Abstract
Melanoma differentiation-associated gene-7 (mda-7)/interleukin-24 (IL-24) has shown potent tumor cell apoptosis inducing capacity in multiple cancers. However, the apoptosis induction capacity of mda-7/IL-24 was low and directly correlated with the adhesion to tumor cells.Cell adhesion molecule integrin αvβ3 expressed on the surface of several types of solid tumor cells, and they bind to arginine-glycine-aspartic acid (RGD) which enhanced the adhesion to tumor cells. This rout was exploited to construct a tumor-targeting gene RGD-IL-24 which can express RGD-MDA-7/IL-24 protein that includes the cell adhesive sequence 164Arg-165Gly-166Asp (A Glycine residue was inserted into the recombinant MDA-7/IL-24 between Arg164 and Asp165 to form a RGD motif). We successfully got the MDA-7/IL-24 mutant by overlapping polymerase chain reaction (PCR) and evaluated its therapeutic efficacy for tumor cell lines MCF-7, HeLa, HepG2, and normal human lung fibroblast (NHLF) line. And we found that the expression of pCDNA3.1/RGD-IL-24 was same to the expression of pCDNA3.1/IL-24. The RGD-IL-24 enhanced the apoptosis-inducing function in tumor cells, but not in normal cells. In tumor cell lines, the apoptosis-inducing activities of RGD-IL-24 was significantly higher than IL-24 detecting by MTT assay, Annexin V, and Hoechst 33258 analysis. Further, pCDNA3.1/RGD-IL-24 showed a significant increase in the ratio of pro-apoptotic (bax) to anti-apoptotic (bcl-2) proteins in tumor cell lines, but not in NHLF cell line. Together, these results suggest that RGD-IL-24 can enhance the apoptosis of tumor cells and may provide a promising drug in tumor therapy.
Introduction
Melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24) gained attention as a potential tumor suppressor which is a cancer-specific, growth-suppressing, and apoptosis-inducing gene with broad-spectrum antitumor activity. When IL-24 is overexpressed via plasmid-mediated gene delivery it inhibits growth of tumor cells and selectively induces apoptosis in cancer cells but not in normal cells (Jiang and others 1996; Madireddi and others 2000; Mhashilkar and others 2001; Sarkar and others 2002). MDA-7 encodes a novel protein of 206 amino acids with a predicted size of 23.8 kDa which was demonstrated to be a secreted glycosylated protein.
MDA-7/IL-24 receptor complexes apparently consist of 2 chains, IL-20R1/IL-20R2 or IL-22R1/IL-20R2, indicating that these receptor complexes can mediate IL-24 signal transduction (Dumoutier and others 2001; Parrish-Novak and others 2002; Wang and others 2002). When overexpressing MDA-7/IL-24, it induces apoptosis in cancers by multiple apoptotic pathways (Su and others 1998, 2001; Madireddi and others 2000; Saeki and others 2000; Lebedeva and others 2002; Fisher and others 2003; Sauane and others 2003b, 2004). Although the precise apoptotic pathways of IL-24 remain to be determined, it is clear from the current literature that IL-24 can function either through its cell-surface receptors as a classical cytokine (Sauane and others 2004; Wang and others 2004) or intracellularly as a cytotoxic agent, in a non-receptor-mediated manner to certain cancer cells (Sauane and others 2003a). Here we shall focus mainly on the receptor-mediated functions of IL-24. In consideration of MDA-7 being a secreted cytokine, the tissue-specific surface expression of the specific combination of receptor subunits is likely to play a key role in determining the function of the MDA-7/IL-24 protein. Considering the absence of IL-24 receptor complexes on the surface of certain cancer cells, we should find a special receptor which is uniquely expressed on the surface of tumor cells.
