Summary
Background
HIV infection and treatment with highly active antiretroviral therapy (HAART) including HIV protease inhibitor ritonavir (RTV) have been associated with endothelial dysfunction and cardiovascular disease including pulmonary arterial hypertension. The objective of this study was to determine if nordihydroguaiaretic acid (NDGA), a natural herbal antioxidant found in the creosote bush Larrea tridentate, can protect vascular tissues against RTV-induced vascular injury.
Material/Methods
Fresh porcine pulmonary artery (PA) rings were treated with a clinically relevant concentration of RTV (15 μmol/L) with or without NDGA for 24 hours, and then subjected to myograph analysis for vasomotor reactivity. Expression of endothelial nitric oxide synthase (eNOS) in both treated PA rings and human pulmonary artery endothelial cells (HPAECs) was analyzed by real-time PCR and immunohistochemistry. Oxidative stress levels were analyzed with the lucigenin-enhanced chemiluminescence and glutathione assay.
Results
In response to bradykinin at 10−10 mol/L, RTV-treated PA rings showed a 39% reduction in endothelium-dependent vasorelaxation compared with the control vessels (P<0.05); when co-cultured with NDGA (1.75 or 3.50 μmol/L), the relaxation increased by 25% and 48%, respectively. RTV also decreased the maximal contraction and endothelium-independent vasorelaxation in RTV-treated vessels, while NDGA improved these vasomotor responses. In addition, treatment of RTV significantly decreased eNOS mRNA levels in both porcine PAs and HPAECs, and reduced eNOS immunoreactivity in porcine PAs, while NDGA significantly inhibited this effect of RTV. Furthermore, NDGA significantly blocked RTV-induced increase of superoxide anion in the PA rings and inhibited RTV-induced decrease of glutathione in HPAECs.
Conclusions
NDGA effectively inhibits the detrimental effects of HIV protease inhibitor RTV on vasomotor functions in porcine PAs. NDGA also blocks RTV-induced decrease of eNOS expression and increase of oxidative stress in both porcine PAs and HPAECs. This study may provide valuable information for the development of effective strategies for the prevention and treatment of HAART-associated cardiovascular complications.
Keywords: nordihydroguaiaretic acid, endothelial dysfunction, ritonavir, endothelial nitric oxide synthase, reactive oxygen species, pulmonary artery
Background
The clinical use of HIV protease inhibitors (PIs) for the treatment of HIV infection has significantly improved survival of patients; so, PIs have become a key component in the highly active antiretroviral therapy (HAART). Meanwhile, HIV infection and treatment with HAART have been associated with a high incidence of endothelial dysfunction and cardiovascular disease including pulmonary arterial hypertension [1–3]. Although the mortality associated with HIV-1 infection has been markedly reduced with the advent of HAART, cardiovascular complications have emerged as a major non-infectious cause of death in HIV-1 patients [4]. Long-term HAART may promote oxidative stress and vascular dysfunction leading to cardiovascular complications [4]. We have recently demonstrated that ritonavir (RTV), one of HIV PIs, causes endothelial dysfunction in porcine coronary and pulmonary arteries and in human endothelial cells, associated with a decrease in endothelial nitric oxide synthase (eNOS) expression and increased oxidative stress [5–8]. Endothelial dysfunction is the initial step in pathogenesis of the vascular lesion formation and is characterized by decreased bioavailability of nitric oxide (NO); this may be due to enhanced NO catabolism secondary to increased superoxide anion (O2−) production or reduced expression and/or activity of eNOS [9,10]. Therefore, using antioxidants that directly scavenge O2− or inhibit its production in cells may effectively reduce the cellular level of oxidative stress. Nordihydroguaiaretic acid (NDGA) is a natural herbal antioxidant found in the creosote bush, Larrea tridentate[11,12]. The objective of this study was to investigate whether NDGA can protect vascular tissues against RTV-induced vascular injury. We used a clinically relevant concentration of RTV as well as low concentrations of NDGA. Porcine pulmonary arteries were used to study the effect of RTV and NDGA on vasomotor functions, eNOS expression, and O2− production. Human pulmonary artery endothelial cells (HPAECs) were also used to elucidate changes in eNOS expression and oxidative stress. This study may provide valuable information leading to the development of effective strategies for the prevention and treatment of cardiovascular and pulmonary vascular complications in HIV-infected patients.
