) | 0.0033 (10) |
| O6 | 0.0560 (12) | 0.0343 (11) | 0.0639 (14) | −0.0033 (9) | 0.0216 (10) | −0.0088 (10) |
| O7 | 0.0644 (14) | 0.0680 (16) | 0.0474 (13) | −0.0105 (11) | 0.0120 (10) | −0.0069 (11) |
| O8 | 0.0547 (13) | 0.0796 (18) | 0.0555 (15) | −0.0104 (12) | 0.0071 (11) | −0.0017 (13) |
| N1 | 0.0337 (11) | 0.0314 (12) | 0.0523 (14) | 0.0070 (9) | 0.0139 (10) | 0.0123 (11) |
| N2 | 0.0346 (11) | 0.0360 (13) | 0.0485 (14) | 0.0070 (9) | 0.0080 (10) | 0.0091 (11) |
| N3 | 0.0459 (14) | 0.0581 (17) | 0.0515 (16) | −0.0072 (12) | 0.0080 (12) | 0.0012 (13) |
| C1 | 0.0348 (13) | 0.0315 (14) | 0.0338 (14) | 0.0018 (10) | 0.0090 (11) | 0.0035 (11) |
| C2 | 0.0366 (14) | 0.0367 (16) | 0.0374 (15) | 0.0023 (11) | 0.0051 (11) | 0.0043 (12) |
| C3 | 0.0331 (13) | 0.0337 (16) | 0.0446 (16) | −0.0044 (11) | 0.0057 (11) | 0.0012 (12) |
| C4 | 0.0390 (14) | 0.0283 (14) | 0.0362 (14) | −0.0019 (10) | 0.0094 (11) | 0.0052 (12) |
| C5 | 0.0348 (14) | 0.0369 (16) | 0.0355 (15) | 0.0049 (11) | 0.0071 (11) | 0.0060 (12) |
| C6 | 0.0323 (13) | 0.0334 (15) | 0.0401 (15) | −0.0014 (10) | 0.0085 (11) | 0.0036 (12) |
| C7 | 0.0325 (13) | 0.0328 (15) | 0.0371 (15) | 0.0008 (10) | 0.0085 (11) | 0.0044 (12) |
| C8 | 0.0364 (16) | 0.068 (2) | 0.086 (3) | −0.0071 (15) | −0.0075 (16) | 0.031 (2) |
| C9 | 0.0386 (16) | 0.069 (2) | 0.083 (3) | −0.0101 (15) | 0.0116 (16) | −0.004 (2) |
| C10 | 0.0341 (13) | 0.0337 (15) | 0.0360 (14) | 0.0015 (11) | 0.0084 (11) | 0.0064 (12) |
| C11 | 0.0307 (13) | 0.0395 (16) | 0.0573 (18) | 0.0095 (11) | 0.0125 (12) | 0.0133 (14) |
| C12 | 0.0294 (13) | 0.0449 (18) | 0.0584 (19) | 0.0023 (11) | 0.0135 (12) | 0.0104 (14) |
| C13 | 0.0342 (13) | 0.0365 (15) | 0.0356 (15) | −0.0004 (11) | 0.0083 (11) | 0.0017 (12) |
| C14 | 0.0392 (14) | 0.0325 (15) | 0.0504 (17) | 0.0048 (11) | 0.0180 (12) | 0.0051 (13) |
| C15 | 0.0369 (14) | 0.0353 (16) | 0.0546 (18) | 0.0033 (11) | 0.0213 (12) | 0.0048 (13) |
| C16 | 0.0369 (14) | 0.0330 (15) | 0.0413 (16) | −0.0035 (11) | 0.0078 (12) | 0.0120 (12) |
| C17 | 0.0379 (15) | 0.0511 (19) | 0.0500 (18) | 0.0008 (12) | 0.0104 (13) | 0.0015 (15) |
| C18 | 0.0560 (19) | 0.052 (2) | 0.0468 (18) | −0.0015 (14) | 0.0180 (15) | 0.0083 (15) |
| C19 | 0.095 (3) | 0.073 (3) | 0.070 (3) | 0.008 (2) | 0.040 (2) | −0.002 (2) |
| C20 | 0.061 (2) | 0.073 (3) | 0.080 (3) | −0.0076 (18) | 0.0143 (19) | −0.003 (2) |
