Abstract
Gammaherpesviruses such as Epstein-Barr virus (EBV, human herpesvirus 4) and Kaposi sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) establish lifelong infection in the host. To further this lifestyle, they encode homologs of cellular cytokines and cytokine receptors with the overarching goal to escape from or to blunt host antiviral defenses. EBV encodes mimics of human interleukin (hIL)-10 and a G protein-coupled receptor protein with sequence similarity to CXCR, whereas KSHV encodes homologs of hIL-6, 3 CC chemokine ligands, and a G protein-coupled receptor with sequence similarity to IL8 receptor alpha. This review focuses on the EBV IL-10 homolog and the KSHV IL-6 homolog with respect to virus biology and pathogenesis of the virus-associated diseases.
Introduction
The host keeps guard against pathogens by deploying innate and adaptive immune responses. The innate immune system defends the host by impeding viral replication and activating the adaptive antiviral responses, and the adaptive immune response provides the host with the ability to neutralize virus particles and to remember a specific virus attack. For successful replication and transmission, viruses must subvert, blunt, or escape host antiviral activities. To accomplish this goal, viruses evolved immune evasion strategies, which include viral mimics of host cytokines and/or cytokines receptors. These strategies are essential for viruses that establish a lifelong presence in the host. This is termed persistent infection if there is consistent evidence of circulating virus or viral shedding of the same strain in the absence of reinfection, and latent infection if the virus can only be intermittently detected in blood, lymph, or body secretions.
Gammaherpesvirus infections are prime examples of latent pathogen infections. The human gammaherpesviruses include Epstein-Barr virus (EBV, also known as human herpesvirus 4) and Kaposi sarcoma-associated herpesvirus (KSHV, or human herpesvirus 8). These are linked to human cancers of the immune compartment. EBV is associated with the development of endemic Burkitt's lymphoma, classic Hodgkin lymphoma, lymphoepithelioma-like nasopharyngeal carcinoma, and a certain subtype of gastric adenocarcinoma (IARC 2010). KSHV is associated with Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and the plasmablastic variant of multicentric Castleman disease (MCD) and in some instances diffuse large B-cell lymphoma (Carbone and others 2009). Both viruses have a large double-stranded DNA genome (∼172 kb for EBV and ∼145 kb for KSHV) and encode over 80 open reading frames (ORFs). Many of the proteins, which are encoded by these ORFs, are highly immunogenic, particularly if present in the virions. Virions are produced during the lytic (productive) cycle, whereas only a very limited set of proteins is translated during the latent infection phase.
EBV encodes 3 host cytokine or chemokine receptor mimics. They are an interleukin (IL)-10 homolog encoded by BamHI-C fragment rightward reading frame 1 (BCRF1) (Moore and others 1990), a CXCR homolog encoded by BILF1 (Paulsen and others 2005), and EBV-induced gene 3 (EBI3), an IL-12p40-related protein, which forms heterodimeric IL-27 with p28 (IL-30), which is itself an IL-12p35–related polypeptide (Pflanz and others 2002).
KSHV encodes an IL-6 homolog (Moore and others 1996; Neipel and others 1997; Nicholas and others 1997), an IL-8 receptor alpha homolog (ORF74) (Cesarman and others 1996; Bais and others 1998), and 3 CC-chemokine ligands (Moore and others 1996; Boshoff and others 1997; Sozzani and others 1998; Dairaghi and others 1999; Stine and others 2000). Other immune evasion strategies by gammaherpesviruses have been reviewed elsewhere (Stevenson 2004; Nicholas 2005; Coscoy 2007; Liang and others 2008; Blake 2010; Lee and others 2010; Rowe and Zuo 2010). In this review, we focus on roles of viral IL (vIL)-6 and vIL-10 with respect to the pathobiology of gammaherpesviruses.
EBV IL-10 Homolog
IL-10 was originally reported as cytokine synthesis inhibitory factor (CSIF) produced by T helper 2 (TH2) cells, which inhibited TH1-derived gamma interferon (IFN-γ) production (Fiorentino and others 1989). It is now known that IL-10 is not just TH2-specific, but expressed by many different kinds of immune cells including TH1, TH17, TReg, B cells, macrophages, and myeloid dendritic cells, indicating its role in controlling diverse immune responses. In addition to IFN-γ, IL-10 inhibits the expression of IL-1α, IL-1β, IL-6, IL-12, IL-18, granulocyte/macrophage colony stimulating factor, tissue necrosis factor, and others. Human IL-10 (hIL-10) has immunosuppressive properties as well as immunostimulatory properties. On the one hand, it functions to deactivate macrophages (Bogdan and others 1991) and it inhibits antigen-specific T-cell proliferation by interfering with the monocytes' antigen-presenting capacity via downregulation of class II major histocompatibility complex (MHC II) expression (de Waal Malefyt and others 1991). On the other hand, hIL-10 cooperates with transforming growth factor β to stimulate anti-CD40-activated naive human B cells to secret immunoglobulin A (Defrance and others 1992).