Increasing amounts of evidence now imply that the integrin signaling plays a key role in tumor angiogenesis and metastasis (Brooks and others 1994; Hood and Cheresh 2002; Kumar 2003). The αvβ3 integrin which is uniquely expressed on the surface of several types of solid tumor cells and on almost all sprouting tumor vasculatures (Ruoslahti 1996) binds to arginine-glycine-aspartic acid (RGD). Plasmid-mediated gene delivery inhibits growth of tumor cells and selectively induces apoptosis in cancer cells but not in normal cells (Su and others 1998; Saeki and others 2000; Parrish-Novak and others 2002; Fisher and others 2003), however, the apoptosis induction efficiency was low (due to low transfer efficiency of plasmid and the absent expression of IL-24 receptor complexes). Studies have shown that apoptosis induction can be significantly enhanced by targeting mda-7/IL-24 protein to cell adhesion molecule integrin αvβ3 (Brooks and others 1994; Hood and Cheresh 2002; Kumar 2003). In this regard, we hypothesized construction of a mutant which expressed IL-24 protein containing RGD peptides that enhanced adhesion of IL-24 protein. When coupled to the anticancer drug, the RGD peptides could develop targeting and enhance the antitumor effect of the drug (Arap and others 1998). Moreover, RGD-containing peptides are able to directly induce apoptosis by triggering conformational changes that promote pro-caspase-3 auto-processing and activation (Buckley and other 1999).
The goal of the present study is to construct a mutant plasmid vector which expresses tumor-targeting protein RGD-IL-24 and further evaluate its enhanced therapeutic efficacy of MDA-7/IL-24 gene therapy by enhancing the adhesion of the secreted IL-24 protein. We choose 3 tumor cell lines (MCF-7, HeLa and HepG2) and normal human lung fibroblast (NHLF) cell line for the study of the enhanced apoptosis-inducing of the mutant.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and Trypsin were from Gibco. Lipofectamine 2000 transfection reagent was from Invitrogen. Total RNA Isolation System Access RT-PCR System and Access RT-PCR System were from Promega. Bradford assay was obtained from Novagen. Rabbit anti-MDA-7 polyclonal antibody was from Santa Cruze. Anti-caspase 3 polyclonal antibody, Anti-bax polyclonal antibody, anti-bcl-2 antibody, and mouse anti-Rabbit secondary antibody were purchased from Cell Signaling. MTT regent, Annexin V-FITC kit and Hoechst 33258 kit were purchased from KeyGen Biotech Co. Ltd.
Cell lines and cell culture
Human tumor cell line MCF-7 and human lung fibroblast (NHLF) were purchased from Chinese Academy of Sciences. The human liver cancer cell line HepG2 and uterine cervix cancer cell line HeLa were purchased from Tongji University. All cell lines were cultured and maintained in DMEM which were supplemented with 10% FBS. All cells were grown in a humidified atmosphere of 5% CO2 at 37°C. The cells were free of mycoplasma and were used in the log phase of growth. Cells were harvested with 0.25% Trypsin.
Construction of pCDNA3.1/RGD-IL-24
The whole cDNA coding region of mda-7/IL-24 gene was kindly provided by Professor Liu (Xin-Yuan Liu, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China), and used as template for the construction of expression vector by means of overlapping PCR. Four primers for PCR were designed as follows based on the mda-7/IL-24 sequence and the pCDNA3.1 vector:
P1: 5′-TGTGAAGCTT ATGAATTTTCAACAGAG-3′
P2: 5′-ACTGTCACCTCTGATGGAAAACATC-3′
P3: 5′-ATCAGAGGTGACAGTGCACACAGGC-3′
P4: 5′-TGTGGGATCCTCAGAGCTTGTAG-3′
The P2 and P3 had the overlapping complementary 15-base sequence shown in italics that resulted in the extra codon ACC encoding the Glycine between Arg164 and Asp165 to form a RGD motif, whereas P1 and P4 had HindIII and BamHI recognition sites (underlined), respectively, for directed cloning into vector. One product was amplified by using the P1 and P2 primers, the other product was amplified by using the P3 and P4 primers. After first round of PCR using P1/P2 and P3/P4, respectively, gel purification was performed for the 2 products. A second round of PCR followed using the 2 purified products as templates and the P1, P4 primers to obtain the fragment encoding mutant RGD-MDA-7. The fragment was digested with HindIII and BamHI, and then inserted inframe into pCDNA3.1 expression vector to construct the pCDNA3.1/RGD-MDA-7. The mutations were confirmed by nucleotide sequencing. Similarly, the wild-type mda-7/IL-24 was inserted with pCDNA3.1 vector to construct the pCDNA3.1/IL-24 plasmid.
DNA transfection assays
Cells were plated 1 day before transfection. Total of 105 cells were seeded per well in 24-well plates and transfected with plasmid vector by using Lipofectamine 2000 transfection reagent according to the manufacturer's instructions. All cell lines were transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24.