Material and Methods
Chemicals and reagents
NDGA was obtained from Cayman Chemical (Ann Arbor, Michigan). Thrombaxane A2 analogue U46619 (9,11-Dideoxy-11, 9-epoxymethanoprosta-glandin F2), bradykinin, sodium nitroprusside (SNP), phosphate buffered saline (PBS) solution were obtained from Sigma Chemical Co. RTV was obtained from AIDS research and Reference Reagent Program (NIH). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Life Technologies, Inc. Antibiotic-antimycotic solution was obtained from Mediatech Inc.
Myograph model
Porcine lungs from freshly slaughtered young adult farm pigs were harvested and placed in cold PBS solution; the main pulmonary arteries were flushed with PBS to prevent clot formation, and the lungs were immediately transported to the laboratory on ice. The pulmonary arteries were cleared of surrounding connective tissue and dissected from the middle and upper lobes. The vessels were cut into 5 mm rings and incubated in culture medium (DMEM with 1% antibiotic solution) for 24 hours at 37°C and 5% CO2 with one of the following treatments: 15 μmol/L RTV alone or both NDGA (1.75 or 3.5 μmol/L) and 15 μmol/L RTV together. DMEM with 0.1% dimethylsulfoxide (DMSO) served as a control because RTV and NDGA were dissolved in DMSO.
The myograph tension system used in our laboratory has been previously described [5–8,13]. Briefly, after 24 hours of incubation in culture medium in the treatment groups described above, the vessel rings were suspended between the wires of the organ bath myograph chamber (Danish Myo Technology Organ Bath 700 MO, Aarhus Denmark) in 6 ml Kreb’s solution at 37°C and bubbled with pure oxygen. The rings were subjected to a slow, stepwise increase in tension until they reached 3 mN. After equilibration, each ring was then precontracted with 20 μl U46619 (10−7 mol/L). After 60 minutes of contraction, the relaxation was generated by adding 60 μl of bradykinin at concentrations of 10−10 mol/L for 3 minutes. Finally 60 μl SNP (10−6 mol/L) was added, and endothelium-independent vasorelaxation was recorded.
Cell culture
HPAECs were purchased from Cambrex (San Diego, CA). The cells were used at passage 4 to 5. When cells grew to 80 to 90% confluence in six-well culture plates, they were treated with DMSO (control) or RTV with and without NDGA at the concentrations described above for 24 hours at 37°C. Cells were then applied to studies of eNOS expression and superoxide anion production.
Real-time PCR
The porcine pulmonary artery endothelial cells were collected by scraping the luminal surface of cultured artery rings with surgical blades. HPAECs were collected from cell culture by trypsin digestion. Total cellular RNA was then extracted using an RNAqueous-4PCR kit (Ambion, Austin, TX), and eNOS mRNA levels were detected by real-time PCR. Primers for eNOS were designed via the Beacon Designer 2.1 software (Bio-Rad, Hercules, CA) as previously reported [5]. Relative mRNA levels of eNOS were normalized to CD31 mRNA levels for porcine vessels, and to glyceraldehydes 3-phosphate dehydrogenase (GAPDH) mRNA levels for HPAECs and presented as 2[Ct(CD31 or GAPDH) _ Ct(eNOS)] as previously described [14].
Immunohistochemistry for eNOS
Porcine pulmonary artery rings treated with RTV and/or NDGA were fixed in 10% formalin and embedded in paraffin. Samples were then sectioned at a 5-μm thickness and slides were incubated with monoclonal antibody against human eNOS (1:500) for 30 min at room temperature. After being washed with PBS, the slides were incubated with biotinylated secondary antibody for 30 min. Then, an avidin-biotin reaction using peroxidase enzyme was used for protein detection (ABC kit; Vector Laboratories, Burlingham, CA). Images were captured using microscopy as previously described [15].