The EBV BCRF1 gene product (vIL-10) shares 70% and 80% amino acid sequence identity with mouse and hIL-10/CSIF, respectively (Moore and others 1990; Vieira and others 1991). The vIL-10 promoter is highly methylated and inactive in latently infected B cells (Niller and others 2001) and the vIL-10 protein is expressed late during the lytic life cycle (Hudson and others 1985). Yet, the vIL-10 is expressed also within the first 6–9 h after infection of human B cells (Miyazaki and others 1993; Touitou and others 1996). This divergent regulation represents a common strategy of both the KSHV and the EBV IL homologs. Even though the vILs as well as the viral chemokine receptor homologs are typically classified as “lytic” cycle viral genes, they are also transcribed in response to specific host cell signaling events outside the rigid framework of the canonical transcriptional cascade that governs herpesvirus lytic replication (Chatterjee and others 2002). Thus, one might speculate that they have a purpose in latent persistence in addition to their role in support of viral replication and progeny production.
The EBV vIL-10 inhibits IFN-γ synthesis by T-cell-dependent antigen-stimulated mouse Th1 cells and phytohemagglutinin-activated human peripheral blood mononuclear cells (PBMCs) analogous to hIL-10 (Hsu and others 1990; Moore and others 1990). The inhibition of IFN-γ from IL-2-activated PBMCs suggests that the vIL-10 may also act on natural killer cells (NK), which are the major source of IL-2 in these assays (Hsu and others 1990). NK and cytotoxic T-cell (CTL) activity was reduced in mice infected with a recombinant vaccinia virus expressing the vIL-10 (Kurilla and others 1993). The EBV vIL-10 cooperates with hIL-10 to downregulate the peptide transporter protein (TAP1), which in turn limits antigen presentation mediated by MHC I molecules. This may influence the CTL's role in recognition of EBV-infected B cells (Zeidler and others 1997).
Both hIL-10 and vIL-10 enhance proliferation and differentiation of EBV-negative, normal, mature B cells, but vIL-10 does not upregulate MHC II expression (Go and others 1990; Rousset and others 1992). Human and mouse IL-10 stimulate the proliferation of mature and immature T cells, whereas their EBV counterpart does not (MacNeil and others 1990). This is probably due to its lower affinity for the IL-10 receptor α (IL-10Rα). A related line of inquiry demonstrated that the EBV vIL-10 had 1,000-fold lower affinity for the IL-10R and this resulted in a much reduced ability to inhibit IL-2 secretion from CD4+ T cells (Liu and others 1997). Thus emerges a picture wherein the EBV vIL-10 displays all the inhibitory phenotypes of hIL-10, but is severely impaired with regard to its pro-proliferative capabilities (Fig. 1).
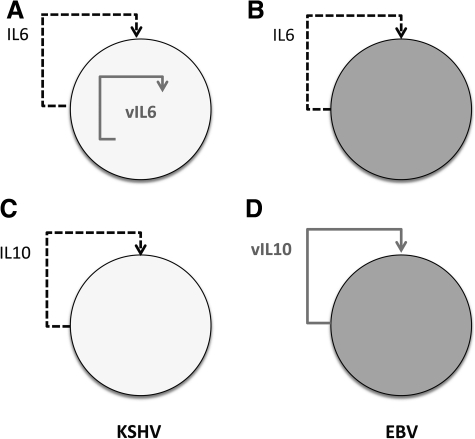
FIG. 1.
Mimicry by viral interleukins. (A) KSHV-encoded vIL-6 activates IL-6R on infected B cells mostly from within the cell (intracrine stimulation). (B) In case of EBV, which encodes no IL-6 homolog, the human IL-6 is induced and required for B-cell proliferation (autocrine and paracrine stimulation). (C) KSHV induces IL-10 as an autocrine effector of infected B cells and perhaps to modulate T-cell responses. (D) EBV encodes its own vIL-10, but with specific defects compared with its host counterpart. KSHV, Kaposi sarcoma-associated herpesvirus; EBV, Epstein-Barr virus; IL, interleukin; vIL, viral IL; IL-6R, IL-6 receptor.
A possible explanation for the lack of pro-proliferative activity toward T cells is a single amino acid change at position 87. An isoleucine is required at position 87 for hIL-10 immunostimulatory functions. When this residue was changed into alanine, the immunostimulatory activity of hIL-10 was destroyed (Ding and others 2000). The EBV vIL-10 has an alanine at the corresponding position in vIL-10. The crystal structure of vIL-10 reveals that two 17-kDa polypeptides form a homodimer-like hIL-10, but with subtle changes in conformation. The resulting orientation change of vIL-10 on a soluble IL-10R1 fragment (sIL-10R1) may cause the reduced affinity of vIL-10 for sIL-10R1 (Zdanov and others 1997; Yoon and others 2005).
The lack of immunostimulatory potency has prompted explorations of vIL-10 as a possible therapeutic for autoimmune diseases or chronic inflammation. In animal models, adenoviral-mediated transfer of vIL-10 was effective in inhibiting collagen-induced arthritis (Apparailly and others 1998; Lehrman and others 2005; Keravala and others 2006), ameliorating symptoms of autoimmune ocular diseases (De Kozak and others 2002; Verwaerde and others 2003; Zhu and others 2004), and suppressing autoimmune diabetes via inhibition of TH1 cell activation (Kawamoto and others 2001; Yang and others 2002b). The EBV IL-10 homolog also reduced IL-1 levels in a particle-associated inflammation mouse air pouch model (Yang and others 2002a) and suppressed crescentic glomerulonephritis in a rat model (Higuchi and others 2003). Because of the lack of immunostimulatory functions, vIL-10 had advantages over hIL-10 in transplantation models including a rat kidney transplantation model, a mouse cardiac allograft model, and a mouse hematopoietic stem cell therapy (Qin and others 1996; Salgar and others 2004; Chen and others 2007).