Geometric parameters (Å, °)
| S1—O5 | 1.426 (2) | C7—C1 | 1.486 (4) |
| S1—O6 | 1.427 (2) | C8—H8C | 0.9600 |
| S1—N2 | 1.641 (3) | C8—H8B | 0.9600 |
| S1—C13 | 1.755 (3) | C8—H8A | 0.9600 |
| O1—C5 | 1.367 (3) | C9—H9C | 0.9600 |
| O1—C8 | 1.420 (4) | C9—H9B | 0.9600 |
| O2—C4 | 1.357 (3) | C9—H9A | 0.9600 |
| O2—H2 | 0.85 (3) | C10—C15 | 1.394 (4) |
| O3—C3 | 1.374 (3) | C10—C11 | 1.388 (4) |
| O3—C9 | 1.421 (4) | C11—H11A | 0.9300 |
| O4—C7 | 1.227 (3) | C11—C12 | 1.375 (4) |
| O7—C18 | 1.347 (4) | C12—H12A | 0.9300 |
| O7—N3 | 1.415 (3) | C13—C12 | 1.389 (4) |
| O8—C20 | 1.410 (4) | C13—C14 | 1.376 (4) |
| O8—H8 | 0.94 (5) | C14—H14A | 0.9300 |
| N1—C7 | 1.350 (3) | C14—C15 | 1.379 (4) |
| N1—C10 | 1.406 (3) | C15—H15A | 0.9300 |
| N1—H1A | 0.8600 | C16—C17 | 1.403 (4) |
| N2—C16 | 1.392 (4) | C16—N3 | 1.301 (4) |
| N2—H2B | 0.8600 | C17—H17A | 0.9300 |
| C2—H2C | 0.9300 | C17—C18 | 1.337 (4) |
| C2—C1 | 1.383 (4) | C18—C19 | 1.482 (4) |
| C2—C3 | 1.379 (4) | C19—H19C | 0.9600 |
| C4—C5 | 1.400 (4) | C19—H19B | 0.9600 |
| C4—C3 | 1.391 (4) | C19—H19A | 0.9600 |
| C6—H6A | 0.9300 | C20—H20C | 0.9600 |
| C6—C1 | 1.394 (4) | C20—H20B | 0.9600 |
| C6—C5 | 1.373 (4) | C20—H20A | 0.9600 |
| S1—N2—H2B | 118.8 | C7—N1—C10 | 127.9 (2) |
| O1—C8—H8A | 109.5 | C10—C11—H11A | 119.7 |
| O1—C8—H8B | 109.5 | C10—C15—H15A | 120.0 |
| O1—C5—C6 | 124.9 (2) | C10—N1—H1A | 116.1 |
| O1—C5—C4 | 114.9 (2) | C11—C12—H12A | 120.1 |
| O1—C8—H8C | 109.5 | C11—C12—C13 | 119.9 (2) |
| O2—C4—C3 | 123.0 (2) | C11—C10—N1 | 117.5 (2) |
| O2—C4—C5 | 117.9 (2) | C11—C10—C15 | 119.0 (2) |
| O3—C9—H9A | 109.5 | C12—C11—H11A | 119.7 |
| O3—C9—H9B | 109.5 | C12—C11—C10 | 120.7 (2) |
| O3—C9—H9C | 109.5 | C12—C13—S1 | 120.2 (2) |
| O3—C3—C2 | 124.1 (2) | C13—C12—H12A | 120.1 |
| O3—C3—C4 | 115.4 (2) | C13—C14—H14A | 119.8 |
| O4—C7—N1 | 122.2 (2) | C13—C14—C15 | 120.4 (2) |
| O4—C7—C1 | 120.4 (2) | C14—C15—H15A | 120.0 |
| O5—S1—O6 | 120.54 (12) | C14—C15—C10 | 120.1 (2) |
| O5—S1—N2 | 107.67 (12) | C14—C13—S1 | 119.8 (2) |
| O6—S1—N2 | 104.17 (13) | C14—C13—C12 | 119.9 (2) |
| O5—S1—C13 | 108.68 (12) | C15—C10—N1 | 123.5 (2) |
| O6—S1—C13 | 108.85 (14) | C15—C14—H14A | 119.8 |
| O7—C18—C19 | 116.7 (3) | C16—N3—O7 | 104.8 (2) |
| O8—C20—H20A | 109.5 | C16—C17—H17A | 127.6 |
| O8—C20—H20B | 109.5 | C16—N2—H2B | 118.8 |