ROS measurement
Levels of O2− produced by the endothelial cells of porcine arteries were detected using the lucigenin-enhanced chemiluminescence method previously described in our studies [5,15]. Time based reading with luminometer was recorded. The data were represented in relative light units per second (RLU/second) for each sample. To normalize the data for each sample, the area of each vessel segment was precisely measured using a digital caliper.
Glutathione (GSH) is an intracellular antioxidant, which reversely correlates with cellular ROS levels. HPAECs were treated with either RTV (15 μmol/L) or pretreated with NDGA for 30 min followed by RTV treatment for 45 min. Cellular GSH levels were measured as per manufacture’s instructions by following a GSH-Glo Glutathione assay kit (Promega, Madison, WI).
Statistical analysis
All data are presented as the mean ±SEM. Differences among three or more groups were analyzed using one way analysis of variance. Student’s t-test was used for comparison between two groups. A P value <0.05 was regarded as significant.
Results
NDGA inhibits RTV-induced endothelial dysfunction in porcine pulmonary arteries
The effects of RTV and NDGA on vasomotor functions in porcine pulmonary arteries were determined using a myograph system which included measurement of vascular contraction (induced by thromboxane A2 analog U46619), endothelium-dependent relaxation (bradykinin) and endothelium-independent relaxation (SNP). Porcine pulmonary artery rings (5 per group) were cultured for 24 hours in DMEM with DMSO as a control or treated with a clinically relevant amount of RTV alone (15 μmol/L) or RTV and NDGA (1.75 or 3.50 μmol/L). In response to U46619 (10−7 M), the control vessels contracted maximally to 25 mN. Treatment with RTV reduced contraction by 36% compared with controls; when cultured together with NDGA (1.75 and 3.50 μmol/L), the contraction increased by 21% and 71%, respectively, compared with RTV-alone group (Figure 1A, P<0.05, n=5). Endothelium-dependent vasorelaxation was induced by the addition of brandykinin (10−10 mol/L) to the organ bath, and the control vessels relaxed by 36%, while RTV-treated vessels relaxed by only 22%, which represented a 39% reduction in endothelium-dependent vasorelaxation; when co-cultured with NDGA (1.75 and 3.50 μmol/L), the relaxation increased by 25% and 48%, respectively, over the relaxation achieved by the RTV-treated vessels (Figure 1B, P<0.05, n=5). In response to SNP, treatment with RTV reduced endothelium-independent vasorelaxation by 17.5%; when NDGA was included with the RTV treatment, endothelium-independent relaxation was restored (Figure 1C, P<0.05, n=5). Taken together, these data demonstrate that RTV induces vasomotor dysfunction in porcine pulmonary arteries, and NDGA effectively inhibits it.
Figure 1.
Effects of RTV and NDGA on vasomotor functions in porcine pulmonary arteries. The vessel rings were cultured with DMEM and DMSO (control) or treated with RTV (15 μmol/L) alone or together with NDGA (1.75 or 3.50 μmol/L) for 24 hours. (A) Vessel contraction. Maximal contraction in response to thromboxane A2 analog U46619 (10−7 mol/L). (B) Endothelium-dependent vasorelaxation in response to bradykinin (10−10 mol/L). (C) Vessel relaxation. Endothelium-independent vasorelaxation in response to SNP (10−6 mol/L). Values are means ±SEM, n=5, *P<0.05.