How the EBV IL-10 homolog contributes to EBV-transformed B-cell proliferation remains to be determined. On the one hand, lymphoblastoid cell lines, which are infected with EBV recombinants devoid of vIL-10, show no difference in latent infection, virus replication, and tumorigenicity in SCID mice (Swaminathan and others 1993). On the other hand, oligonucleotides against vIL-10 mRNA inhibit aspects of EBV-dependent B-cell transformation (Miyazaki and others 1993), and the addition of exogenous vIL-10 enhances growth transformation of B cells by EBV (Stuart and others 1995) as well as the initial immortalization of EBV-infected B lymphocytes, which is dependent on vIL-10 (Irons and Le 2008).
KSHV IL-6 Homolog
IL-6 is a cytokine with pro- and anti-inflammatory functions. It was originally reported as a B-cell stimulating and differentiating factor secreted by T cells, which induces terminal maturation into plasma cells (Okada and others 1983; Hirano and others 1986; Muraguchi and others 1988). It is now known that IL-6 is not only secreted by T-cells, but also by a variety of other cells including macrophages, fibroblasts, synovial cells, endothelial cells, glial cells, and keratinocytes. IL-6 folds into a 4-helix bundle, which is the common motif for helical cytokines (Bazan 1990). The IL-6 family of cytokines comprises IL-11, oncostatin M, and leukemia inhibitory factor in addition to IL-6. Cytokines of the IL-6 family act via receptor complexes containing a signal-transducing protein, gp130. hIL-6 binds to 2 domains of gp130 and a specific α subunit of IL-6R (IL-6Rα/gp80) to initiate intracellular signal transduction (Grotzinger and others 1997). Dysregulated expression of IL-6 is involved in inflammation and hematopoietic malignancies, such as cardiac myxoma, Castleman disease (CD), rheumatoid arthritis (RA), and systemic-onset juvenile idiopathic arthritis (SoJIA). Therapeutic approaches targeted against IL-6 and/or IL-6R complexes are in clinical testing (eg, humanized anti-IL6: Sirukumab, ALD518, and others). A humanized anti-IL-6R antibody, tocilizumab, has shown efficacy against immune-mediated inflammatory diseases such as RA, SoJIA, systemic lupus erythematous, adult-onset Still disease, Takayasu arthritis, and systemic sclerosis as well as MCD (Murakami and Nishimoto 2011).
The KSHV IL-6 homolog (vIL-6) shows only 24.8% amino acid identity to hIL-6 (Moore and others 1996; Neipel and others 1997; Nicholas and others 1997). However, its crystal structure follows the canonical 4-helix bundle fold (Chow and others 2001; Boulanger and others 2003). The vIL-6 is an early lytic gene; however, the vIL-6 can also respond to cell signaling events directly and discordantly from other viral lytic genes, suggesting vIL-6's roles in survival of KSHV-infected B cells (Chatterjee and others 2002; Chang and others 2005; Chandriani and Ganem 2010). In MCD, the vIL-6 is constitutively expressed in virally infected B cells and can thus be considered a latent gene in this population. Host IFN-α signaling is inhibited by vIL-6. However, IFN-α itself can activate vIL-6 through an IFN-stimulated response element in the vIL-6 promoter. Subsequently, the expression of vIL-6 acts in a negative feedback loop to block IFN-α antiviral signaling (Chatterjee and others 2002).
Analogous to its cellular counterpart, vIL-6 functions to transduce signals via gp130 (Molden and others 1997) and activates CCAAT/enhancer-binding protein transcription factors, Janus kinase/signal transducer and activator of transcription, and mitogen-activated protein kinase signaling pathways (Osborne and others 1999; Hideshima and others 2000).
The first important difference between hIL-6 and vIL-6 is that vIL-6 requires only gp130 for signaling. This is unlike hIL-6, which needs the high-affinity coreceptor IL-6R (gp80) in complex with gp130 (Molden and others 1997; Chow and others 2001). The crystal structure of the vIL-6 signaling complex shows the vIL-6 dimer forming a tetramer with gp130 (vIL-62:gp1302) (Chow and others 2001). This led to advanced structural and functional studies (Li and others 2001; Li and Nicholas 2002; Boulanger and others 2004; Dela Cruz and others 2004, 2009; Chen and Nicholas 2006; Hu and Nicholas 2006). The replacement of hIL-6 with the corresponding residues of the gp130 contact site III and BC loop of vIL-6 confers gp80 independence to the hIL-6 (Adam and others 2009). Based on these observations, one can speculate that the conformational change between hIL-6 and vIL-6 is a key factor in determining the gp80-independent binding of the vIL-6 to gp130. In fact, vIL-6 may exist in multiple signaling complexes, a gp80-independent tetramer (vIL-62:gp1302) and gp80-dependent hexamer (gp1302:gp802:vIL-62).
The second important difference between hIL-6 and vIL-6 is that most vIL-6 is retained in endoplasmic reticulum (ER), implying autocrine stimulation as the dominant mode of action (Chen and others 2009b, Fig. 1). An ER chaperone protein, calnexin, interacts with vIL-6 to mediate proper protein folding (Chen and others 2009a). This is in contrast to hIL-6, which is quickly secreted (Rose-John and others 1993).