| O8—C20—H20C | 109.5 | C16—N2—S1 | 122.30 (18) |
| N1—C7—C1 | 117.3 (2) | C17—C18—C19 | 133.6 (3) |
| N2—S1—C13 | 105.97 (12) | C17—C18—O7 | 109.7 (3) |
| N2—C16—C17 | 129.2 (3) | C18—C19—H19C | 109.5 |
| N3—C16—N2 | 118.5 (2) | C18—C19—H19B | 109.5 |
| N3—C16—C17 | 112.2 (3) | C18—C19—H19A | 109.5 |
| C1—C2—H2C | 119.9 | C18—C17—H17A | 127.6 |
| C1—C6—H6A | 119.8 | C18—C17—C16 | 104.9 (3) |
| C2—C3—C4 | 120.5 (2) | C18—O7—N3 | 108.4 (2) |
| C2—C1—C7 | 118.0 (2) | C20—O8—H8 | 108 (3) |
| C2—C1—C6 | 119.7 (2) | H8A—C8—H8C | 109.5 |
| C3—C2—H2C | 119.9 | H8A—C8—H8B | 109.5 |
| C3—C2—C1 | 120.2 (2) | H8B—C8—H8C | 109.5 |
| C3—O3—C9 | 115.7 (2) | H9A—C9—H9C | 109.5 |
| C3—C4—C5 | 119.1 (2) | H9A—C9—H9B | 109.5 |
| C4—O2—H2 | 110 (2) | H9B—C9—H9C | 109.5 |
| C5—C6—H6A | 119.8 | H19A—C19—H19C | 109.5 |
| C5—C6—C1 | 120.3 (2) | H19A—C19—H19B | 109.5 |
| C5—O1—C8 | 116.1 (2) | H19B—C19—H19C | 109.5 |
| C6—C5—C4 | 120.2 (2) | H20A—C20—H20C | 109.5 |
| C6—C1—C7 | 122.0 (2) | H20A—C20—H20B | 109.5 |
| C7—N1—H1A | 116.1 | H20B—C20—H20C | 109.5 |
| S1—C13—C12—C11 | 175.8 (2) | C1—C6—C5—C4 | −0.9 (4) |
| S1—C13—C14—C15 | −175.4 (2) | C1—C6—C5—O1 | 177.9 (2) |
| S1—N2—C16—C17 | 39.3 (4) | C3—C4—C5—C6 | 0.7 (4) |
| S1—N2—C16—N3 | −144.2 (2) | C3—C4—C5—O1 | −178.1 (2) |
| O2—C4—C3—C2 | −178.8 (2) | C3—C2—C1—C7 | 173.9 (2) |
| O2—C4—C3—O3 | 1.2 (4) | C3—C2—C1—C6 | −0.6 (4) |
| O2—C4—C5—C6 | 179.1 (2) | C5—C4—C3—C2 | −0.5 (4) |
| O2—C4—C5—O1 | 0.2 (4) | C5—C4—C3—O3 | 179.5 (2) |
| O4—C7—C1—C6 | 151.8 (3) | C5—C6—C1—C7 | −173.5 (2) |
| O4—C7—C1—C2 | −22.6 (4) | C5—C6—C1—C2 | 0.8 (4) |
| O5—S1—C13—C12 | 16.6 (3) | C7—N1—C10—C15 | −15.7 (4) |
| O5—S1—C13—C14 | −167.0 (2) | C7—N1—C10—C11 | 165.2 (3) |
| O5—S1—N2—C16 | −42.3 (2) | C8—O1—C5—C4 | 174.2 (3) |
| O6—S1—C13—C12 | 149.6 (2) | C8—O1—C5—C6 | −4.6 (4) |
| O6—S1—C13—C14 | −34.0 (3) | C9—O3—C3—C4 | −137.6 (3) |
| O6—S1—N2—C16 | −171.4 (2) | C9—O3—C3—C2 | 42.4 (4) |
| N1—C10—C11—C12 | −178.7 (3) | C10—C11—C12—C13 | −1.0 (4) |
| N1—C10—C15—C14 | 179.1 (3) | C10—N1—C7—C1 | 177.2 (2) |
| N1—C7—C1—C6 | −27.2 (4) | C10—N1—C7—O4 | −1.8 (4) |
| N1—C7—C1—C2 | 158.4 (2) | C11—C10—C15—C14 | −1.8 (4) |
| N2—C16—N3—O7 | −177.1 (2) | C12—C13—C14—C15 | 0.9 (4) |
| N2—C16—C17—C18 | 177.1 (3) | C13—C14—C15—C10 | 0.3 (4) |
| N2—S1—C13—C12 | −98.8 (2) | C13—S1—N2—C16 | 73.8 (2) |