Reverse transcription PCR
The expression of mda-7/IL-24 gene was analyzed by reverse transcription-PCR using Access RT-PCR System. Total cellular RNA was isolated from cells 36 h after transfection using Total RNA Isolation System according to the manufacturer's introductions. Relative RNA integrity was then assessed by fractionating 2 μg of total RNA in a glyoxal/dimethyl sulfoxide gel and visualizing the 28s and the 18s ribosomal RNA bands. The upstream and downstream primers were P1 and P4 which were described in construction of pCDNA3.1/RGD-IL-24. Reverse transcription polymerase chain reaction (RT-PCR) was then done in a reaction volume of 25 μL, including 1 μL primer (P1 and P4) or 1 μL glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer. First Strand cDNA synthesis conditions were 45°C for 45 min and 94°C for 2 min, followed by second strand cDNA synthesis and PCR amplification, the conditions for them were 36 cycles at 94°C for 30 s, 55°C for 30 s, 68°C for 30 s, and 1 cycle at 68°C for 7 min for final extension. Each plate included a negative template control. GAPDH was used as an internal reference gene to normalize the expression of mda-7/IL-24. Quantitation was performed with an image analyzer (Labworks Software 3.0; UVP).
Western blotting assay
Cells were seeded in appropriate culture vessels and transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24 when they were grown to approximately 80% density. Cells receiving no treatment served as control group. At 48 h after transfection, they were washed with cold phosphate-buffered saline (PBS), followed by lysed in lysis buffer (1% Triton X-100, 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 10 mM PMSF, 1 mM benzaminidine, 5 mg/mL aprotinin, 3 mg/mL pepstatin, 5 mg/mL leupetpin) and the concentration of total cell lysates was determined by Bradford assay. Two thousand fifty micrograms of total lysates were with mixed equal volumes of laemmli sample buffer, boiled for 5 min at 95°C, loaded on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, and transferred onto a NC membrane. Filters were blocked for 4 h at room temperature in blocking buffer [3% nonfat milk power in TBS-T: 10 mm Tris-HCl (pH 8.0) 150 mm NaCl, 0.05% Tween 20], and then incubated for 4 h at room temperature in blocking buffer containing a 1:1,000 dilution of rabbit anti MDA-7 polyclonal antibody, anti-caspase-3, anti-bcl-2, or anti-bax polyclonal antibodies. After washing in TBS-T buffer (3×5 min, RT), filters were incubated for 2 h at room temperature in blocking buffer containing a 1:10,000 dilution of peroxidase conjugated mouse anti-rabbit secondary antibody. After washing in TBS-T, membranes were developed using NBT/BCIP color substrate. The bands on the membrane were scanned for the density and analyzed with the image analyzer (Labworks Software).
Cell adhesion assay
Polyvinyl chloride microtiter plates (96 well) were coated respectively with RGD-mda-7 (8 μg/mL) and wtmda-7/IL-24 solutions (8 μg/mL) at 4°C overnight. After being washed with 0.9% sodium chloride, each well was filled with RPMI 1640 medium containing 1% bovine serum albumin for 2 h at 37°C and washed again. For adhesion assay, cells were detached using trypsin digestion, washed 3 times with 0.9% sodium chloride, and resuspended in incomplete RPMI 1640 medium; then added cells to RGD-mda-7 or wtmda-7/IL-24 coated plates with 2×104 cells/well in 100 μL. After incubation for 1 h at 37°C in 5% CO2, unbound cells were removed by washing with incomplete RPMI 1640 medium. Adherent cells were fixed with a solution of 4% paraformaldehyde in PBS (pH 7.2) and stained with 0.5% crystal violet, then observed using a microscope. Absorbance from the plates was read on an ELX-800 spectrometer reader (Bio-Tek Instruments, Inc.) at 490 nm.
Cell viability assay
Cells were seeded at 5×103 cells per well in 96-well tissue culture plates. Twenty-four hours after plating transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24, or treated with complete medium. Twenty-four hours after transfection treatment, the cytotoxicity of the various groups was assessed at different time points (2,496 h) following treatment, 20 μL of the dye MTT (5 mg/mL) was added to each well. Four hours later, the cells were given 100 μL of dimethyl sulfoxide per dish and the absorption (A) was determined on an ELX-800 spectrometer reader (Bio-Tek Instruments, Inc.) at 490 nm. All data were normalized relative to the control, untreated cells of the corresponding cell type.