These 2 biochemical differences translate into functional and biological differences. The vIL-6 stimulates intracellular signaling in human B cells, which are IL-6R (gp80) negative and respond poorly or not at all to hIL-6 (Breen and others 2001). In the presence of gp80, the vIL-6 can support growth of IL-6/IL-3-dependent gp80−/gp130+ BAF-130 cells better than its human counterpart (Hu and Nicholas 2006).
The KSHV vIL-6 augments growth and survival of PEL cell lines (Jones and others 1999; Chatterjee and others 2002) and increases tumorigenicity in athymic nude mice (Aoki and others 1999). The vIL-6 is also important for PEL tumorigenesis, because it induces VEGF-1, a paracrine factor that has been implicated in the pathogenesis of PEL and KS (Aoki and others 1999; Aoki and Tosato 1999; Jones and others 1999). Thus, it contributes to the transforming potency of KSHV. Neutralizing antibodies against vIL-6, IL-6R, or gp130 reduced the growth of some PEL cell lines (Drexler and others 1999; Jones and others 1999), and a genetic knockdown of vIL-6 expression using short hairpin RNA (shRNA) or antisense peptide-conjugated oligomers leads to the reduced growth of PEL (Zhang and others 2008; Chen and others 2009b). Conversely, exogenously supplied hIL-6 (or hIL-10) is able to counteract the rapamycin-induced growth arrest in PEL (Sin and others 2007). In other tissue culture models, IL-6 may not be required for the KSHV lifecycle, as a KSHV isolate devoid of vIL-6 showed no significant difference in establishment, maintenance, and reactivation from latency in transformed B cells (Chen and Lagunoff 2007).
We speculate that vIL-6 (and the induction of hIL6) contributes to the initial steps in B-cell transformation toward the hyperplastic KSHV-associated B-cell disease, MCD, and the neoplastic KSHV-associated lymphoma PEL. For MCD, the dependence on IL-6 signaling persists, whereas some PEL eventually develop IL-6 independence. Such a model would be akin to gammaherpesvirus Saimiri-associated T-cell transformation. Here, transformed T-cell clones initially require IL-2 for survival, but over time evolve IL-2 independence.
A homolog of IL-6 was identified in rhesus rhadinovirus (RRV), which is closely related to KSHV. The RRV homolog of IL-6 (RvIL-6) shows 35.6% and 27.4% amino acid sequence similarity to rhesus IL-6 and KSHV vIL-6, respectively. One can expect that the RvIL-6 also adopts the 4-helix bundle fold, although the crystal structure of RvIL-6 has not been solved. The RvIL-6 supports B-cell growth, and antibodies against RvIL-6 neutralize the proliferating activity of the RvIL-6 (Kaleeba and others 1999). RvIL-6 is associated with lymphoproliferative disorder in rhesus macaques (Orzechowska and others 2009). Thus, all IL-6 functions seem to be conserved among primates and primate viruses.
CD is a B-cell lymphoproliferative disorder characterized by lymph node hyperplasia, plasma cell infiltration between the lymphoid follicles, and hypergammaglobulinemia. The main symptoms include fever, anemia, weight loss, and loss of appetite. Elevated expression of host IL-6 has been implicated in disease progression (Yoshizaki and others 1989; Leger-Ravet and others 1991; Mandler and others 1992; Hsu and others 1993). Unicentric CD is localized to a single site, whereas the multicentric form is characterized by lymphadenopathic presentation and systemic symptoms in more than 1 site of the body. The multicentric form of CD is strongly associated with KSHV (Soulier and others 1995). Treatment option for the unicentric form is surgical removal, but there is no standard therapy for the multicentric form. Consistent with the biology of IL-6 blockade of IL-6 signaling by an anti-IL-6R antibody is effective in alleviating MCD symptoms such as fatigue, fever, anemia, hypergammaglobulinemia, and lymphadenopathy (Nishimoto and others 2000; Song and others 2010).
Conclusions
EBV and KSHV are 2 human gammaherpesviruses. They establish long-term latency in B cells and induce hematologic malignancies. In this review, we have discussed functions and activities of EBV's IL-10 homolog and KSHV's IL-6 homolog. These viral proteins elicit many of the same phenotypes as their human counterparts and therefore can be thought of as providing functionality in virally infected cell types that do not normally express the corresponding host IL. However, these 2 viral homologs do more than merely filling in for their host counterparts. EBV vIL-10 has lost the immunostimulatory properties of hIL-10. With regard to this phenotype, it represents a hypomorphic variant. How this would benefit the virus is at present unclear, but one could utilize vIL-10 in chronic inflammation and autoimmune diseases. KSHV IL-6 can signal independent of the IL-6 high-affinity receptor gp80. With regard to this phenotype, it represents a hypermorphic variant and potential oncogene. Therapeutic strategies targeting vIL-6 using humanized anticytokine antibodies or soluble decoy receptors could be as effective against PEL and MCD as strategies targeting the hIL-6 (or IL-6R), were it not for the observation that the majority of KSHV vIL-6 does not leave the ER and thus may be more difficult to target.