| N2—S1—C13—C14 | 77.5 (2) | C14—C13—C12—C11 | −0.6 (4) |
| N3—O7—C18—C17 | 0.6 (3) | C15—C10—C11—C12 | 2.1 (4) |
| N3—C16—C17—C18 | 0.4 (3) | C16—C17—C18—C19 | 177.9 (3) |
| N3—O7—C18—C19 | −178.2 (3) | C16—C17—C18—O7 | −0.6 (3) |
| C1—C2—C3—C4 | 0.5 (4) | C17—C16—N3—O7 | −0.1 (3) |
| C1—C2—C3—O3 | −179.5 (3) | C18—O7—N3—C16 | −0.3 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···N3i | 0.85 (3) | 2.05 (3) | 2.852 (4) | 157 (3) |
| O8—H8···O2ii | 0.94 (5) | 2.12 (6) | 3.004 (4) | 156 (5) |
| O8—H8···O1ii | 0.94 (5) | 2.44 (5) | 3.133 (4) | 131 (4) |
Symmetry codes: (i) −x+1, −y+2, −z+2; (ii) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2401).
References
- Bruker (2004). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Camerman, N., Chan, L. Y. Y., Yeung, H. W. & Mak, T. C. W. (1979). Acta Cryst. B35, 3004–3007.
- Hida, S., Yoshida, M., Nakabayashi, I., Miura, N. N., Adachi, Y. & Ohno, N. (2005). Biol. Pharm. Bull. 28, 773–778. [DOI] [PubMed]
- Itoh, A., Isoda, K., Kondoh, M., Kawase, M., Kobayashi, M., Tamesada, M. & Yagi, K. (2009). Biol. Pharm. Bull. 32, 1215–1219. [DOI] [PubMed]
- Itoh, A., Isoda, K., Kondoh, M., Kawase, M., Watari, A., Kobayashi, M., Tamesada, M. & Yagi, K. (2010). Biol. Pharm. Bull. 33, 983–987. [DOI] [PubMed]
- Liu, Y.-H., Fang, J.-G., Lei, T., Wang, W.-Q. & Lin, A.-H. (2003). J. Huazhong Univ. Sci. Technol. Med. Sci. 23, 206–208. [DOI] [PubMed]
- Ma, M.-L., Cheng, Y.-Y., Xu, Z.-H., Xu, P., Qu, H.-O., Fang, Y.-J., Xu, T.-W. & Wen, L.-P. (2007). Eur. J. Med. Chem. 42, 93–98. [DOI] [PubMed]
- Ramachandran, V. & Raja, B. (2010). J. Basic Clin. Physiol. Pharmacol. 21, 369–385. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wu, H.-S., Luo, J., Liu, Y.-X., Chen, A.-Q., Tang, Z., Cao, Y., Chen, G., Mao, Z.-S., Huang, Q.-W. & Shen, Q.-R. (2009). J. Eukaryot. Microbiol. 56, 386–387. [DOI] [PubMed]
- Yan, Y.-X., Hu, X.-D., Chen, J.-C., Sun, Y., Zhang, X.-M., Qing, C. & Qiu, M.-H. (2009). J. Nat. Prod. 72, 308–311. [DOI] [PubMed]
- Yasmeen, S., Murtaza, S., Akkurt, M., Khan, I. U. & Sharif, S. (2010). Acta Cryst. E66, o2264. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811055991/bh2401sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811055991/bh2401Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811055991/bh2401Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