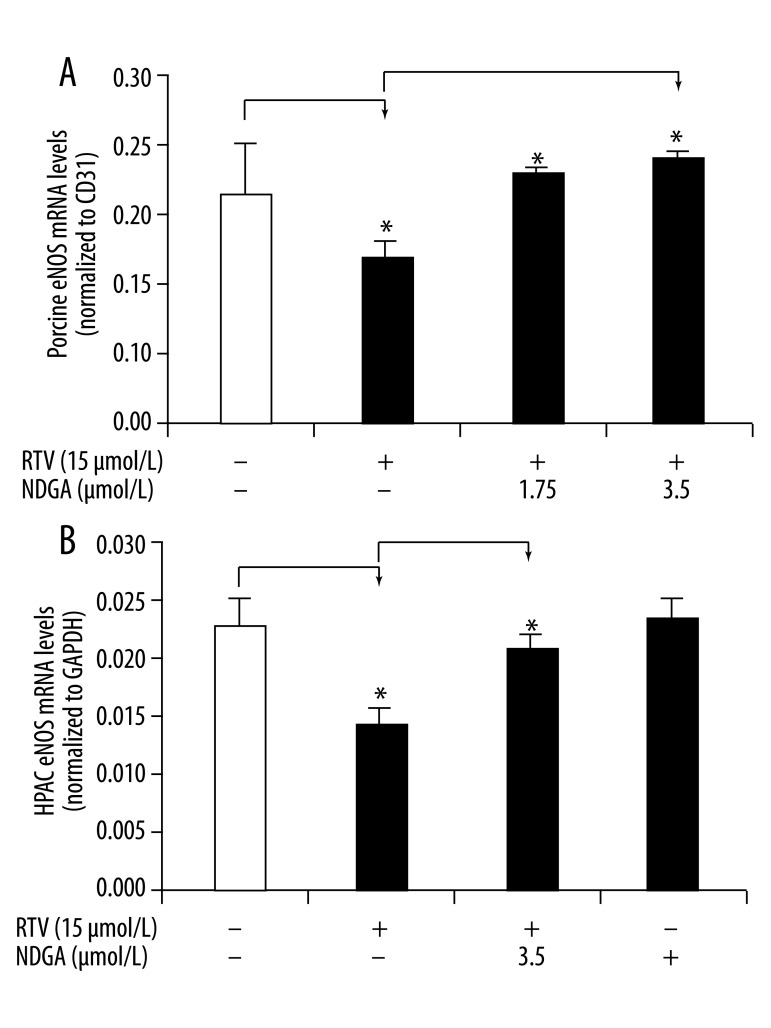
NDGA inhibits the RTV-induced decrease of eNOS expression in porcine pulmonary arteries and HPAECs
In our previous studies, we demonstrated that eNOS protein is involved in RTV-induced vasomotor dysfunction in porcine pulmonary arteries; RTV treatment significantly decreases eNOS expression in a concentration-dependent manner in both porcine pulmonary artery cells and HPAECs [5,6,8]. In this study, we investigated whether NDGA can inhibit RTV-induced reductions in eNOS expression. After treatment with 15 μmol/L of RTV, there was a reduction in eNOS mRNA levels by 21% in arterial rings and by 38.5% in HPAECs compared with their respective DMSO controls. However, after co-treatment with RTV and NDGA (1.75 and 3.50 μmol/L), eNOS mRNA levels in the vessel rings and HPAECs significantly increased compared with RTV only group (Figure 2A, B, P<0.05, n=3).
Figure 2.
Effects of RTV and NDGA on eNOS mRNA levels in porcine pulmonary arteries and HPAECs. The vessel rings or HPAECs were cultured with DMEM and DMSO (control) or treated with RTV (15 μmol/L) alone or together with NDGA (1.75 or 3.50 μmol/L) for 24 hours. eNOS mRNA levels were determined by real-time PCR and normalized to CD31 or GAPDH mRNA. (A) eNOS mRNA levels in porcine artery rings. (B) eNOS mRNA levels in HPAECs. Values are means ±SEM, n=3, * P<0.05.