Annexin V-FITC binding assay
All cell lines were analyzed for apoptosis, using the Annexin V-FITC kit. Briefly, plasmid transfected cells were seeded in 24-well plates; 24 h after transfection, cells were washed twice with medium, then incubated with Annexin V-FITC, and then visualized under a fluorescence microscope. Under microscopy, 6 fields were randomly selected from every sample and independent observers performed cell counting in a blind fashion. The apoptotic rate=(number of total apoptotic cells/total number of cells)×100%.
Hoechst 33258 assay
Each cell line was plated in 24-well plates, which had 3×104 cells per well and until 80% confluence they were transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24. PBS (pH 7.2) was used in control wells. Seventy-two hours after transfection, supernatant medium was removed and cells were washed with washing buffer and fixed with freshly made 4% formaldehyde for 10 min at 4°C. Cells were then washed twice with washing buffer and Hoechst strain 33258 was added at room temperature which would be sufficiently washed 10 min later. Cells were then visualized under a fluorescence microscope. Under microscopy, 6 fields were randomly selected from every sample and independent observers performed cell counting in a blind fashion. The apoptotic rate=(number of total apoptotic cells/total number of cells)×100%.
Statistical analyses
All of the experiments were performed at least 3 times. Results are expressed as mean±SE. Statistical comparisons were made using an unpaired 2-tailed Student's t-test. Differences with P value of <0.05 was considered significant.
Results
Construction of pCDNA3.1/RGD-IL-24
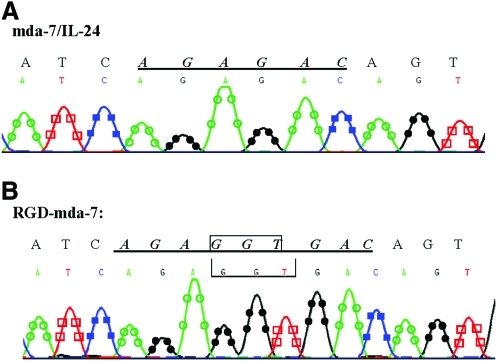
We successfully constructed pCDNA3.1/RGD-IL-24 by means of overlapping PCR. The fragment encoded RGD-IL-24 was confirmed by sequencing (Fig. 1). The results indicated that extra codon ACC encoding the Glycine was inserted into wild-type MDA-7. For the special site of the Glycine, between Arg164 and Asp165, it can form a RGD motif. The mutant RGD-IL-24 fragment was then inserted into pCDNA3.1 expression vector to construct the pCDNA3.1/RGD-MDA-7.
FIG. 1.
DNA sequence alignment of IL-24 (A) and RGD-mda-7 (B). (B) The sequencing shows the codon GGT (Gly) was inserted between codon AGA (Arg) and GAC (Asp), and successfully formed RGD domain of RGD-IL-24. IL, interleukin-24; mda-7, melanoma differentiation-associated gene-7. Color images available online at www.liebertonline.com/jir
Expression of RGD-IL-24 gene
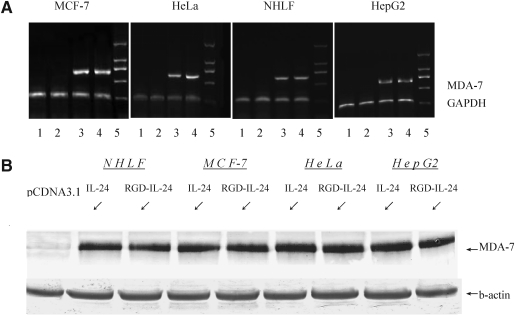
To determine the transcription of pCDNA3.1/RGD-IL-24, RT-PCR assay was performed with the total RNA of tumor and normal cells transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24. These lines were harvested 36 h after transfection, we found mda-7/IL-24 gene was expressed at a relatively high level in all tumor cell lines transfected with pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24, and the results suggested that the modifying of MDA-7 gene did not affect the mRNA expression. The expression of mda-7/IL-24 gene was undetectable in the control group or groups which was transfected with pCDNA3.1. In normal cell line, we can detect the expression of mda-7/IL-24 gene, but it expressed at a relatively low level compared with the tumor cell lines (Fig. 2A).
FIG. 2.