Acknowledgments
The authors apologize for not citing all the literature on this topic because of space limitations. The authors thank Dr. B. Damania for critical reading and members of the Dittmer and Damania labs for helpful discussions. D.P.D. was supported by funding from the Lymphoma and Leukemia Society (LLS), the National Institutes of Health (NIH), and the University Cancer Research Fund (UCRF).
Author Disclosure Statement
No competing financial interests exist.
References
- Adam N. Rabe B. Suthaus J. Grotzinger J. Rose-John S. Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117–5126. doi: 10.1128/JVI.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y. Jaffe ES. Chang Y. Jones K. Teruya-Feldstein J. Moore PS. Tosato G. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93(12):4034–4043. [PubMed] [Google Scholar]
- Aoki Y. Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94(12):4247–4254. [PubMed] [Google Scholar]
- Apparailly F. Verwaerde C. Jacquet C. Auriault C. Sany J. Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160(11):5213–5220. [PubMed] [Google Scholar]
- Bais C. Santomasso B. Coso O. Arvanitakis L. Raaka EG. Gutkind JS. Asch AS. Cesarman E. Gerhengorn MC. Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Bazan JF. Haemopoietic receptors and helical cytokines. Immunol Today. 1990;11:350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Blake N. Immune evasion by gammaherpesvirus genome maintenance proteins. J Gen Virol. 2010;91(4):829–846. doi: 10.1099/vir.0.018242-0. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Vodovotz Y. Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C. Endo Y. Collins PD. Takeuchi Y. Reeves JD. Schweickart VL. Siani MA. Sasaki T. Williams TJ. Gray PW. Moore PS. Chang Y. Weiss RA. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ. Chow D-c. Brevnova E. Martick M. Sandford G. Nicholas J. Garcia KC. Molecular mechanisms for viral mimicry of a human cytokine: activation of gp130 by HHV-8 interleukin-6. J Mol Biol. 2004;335(2):641–654. doi: 10.1016/j.jmb.2003.10.070. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ. Chow D-c. Brevnova EE. Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science. 2003;300(5628):2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- Breen EC. Gage JR. Guo B. Magpantay L. Narazaki M. Kishimoto T. Miles S. Martínez-Maza O. Viral interleukin 6 stimulates human peripheral blood B cells that are unresponsive to human interleukin 6. Cell Immunol. 2001;212(2):118–125. doi: 10.1006/cimm.2001.1852. [DOI] [PubMed] [Google Scholar]
- Carbone A. Cesarman E. Spina M. Gloghini A. Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113(6):1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- Cesarman E. Nador R. Bai F. Bohenzky R. Russo J. Moore P. Chang Y. Knowles D. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70(11):8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandriani S. Ganem D. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi's sarcoma-associated herpesvirus. J Virol. 2010;84(11):5565–5573. doi: 10.1128/JVI.02723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Dittmer DP. Shin YC. Hong Y. Jung JU. Role of Notch signal transduction in Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 2005;79(22):14371–14382. doi: 10.1128/JVI.79.22.14371-14382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M. Osborne J. Bestetti G. Chang Y. Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298(5597):1432–1435. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- Chen B. Kapturczak MH. Joseph R. George JF. Campbell-Thompson M. Wasserfall CH. Atkinson MA. Tisher CC. Flotte TR. Agarwal A. Chen S. Adeno-associated viral vector-mediated interleukin-10 prolongs allograft survival in a rat kidney transplantation model. Am J Transplant. 2007;7(5):1112–1120. doi: 10.1111/j.1600-6143.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Chen D. Choi YB. Sandford G. Nicholas J. Determinants of secretion and intracellular localization of human herpesvirus 8 interleukin-6. J Virol. 2009a;83(13):6874–6882. doi: 10.1128/JVI.02625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Nicholas J. Structural requirements for gp80 independence of human herpesvirus 8 interleukin-6 (vIL-6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL-6. J Virol. 2006;80(19):9811–9821. doi: 10.1128/JVI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Sandford G. Nicholas J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J Virol. 2009b;83(2):722–733. doi: 10.1128/JVI.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Lagunoff M. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology. 2007;359(2):425–435. doi: 10.1016/j.virol.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D-c. He X-l. Snow AL. Rose-John S. Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291(5511):2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- Coscoy L. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat Rev Immunol. 2007;7(5):391–401. doi: 10.1038/nri2076. [DOI] [PubMed] [Google Scholar]