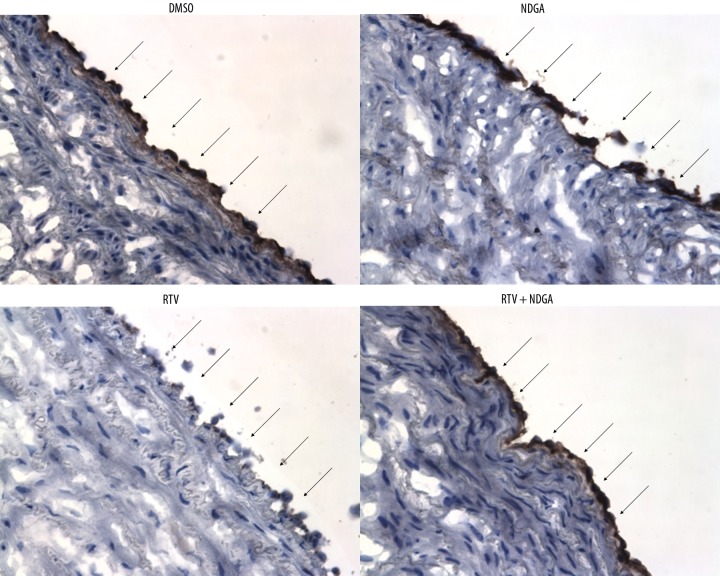
eNOS protein levels in the porcine pulmonary arteries were analyzed using immunohistochemistry staining (Figure 3). Levels of the dark brown-staining eNOS protein were reduced at the luminal endothelial layer in the RTV alone group compared with the DMSO control group. When co-treated with NDGA and RTV, eNOS immunoreactivity was significantly increased to the control level; meanwhile NDGA alone did not alter the eNOS protein level compared with controls. Therefore, NDGA inhibits the RTV-induced decrease of eNOS expression at the protein level in porcine pulmonary arteries.
Figure 3.
Effects of RTV and NDGA on eNOS immunoreactivity in porcine pulmonary arteries. The vessel rings were treated with DMEM and DMSO (control), NDGA (3.5 μmol/L), RTV (15 μmol/L), and RTV (15 μmol/L) together with NDGA (3.50 μmol/L) for 24 hours. The eNOS protein expression was determined by immunohistochemistry. Dark brown color represents positive staining of eNOS at the luminal endothelial layer (arrowheads). Original magnification, ×200.
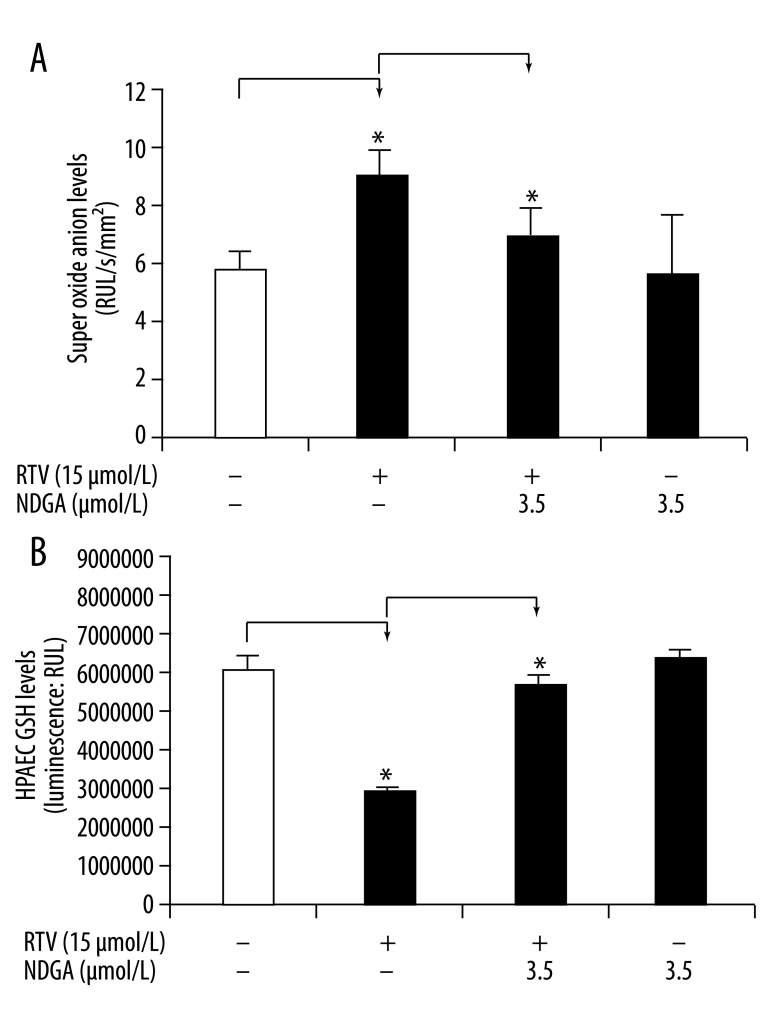
NDGA inhibits RTV-induced ROS production in porcine pulmonary arteries and HPAECs
Oxidative stress has been shown to cause endothelial dysfunction and vascular injury. The HIV protease inhibitor RTV has been shown to significantly increase superoxide anion production in porcine pulmonary arteries [5,6,8]. To investigate whether NDGA can block the superoxide production induced by RTV, superoxide anion production in porcine pulmonary arterial rings and HPAECs was analyzed using lucigenin-enhanced chemiluminescence and GSH-mediated quantification of ROS, respectively. Superoxide anion levels in the arterial rings were significantly increased in response to RTV treatment. RTV (15 μmol/L) treatment of the vessel rings increased the chemiluminescence signal to 8.55±1.32 RLU/sec/mm2, compared with the control value of 5.66±0.66 RLU/sec/mm2 (Figure 4A, P<0.05, n=4). This indicates a 51% increase of superoxide anion production in the RTV-treated rings over controls. Co-treatment with NDGA (3.5 μmol/L) in addition to RTV led to a reduction in the chemiluminescence signal to 6.36±1.07 RLU/sec/mm2, indicating a partial reversal of the effects of RTV to increase ROS.
Figure 4.