Analysis of mda-7/IL-24 transcription and expression by RT-PCR and Western blot. (A) Tumor cells transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24 were harvested and total RNA was extracted, then analyzed for the mRNA of IL-24, 24 h after transfection. Lane 1, RT-PCR product of untransfected group; lane 2, RT-PCR product of pCDNA3.1 group; lane 3, RT-PCR product of pCDNA3.1/IL-24 group; lane 4, RT-PCR product of pCDNA3.1/RGD-IL-24 group; lane 5, marker 1 kb ladder. (B) Tumor cells and normal cells transfected with pCDNA3.1, pCDNA3.1/IL-24, or pCDNA3.1/RGD-IL-24 were observed and analyzed for MDA-7 expression by Western blot analysis. After 48 h, the cell lysates were collected, and expression of mda-7 was detected by Western blotting using rabbit anti-mda-7 polyclonal antibody. Actin was used as loading control. PCR, polymerase chain reaction; RT, reverse transcription.
Protein expression of RGD-IL-24
To confirm the expression of MDA-7 protein in pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 group, lysates of the 3 tumor cell lines and the normal cell line were subjected to Western blot analysis. Rabbit anti-mda-7 polyclonal antibody was used as primary antibody. Both in tumor cells and normal cells, the results showed the expression of MDA-7 protein in pCDNA3.1/RGD-IL-24 group was same as pCDNA3.1/IL-24 group, which confirmed MDA-7 protein expression in pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 group (Fig. 2B).
RGD motif targets mda-7/IL-24 to cancer cells
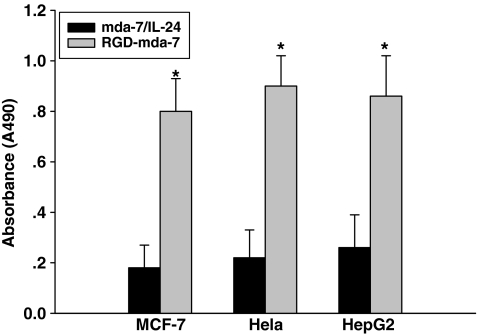
To assess whether the RGD domain of RGD-mda-7 is functional and accessible to integrin αvβ3, αvβ5, we compared cell adhesion promoted by RGD-mda-7 to wtmda-7/IL-24. Crystal violet staining showed adhesion and spreading of MCF-7 cells, HepG-2 cells, and HeLa cells on plates coated with RGD-mda-7, but not on plates coated with wtmda-7/IL-24 (Fig. 3). These results indicate that RGD-mda-7 is able to interact with adhesion receptors and increase adhesion to cancer cells.
FIG. 3.
The binding specificity of RGD-mda-7 to cancer cells. Microtiter wells were coated with RGD-mda-7 (8 μg/mL) or wtmda-7/IL-24, and seeded with MCF-7, HeLa and HepG2 cells. Absorbance was read at 490 nm after staining with crystal violet. Data were expressed as mean±SE from 6 independent groups (n=6). *P<0.05 versus wild-type mda-7/IL-24.
Growth suppression and viability assays
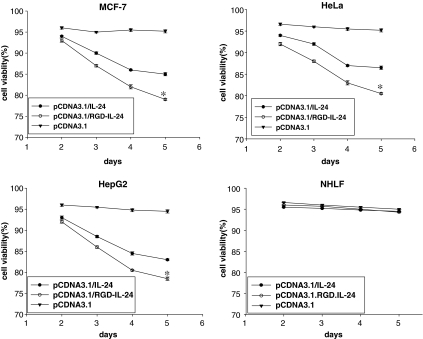
Treatment of the tumor cell lines with pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 caused growth inhibition in cell proliferation, as judged by MTT assays (Fig. 4). And compared with pCDNA3.1/IL-24 group, the growth inhibition function of pCDNA3.1/RGD-IL-24 group was higher, and the inhibition ratio was 24%. Treatment of the 3 tumor cell lines resulted in a time-dependent increasing in cell death. In agreement with pCDNA3.1/IL-24, pCDNA3.1/RGD-IL-24 had no effect on normal cell line NHLF (Fig. 4).
FIG. 4.
Cell viability was measured by MTT assay. Tumor cells (MCF-7, HeLa, and HepG2) and NHLF were transfected with pCDNA3.1/IL-24, pCDNA3.1/RGD-IL-24, pCDNA3.1, or untransfected (control) and cell viability was determined on days 2, 3, 4, and 5 after transfection by MTT assay. Experiments were repeated 3 times in quadruplicates, and the results were presented as mean±SE, *P<0.05 versus pCDNA3.1/IL-24. NHLF, normal human lung fibroblast.