- Dairaghi DJ. Fan RA. McMaster BE. Hanley MR. Schall TJ. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. J Biol Chem. 1999;274(31):21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- De Kozak Y. Thillaye-Goldenberg B. Naud MC. Da Costa AV. Auriault C. Verwaerde C. Inhibition of experimental autoimmune uveoretinitis by systemic and subconjunctival adenovirus-mediated transfer of the viral IL-10 gene. Clin Exp Immunol. 2002;130(2):212–223. doi: 10.1046/j.1365-2249.2002.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R. Haanen J. Spits H. Roncarolo MG. te Velde A. Figdor C. Johnson K. Kastelein R. Yssel H. de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T. Vanbervliet B. Brière F. Durand I. Rousset F. Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz CS. Lee Y. Viswanathan SR. El-Guindy AS. Gerlach J. Nikiforow S. Shedd D. Gradoville L. Miller G. N-linked glycosylation is required for optimal function of Kaposi's sarcoma herpesvirus–encoded, but not cellular, interleukin 6. J Exp Med. 2004;199(4):503–514. doi: 10.1084/jem.20031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz CS. Viswanathan SR. El-Guindy AS. Shedd D. Miller G. Complex N-linked glycans on Asn-89 of Kaposi sarcoma herpes virus-encoded interleukin-6 mediate optimal function by affecting cytokine protein conformation. J Biol Chem. 2009;284(43):29269–29282. doi: 10.1074/jbc.M109.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y. Qin L. Kotenko SV. Pestka S. Bromberg JS. A single amino acid determines the immunostimulatory activity of interleukin 10. J Exp Med. 2000;191(2):213–224. doi: 10.1084/jem.191.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler HG. Meyer C. Gaidano G. Carbone A. Constitutive cytokine production by primary effusion (body cavity-based) lymphoma-derived cell lines. Leukemia. 1999;13:634–640. doi: 10.1038/sj.leu.2401371. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF. Bond MW. Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go NF. Castle BE. Barrett R. Kastelein R. Dang W. Mosmann TR. Moore KW. Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger J. Kurapkat G. Wollmer A. Kalai M. Rose-John S. The family of the IL-6-Type cytokines: Specificity and promiscuity of the receptor complexes. Proteins Struct Funct Bioinform. 1997;27(1):96–109. [PubMed] [Google Scholar]
- Hideshima T. Chauhan D. Teoh G. Raje N. Treon SP. Tai Y-T. Shima Y. Anderson KC. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi's sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res. 2000;6(3):1180–1189. [PubMed] [Google Scholar]
- Higuchi N. Maruyama H. Kuroda T. Kameda S. Iino N. Kawachi H. Nishikawa Y. Hanawa H. Tahara H. Miyazaki J. Gejyo F. Hydrodynamics-based delivery of the viral interleukin-10 gene suppresses experimental crescentic glomerulonephritis in Wistar-Kyoto rats. Gene Ther. 2003;10(16):1297–1310. doi: 10.1038/sj.gt.3301988. [DOI] [PubMed] [Google Scholar]
- Hirano T. Yasukawa K. Harada H. Taga T. Watanabe Y. Matsuda T. Kashiwamura S-i. Nakajima K. Koyama K. Iwamatsu A. Tsunasawa S. Sakiyama F. Matsui H. Takahara Y. Taniguchi T. Kishimoto T. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hsu D. de Waal Malefyt R. Fiorentino D. Dang M. Vieira P. de Vries J. Spits H. Mosmann T. Moore K. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Hsu S-M. Waldron JA. Xie S-S. Barlogie B. Expression of interleukin-6 in Castleman's disease. Hum Pathol. 1993;24(8):833–839. doi: 10.1016/0046-8177(93)90132-z. [DOI] [PubMed] [Google Scholar]
- Hu F. Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J Virol. 2006;80(21):10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson GS. Bankier AT. Satchwell SC. Barrell BG. The short unique region of the B95-8 Epstein-Barr virus genome. Virology. 1985;147(1):81–98. doi: 10.1016/0042-6822(85)90229-6. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the evaluation of carcinogenic risks to humans. 100B. Lyon, France: IARC; 2011. [Google Scholar]
- Irons RD. Le AT. Dithiocarbamates and viral IL-10 collaborate in the immortalization and evasion of immune response in EBV-infected human B lymphocytes. Chem Biol Interact. 2008;172(1):81–92. doi: 10.1016/j.cbi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD. Aoki Y. Chang Y. Moore PS. Yarchoan R. Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94(8):2871–2879. [PubMed] [Google Scholar]
- Kaleeba JAR. Bergquam EP. Wong SW. A Rhesus macaque rhadinovirus related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J Virol. 1999;73(7):6177–6181. doi: 10.1128/jvi.73.7.6177-6181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S. Nitta Y. Tashiro F. Nakano A. Yamato E. Tahara H. Tabayashi K. Miyazaki J-i. Suppression of Th1 cell activation and prevention of autoimmune diabetes in NOD mice by local expression of viral IL-10. Int Immunol. 2001;13(5):685–694. doi: 10.1093/intimm/13.5.685. [DOI] [PubMed] [Google Scholar]
- Keravala A. Lechman E. Nash J. Mi Z. Robbins P. Human, viral or mutant human IL-10 expressed after local adenovirus-mediated gene transfer are equally effective in ameliorating disease pathology in a rabbit knee model of antigen-induced arthritis. Arthritis Res Ther. 2006;8(4):R91. doi: 10.1186/ar1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla MG. Swaminathan S. Welsh RM. Kieff E. Brutkiewicz RR. Effects of virally expressed interleukin-10 on vaccinia virus infection in mice. J Virol. 1993;67(12):7623–7628. doi: 10.1128/jvi.67.12.7623-7628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-R. Lee S. Chaudhary PM. Gill P. Jung JU. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Future Microbiol. 2010;5(9):1349–1365. doi: 10.2217/fmb.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger-Ravet M. Peuchmaur M. Devergne O. Audouin J. Raphael M. Van Damme J. Galanaud P. Diebold J. Emilie D. Interleukin-6 gene expression in Castleman's disease. Blood. 1991;78(11):2923–2930. [PubMed] [Google Scholar]