Effects of RTV and NDGA on ROS production in porcine pulmonary arteries and HPAECs. The vessel rings or HPAECs were treated with DMEM and DMSO (control), RTV (15 μmol/L), RTV (15 μmol/L) together with NDGA (3.50 μmol/L), or NDGA (3.5 μmol/L) for 24 hours. (A) Superoxide anion levels at the endothelial layer were determined with the lucigenin-enhanced chemiluminescence assay. (B) GSH levels in HPAECs were assayed using a GSH-mediated quantification kit. GHS levels reversely correlate to cellular ROS levels. Values are means ±SEM, n=4, * P<0.05.
Superoxide production was assessed in HPAECs indirectly using a GSH-mediated quantification kit. GSH levels reversely correlate to ROS levels in the cells. RTV treatment led to a significant decrease in GSH levels by 56% in HPAECs compared to controls (Figure 4B, P<0.05, n=4); this indicates that ROS production increased by 56%. As before, this RTV-induced increase in ROS, was blocked by NDGA. In this case, the NDGA alone group showed a higher GSH level than the control group, indicating its capacity to further reduce ROS levels over baseline control levels.
Discussion
The major finding of this study is that the natural herbal antioxidant NDGA can effectively inhibit the reduction in vasocontractility and endothelium-dependent vasorelaxation induced by HIV protease inhibitor RTV in porcine pulmonary arteries. This may be mediated by restoration of eNOS expression, as we found NDGA blocked the RTV-induced decrease of eNOS expression in both porcine pulmonary arteries and HPAECs. Furthermore, NDGA inhibits RTV-induced superoxide anion production in porcine pulmonary arteries and HPAECs. As such, this study suggests that NDGA may be a useful therapy in HIV-infected patients.
The endothelium is considered an active biologic interface between the blood and all other tissues. Functions of the endothelium include control of vascular tone, modulation of inflammation, promotion or inhibition of vascular growth, and regulation of thrombosis. Endothelial dysfunction can be broadly defined as an imbalance between vasodilating and vasoconstricting substances produced by the endothelium [16,17]. This endothelial dysfunction is an important mechanism underlying idiopathic pulmonary arterial hypertension. In addition, it has been seen in a variety of pathological conditions such as atherosclerosis, hypercholesterolemia, diabetes, hypertension, heart failure, cigarette smoking and aging. It is characterized by decreased bioavailability of NO, which may be due to enhance NO catabolism secondary to increased superoxide anion production or reduced expression and/or activity of eNOS [9,10].