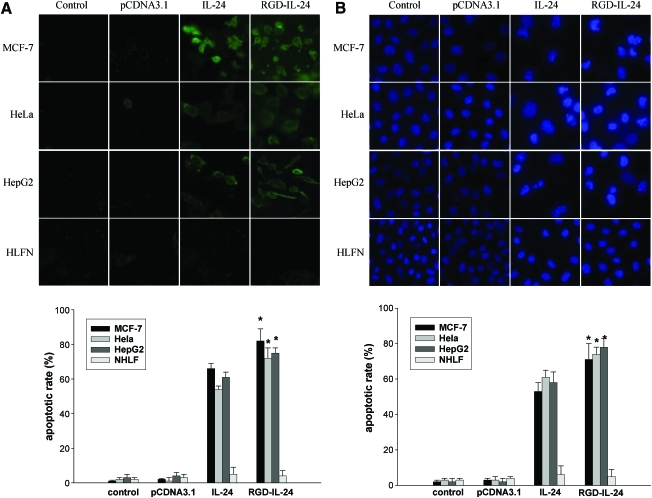
Evaluation of prophase apoptosis by Annexin V-FITC binding assays
Transfected with pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 48 h later, Annexin V-FITC staining assays showed high levels of Annexin V-FITC positive cells in pCDNA3.1/RGD-IL-24 group (Fig. 5A). However, no changes were observed in cells transfected with pCDNA3.1 or in the control group. In contrast, normal cells transfected with either pCDNA3.1/IL-24 or pCDNA3.1/RGD-IL-24 did not show any change in the number of apoptotic cells.
FIG. 5.
MDA-7- induced apoptosis in tumor cells but not normal cells. Tumor cells (MCF-7, HeLa, HepG2) and NHLF cells were transfected with pCDNA3.1, pCDNA3.1/IL-24, pCDNA3.1/RGD-IL-24, or treated with the medium (control). Cells were analyzed for apoptosis by Annexin V assay and Hoechst 33258. (A) Prophase apoptotic cells were recognized by binding with FITC on the membrane. (B) Seventy-two hours after transfection, apoptotic cells were recognized by condensation of nuclear chromation and its fragmentation. *P<0.05 versus pCDNA3.1/IL-24. Color images available online at www.liebertonline.com/jir
Evaluation of advanced apoptosis by Hoechst 33258 staining
After 72 h transfection of pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24, we observed pronounced apoptosis in tumor cells transfected with pCDNA3.1/RGD-IL-24 that showed Hoechst 33258 fluorescence staining in the nucleus (Fig. 5B). pCDNA3.1/IL-24 group had a relatively lower apoptotic activity and no apparent changes were observed in normal cells.
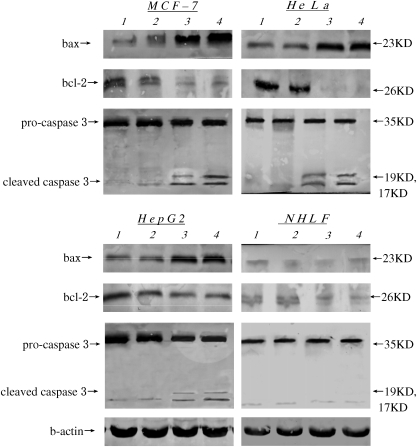
Detection of bax and bcl-2 protein levels by Western blot
The pro-apoptotic protein bax and anti-apoptotic protein bcl-2 was detected in the study. To determine if these apoptosis-associated molecules contribute to programmed cell death in tumor cells, the levels of the various proteins were determined by Western blotting 48 h after transfection with pCDNA3.1/IL-24 or pCDNA3.1/RGD-IL-24. In the 3 tumor cell lines, variable changes were observed in Bax and Bcl-2 protein levels, whereas there was no change in normal cell line NHLF (Fig. 6). Although pCDNA3.1/IL-24 or pCDNA3.1/RGD-IL-24 group could detect upregulated ratio of Bax to Bcl-2, pCDNA3.1/RGD-IL-24 group showed a higher ratio of Bax to Bcl-2 (Fig. 6).
FIG. 6.