- Lehrman G. Hogue IB. Palmer S. Jennings C. Spina CA. Wiegand A. Landay AL. Coombs RW. Richman DD. Mellors JW. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Nicholas J. Identification of amino acid residues of gp130 signal transducer and gp80 {alpha} receptor subunit that are involved in ligand binding and signaling by human herpesvirus 8-encoded interleukin-6. J Virol. 2002;76(11):5627–5636. doi: 10.1128/JVI.76.11.5627-5636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Wang H. Nicholas J. Detection of direct binding of human herpesvirus 8-encoded interleukin-6 (vIL-6) to both gp130 and IL-6 receptor (IL-6R) and identification of amino acid residues of vIL-6 important for IL-6R-dependent and -independent signaling. J Virol. 2001;75(7):3325–3334. doi: 10.1128/JVI.75.7.3325-3334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. Lee J-S. Jung JU. Immune evasion in Kaposi's sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol. 2008;18(6):423–436. doi: 10.1016/j.semcancer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. de Waal Malefyt R. Briere F. Parham C. Bridon J. Banchereau J. Moore K. Xu J. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J Immunol. 1997;158(2):604–613. [PubMed] [Google Scholar]
- MacNeil I. Suda T. Moore K. Mosmann T. Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145(12):4167–4173. [PubMed] [Google Scholar]
- Mandler RN. Kerrigan DP. Smart J. Kuis W. Villiger P. Lotz M. Castleman's disease in poems syndrome with elevated interleukin-6. Cancer. 1992;69(11):2697–2703. doi: 10.1002/1097-0142(19920601)69:11<2697::aid-cncr2820691112>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki I. Cheung RK. Dosch HM. Viral interleukin 10 is critical for the induction of B cell growth transformation by Epstein-Barr virus. J Exp Med. 1993;178(2):439–447. doi: 10.1084/jem.178.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molden J. Chang Y. You Y. Moore PS. Goldsmith MA. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272(31):19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- Moore KW. Vieira P. Fiorentino DF. Trounstine ML. Khan TA. Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Moore PS. Boshoff C. Weiss RA. Chang Y. Molecular Mimicry of Human Cytokine and Cytokine Response Pathway Genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Muraguchi A. Hirano T. Tang B. Matsuda T. Horii Y. Nakajima K. Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M. Nishimoto N. The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Curr Opin Rheumatol. 2011;23(3):273–277. doi: 10.1097/BOR.0b013e3283456797. [DOI] [PubMed] [Google Scholar]
- Neipel F. Albrecht J. Ensser A. Huang Y. Li J. Friedman-Kien A. Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71(1):839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. Review: human gammaherpesvirus cytokines and chemokine receptors. J Interferon Cytokine Res. 2005;25(7):373–383. doi: 10.1089/jir.2005.25.373. [DOI] [PubMed] [Google Scholar]
- Nicholas J. Ruvolo VR. Burns WH. Sandford G. Wan X. Ciufo D. Hendrickson SB. Guo H-G. Hayward GS. Reixz MS. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3(3):287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- Niller HH. Salamon D. Takacs M. Uhlig J. Wolf H. Minarovits J. Protein-DNA interaction and CpG methylation at rep*/vIL-10p of latent Epstein-Barr virus genomes in lymphoid cell lines. Biol Chem. 2001;382(10):1411–1419. doi: 10.1515/BC.2001.174. [DOI] [PubMed] [Google Scholar]
- Nishimoto N. Sasai M. Shima Y. Nakagawa M. Matsumoto T. Shirai T. Kishimoto T. Yoshizaki K. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95(1):56–61. [PubMed] [Google Scholar]
- Okada M. Sakaguchi N. Yoshimura N. Hara H. Shimizu K. Yoshida N. Yoshizaki K. Kishimoto S. Yamamura Y. Kishimoto T. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med. 1983;157(2):583–590. doi: 10.1084/jem.157.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzechowska BU. Manoharan M. Sprague J. Estep RD. Axthelm MK. Wong SW. Viral interleukin-6 encoded by rhesus macaque rhadinovirus is associated with lymphoproliferative disorder (LPD) J Med Primatol. 2009;38:2–7. doi: 10.1111/j.1600-0684.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne J. Moore PS. Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60(10):921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- Paulsen SJ. Rosenkilde MM. Eugen-Olsen J. Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79(1):536–546. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S. Timans JC. Cheung J. Rosales R. Kanzler H. Gilbert J. Hibbert L. Churakova T. Travis M. Vaisberg E. Blumenschein WM. Mattson JD. Wagner JL. To W. Zurawski S. McClanahan TK. Gorman DM. Bazan JF. de Waal Malefyt R. Rennick D. Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Qin L. Chavin K. Ding Y. Tahara H. Favaro J. Woodward J. Suzuki T. Robbins P. Lotze M. Bromberg J. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J Immunol. 1996;156(6):2316–2323. [PubMed] [Google Scholar]