In this study, we used a clinically relevant concentration of RTV (15 μmol/L) and a well-defined myograph tension system to test our hypothesis that the natural herbal antioxidant NDGA can protect vasomotor function against oxidative damage in porcine pulmonary arteries. Our data demonstrate that RTV significantly decreases endothelium-dependent vasorelaxation in response to bradykinin in porcine pulmonary arteries, as expected. In addition, endothelium-independent vasorelaxation was reduced by RTV. These changes indicate that RTV has direct effects on the vascular endothelium and are consistent with several clinical studies [18]. Meanwhile, when the pulmonary artery rings were cultured with NDGA (1.75 or 3.50 μmol/L) in addition to RTV, there was a significant reversal of the detrimental effects of RTV on vasomotor reactivity in a concentration-dependent manner. In other studies, natural antioxidants such as ginsenosides, ginkgo [5], soybean isoflavonoid equol [19], capsaicin [6], and dihydroxybenzyl alcohol [8] have been shown to block RTV-induced endothelial dysfunction. Like these antioxidants, NDGA showed a strong effect against RTV-induced vasomotor dysfunction.
In the endothelium, NO is constitutively generated from the conversion of L-arginine to L-citrulline by the enzymatic action of eNOS. This NO is critical in the maintenance of vascular tone. However, eNOS may become dysfunctional or its expression may decrease under various pathological conditions [20]. Dysfunctional eNOS or low levels of eNOS reduce NO availability, which results in impaired endothelium-dependent vasorelaxation and accelerated vascular lesion formation [21]; the development of pulmonary arterial hypertension is likewise promoted. In the current study, real-time PCR analysis showed that there was reduced eNOS mRNA expression in the RTV-treated porcine pulmonary arteries and cultured HPAECs, and immunohistochemistry showed a significant decrease of the eNOS protein levels in the endothelium of RTV treated vessels. However, NDGA effectively blocked these effects of RTV on downregulating eNOS at both the mRNA and protein levels, suggesting a potential therapeutic role for NDGA.
In a previous paper, it was reported that the two HAART drugs RTV and AZT substantially increased the phosphorylation of ERK2 in HPAECs, and a MEK/ERK inhibitor effectively blocked RTV- or AZT-induced reductions in eNOS protein levels in HPAECs; this indicated that MAPK signaling is involved in the development of endothelial dysfunction induced by HAART drugs [5]. In this study, we found that NDGA reversed the decrease of eNOS protein level; this may be due to inhibition of the ERK 1/2 activation induced by RTV. NDGA is a general lipoxygenase (LOX) enzyme inhibitor. Of note, it has been reported that treatment with NDGA reduces ERK 1/2 phosphorylation in 15-LOX-1 overexpressing HCT-116 cells [22].
Oxidative stress plays a critical role in pulmonary arterial hypertension. Our previous studies have demonstrated that some HAART drugs including RTV significantly increase superoxide anion production in porcine pulmonary arteries and HPAECs [5,6,8]. Increased ROS contribute to endothelial dysfunction and vascular pathology. Superoxide can influence NO bioavailability by binding to NO to form the highly reactive intermediate ONOO−. The speed of this reaction is about 10 times faster than the dismutation of superoxide by the superoxide dismutase [23]. Thus, excessive amount of superoxide may substantially limit NO bioavailability and its effects. Furthermore, increased superoxide and ONOO− may impair the function of eNOS by reducing the bioavailability of its coenzyme tetrahydrobiopterin. ONOO− may also influence the synthesis of other endothelial mediators, such as the enzyme prostacyclin synthase, therefore leading to reduced production of vasodilator, anti-aggregant and anti-inflammatory prostaglandins [24]. Our study demonstrates that NDGA also effectively abolishes the oxidative stress induced by RTV in both porcine pulmonary arteries and HPAECs; NDGA either blocks the superoxide anion production or directly scavenges superoxide anion. The latter is more likely, considering the NDGA alone treatment group led to a higher GSH level than the control group, indicating a lower superoxide anion level. Also, it has been reported that NDGA is a strong superoxide anion scavenger by Floriano-Sanchez [12].
Conclusions
In summary, the natural herbal compound NDGA could potentially have a protective role against RTV-induced vascular dysfunction. NDGA effectively inhibits the detrimental effects of HIV protease inhibitor RTV on the vasomotor functions of endothelial and smooth muscle cells in porcine pulmonary arteries. NDGA also blocks the decrease in eNOS expression and the increase in superoxide anion production induced by RTV. These findings suggest that NDGA may have clinical applications in the prevention of systemic and pulmonary vascular complications associated with the long term use of HAART drugs in HIV patients.
Acknowledgements
Authors would like to thank Dr. Xinwen Wang for his technique assistance. We thank the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing ritonavir (RTV).
Footnotes
Source of support: This work is partially supported by a research grant from the National Institutes of Health (R01 HL083471 to C.C.). S.M.W. was supported by a training grant from NIH (T32HL083774)
References
- 1.Cotter BR. Endothelial dysfunction in HIV infection. Curr HIV/AIDS Rep. 2006;3:126–31. doi: 10.1007/BF02696656. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–99. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 3.Rudich A, Ben-Romano R, Etzion S, Bashan N. Cellular mechanisms of insulin resistance, lipodystrophy and atherosclerosis induced by HIV protease inhibitors. Acta Physiol Scand. 2005;183:75–88. doi: 10.1111/j.1365-201X.2004.01383.x. [DOI] [PubMed] [Google Scholar]
- 4.Barbaro G. HIV infection, highly active antiretroviral therapy and the cardiovascular system. Cardiovasc Res. 2003;60:87–95. doi: 10.1016/s0008-6363(02)00828-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Chai H, Lin PH, et al. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–81. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhadwal AK, Wang X, Annambhotla S, et al. Capsaicin blocks HIV protease inhibitor ritonavir-induced vascular dysfunction in porcine pulmonary arteries. Med Sci Monit. 2009;15(1):BR1–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Bismuth J, Chai H, Lin PH, et al. Lactosylceramide causes endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Med Sci Monit. 2009;15(9):BR270–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Weakley SM, Jiang J, Lu J, et al. Natural antioxidant dihydroxybenzyl alcohol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human endothelial cells. Med Sci Monit. 2011;17(9):BR235–41. doi: 10.12659/MSM.881926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–78. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Lu JM, Nurko J, Weakley SM, et al. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Med Sci Monit. 2010;16(5):RA93–100. [PMC free article] [PubMed] [Google Scholar]
- 12.Floriano-Sanchez E, Villanueva C, Medina-Campos ON, et al. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic Res. 2006;40:523–33. doi: 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Chai H, Courson A, et al. Ginkgolide A attenuates homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2006;44:853–62. doi: 10.1016/j.jvs.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Mu H, Chai H, et al. Human immunodeficiency virus protease inhibitor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am J Pathol. 2007;171:304–14. doi: 10.2353/ajpath.2007.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy P, Wang X, Lin PH, et al. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res. 2009;156:257–64. doi: 10.1016/j.jss.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Limsukon A, Saeed AI, Ramasamy V, et al. HIV-related pulmonary hypertension. Mt Sinai J Med. 2006;73:1037–44. [PubMed] [Google Scholar]
- 18.Cotter BR, Torriani FJ. Cardiovascular effects of antiretroviral therapy and noninvasive assessments of cardiovascular disease in HIV infection. Cardiovasc Toxicol. 2004;4:281–86. doi: 10.1385/ct:4:3:281. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C, Wang X, Weakley SM, et al. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Nutr. 140:12–17. doi: 10.3945/jn.109.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 21.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–29. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga M, Buchanan FG, DuBois RN. 15-LOX-1 inhibits p21 (Cip/WAF 1) expression by enhancing MEK-ERK 1/2 signaling in colon carcinoma cells. Prostaglandins Other Lipid Mediat. 2004;73:111–22. doi: 10.1016/j.prostaglandins.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Lu JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–60. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munzel T, Sinning C, Post F, et al. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–96. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]