- Rose-John S. Schooltink H. Schmitz-Van de Leur H. Müllberg J. Heinrich PC. Graeve L. Intracellular retention of interleukin-6 abrogates signaling. J Biol Chem. 1993;268(29):22084–22091. [PubMed] [Google Scholar]
- Rousset F. Garcia E. Defrance T. Péronne C. Vezzio N. Hsu DH. Kastelein R. Moore KW. Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci. 1992;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. Zuo J. Immune responses to Epstein-Barr virus: molecular interactions in the virus evasion of CD8+ T cell immunity. Microbes Infect. 2010;12(3):173–181. doi: 10.1016/j.micinf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgar SK. Yang D. Ruiz P. Miller J. Tzakis AG. Viral interleukin-10–engineered autologous hematopoietic stem cell therapy: a novel gene therapy approach to prevent graft rejection. Hum Gene Ther. 2004;15(2):131–144. doi: 10.1089/104303404772679940. [DOI] [PubMed] [Google Scholar]
- Sin S-H. Roy D. Wang L. Staudt MR. Fakhari FD. Patel DD. Henry D. Harrington WJ., Jr. Damania BA. Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109(5):2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-NJ. Tomosugi N. Kawabata H. Ishikawa T. Nishikawa T. Yoshizaki K. Down-regulation of hepcidin resulting from long-term treatment with an anti–IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116(18):3627–3634. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- Soulier J. Grollet L. Oksenhendler E. Cacoub P. Cazals-Hatem D. Babinet P. d'Agay M. Clauvel J. Raphael M. Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- Sozzani S. Luini W. Bianchi G. Allavena P. Wells TNC. Napolitano M. Bernardini G. Vecchi A. D'Ambrosio D. Mazzeo D. Sinigaglia F. Santoni A. Maggi E. Romagnani S. Mantovani A. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92(11):4036–4039. [PubMed] [Google Scholar]
- Stevenson PG. Immune evasion by gamma-herpesviruses. Curr Opin Immunol. 2004;16(4):456–462. doi: 10.1016/j.coi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Stine JT. Wood C. Hill M. Epp A. Raport CJ. Schweickart VL. Endo Y. Sasaki T. Simmons G. Boshoff C. Clapham P. Chang Y. Moore P. Gray PW. Chantry D. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95(4):1151–1157. [PubMed] [Google Scholar]
- Stuart AD. Stewart JP. Arrand JR. Mackett M. The Epstein-Barr virus encoded cytokine viral interleukin-10 enhances transformation of human B lymphocytes. Oncogene. 1995;11(9):1711–1719. [PubMed] [Google Scholar]
- Swaminathan S. Hesselton R. Sullivan J. Kieff E. Epstein-Barr virus recombinants with specifically mutated BCRF1 genes. J Virol. 1993;67(12):7406–7413. doi: 10.1128/jvi.67.12.7406-7413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou R. Cochet C. Joab I. Transcriptional analysis of the Epstein—Barr virus interleukin-10 homologue during the lytic cycle. J Gen Virol. 1996;77(6):1163–1168. doi: 10.1099/0022-1317-77-6-1163. [DOI] [PubMed] [Google Scholar]
- Verwaerde C. Naud MC. Delanoye A. Wood M. Thillaye-Goldenberg B. Auriault C. de Kozak Y. Ocular transfer of retinal glial cells transduced ex vivo with adenovirus expressing viral IL-10 or CTLA4-Ig inhibits experimental autoimmune uveoretinitis. Gene Ther. 2003;10(23):1970–1981. doi: 10.1038/sj.gt.3302101. [DOI] [PubMed] [Google Scholar]
- Vieira P. de Waal-Malefyt R. Dang MN. Johnson KE. Kastelein R. Fiorentino DF. deVries JE. Roncarolo MG. Mosmann TR. Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci. 1991;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Wu B. Mayton L. Evans CH. Robbins PD. Wooley PH. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res. 2002a;51(7):342–350. doi: 10.1007/pl00000313. [DOI] [PubMed] [Google Scholar]
- Yang Z. Chen M. Wu R. Fialkow LB. Bromberg JS. McDuffie M. Naji A. Nadler JL. Suppression of autoimmune diabetes by viral IL-10 gene transfer. J Immunol. 2002b;168(12):6479–6485. doi: 10.4049/jimmunol.168.12.6479. [DOI] [PubMed] [Google Scholar]
- Yoon SI. Jones BC. Logsdon NJ. Walter MR. Same structure, different function: crystal structure of the Epstein-Barr virus IL-10 bound to the soluble IL-10R1 chain. Structure. 2005;13(4):551–564. doi: 10.1016/j.str.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K. Matsuda T. Nishimoto N. Kuritani T. Taeho L. Aozasa K. Nakahata T. Kawai H. Tagoh H. Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360–1367. [PubMed] [Google Scholar]
- Zdanov A. Schalk-Hihi C. Menon S. Moore KW. Wlodawer A. Crystal structure of epstein-barr virus protein BCRF1, a homolog of cellular interleukin-10. J Mol Biol. 1997;268(2):460–467. doi: 10.1006/jmbi.1997.0990. [DOI] [PubMed] [Google Scholar]
- Zeidler R. Eissner G. Meissner P. Uebel S. Tampé R. Lazis S. Hammerschmidt W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus–encoded interleukin-10. Blood. 1997;90(6):2390–2397. [PubMed] [Google Scholar]
- Zhang Y-J. Bonaparte RS. Patel D. Stein DA. Iversen PL. Blockade of viral interleukin-6 expression of Kaposi's sarcoma–associated herpesvirus. Mol Cancer Ther. 2008;7(3):712–720. doi: 10.1158/1535-7163.MCT-07-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Stevenson D. Schechter JE. Mircheff AK. Ritter T. Labree L. Trousdale MD. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Investig Ophthalmol Vis Sci. 2004;45(5):1375–1381. doi: 10.1167/iovs.03-0755. [DOI] [PubMed] [Google Scholar]