Determination of BAX, BCL-2 and cleaved caspase-3 protein levels in NHLF and in tumor cell lines as a consequence of transfection with pCDNA3.1, pCDNA3.1/IL-24, pCDNA3.1/RGD-IL-24, or untransfection. Cells were transfected as described in Materials and Methods section, and protein lysates were prepared at the specified time pointes, samples of 50 μg of total protein were run on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a NC membrane, and stained with different antibodies as described in Materials and Methods section. Actin was used as a loading control. Lane 1, control group; lane 2, pCDNA3.1 group; lane3, pCDNA3.1/IL-24 group; lane 4, pCDNA3.1/RGD-IL-24 group.
Detection of cleaved caspase 3 levels by Western blot
Since pCDNA3.1/RGD-IL-24 enhanced the inducing of apoptosis in tumor cells, the activation of caspase 3 was examined by Western blot analysis. At the 48 h time point, cleavage of pro-caspase 3 was observed in pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 groups; pCDNA3.1/RGD-IL-24 group showed a higher cleaved level of caspase 3. However, caspase 3 were not cleaved in NHLF cells (Fig. 6).
Discussion
To determine the therapeutic utility of RGD-IL-24 for tumor cells, we have performed in vitro apoptosis assays in a panel of tumor cell lines. In this study, we have demonstrated that pCDNA3.1/Il-24 and pCDNA3.1/RGD-IL-24 gene therapy induced apoptosis in 3 tumor cell lines (MCF-7, HeLa, and HepG2), but did not do harm to NHLF. We have also shown that pCDNA3.1/RGD-IL-24 significantly enhanced inhibition of tumor growth in vitro in human cancer cells compared with unmodified pCDNA3.1/IL-24.
Although mda-7 was shown to induce apoptosis by multiple pathways, but 2 primary apoptosis pathways are presently recognized, one mediated by death receptors and controlled by caspase-8 and/or −10, and the other regulated by the release of cytochrome c and activation of caspase-9/Apafl/cytochrome c “apoptosome” (Nunez and others 1998). The particular mechanism(s) by which overexpression of mda-7/IL-24 induces cancer specific apoptosis is unknown; nevertheless the mechanism of cancer cell killing appears to follow one of several alternative pro-apoptotic pathways, likely depending on the cell context. Studies using tumor cell lines MCF-7, HeLa, and HepG2 indicated that ectopic expression of mda-7/IL-24 induces apoptosis which correlates with a selective upregulation of the pro-apoptotic protein Bax and an increase in the ratio of Bax to Bcl-2 protein (Su and others 1998, 2001).
In the present study, we constructed pCDNA3.1/RGD-IL-24 by means of overlapping PCR which encodes RGD-IL-24 protein. To confirm the expression of mda-7 gene by the plasmid pCDNA3.1/RGD-IL-24, we detected the transcription of mda-7 by RT-PCR, and the expression of mda-7/IL-24 protein was detected by Western blotting assay. The expression levels appear to be the same. This result shows that the mutant pCDNA3.1/RGD-IL-24 does not affect the expression of mda-7/Il-24. However, in all the cell lines tested, pCDNA3.1/RGD-IL-24 showed enhanced cell killing compared with unmodified pCDNA3.1/IL-24. Studies have showed that mda-7 induced apoptosis by both secretory and nonsecretory pathways (Sauane and others 2004; Sieger and others 2004). It is unknown whether pCDNA3.1/RGD-IL-24 directly or indirectly induced tumor cell killing via some alternative mechanisms. One possibility is that the enhanced apoptosis-inducing of the mutant is correlated with the adhesion effect of the RGD-IL-24 protein. Therefore, enhanced cell killing effect showed by pCDNA3.1/RGD-IL-24 demonstrates the significance of expression of adhesion-enhanced protein in further potentiating the bystander effects of mda-7. Our analysis of the effects of pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 on normal lung fibroblast cells demonstrated that pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24 did not inhibit cell growth in NHLF cells. The difference in toxicity of mda-7 between normal cells and tumor cells remain unknown.
To understand further the underlying mechanism of tumor killing mediated by RGD-IL-24 expression, we analyzed the effects of such expression on the pro-apoptotic gene Bax and anti-apoptotic gene Bcl-2. Following transfection with pCDNA3.1/IL-24 and pCDNA3.1/RGD-IL-24, in tumor cells we detected an increased expression of the tumor suppressor gene Bax, whose expression induces apoptosis, but it was undetected in normal cell. Moreover, since we observed more increase in the ratio of Bax to Bcl-2 protein in pCDNA3.1/RGD-IL-24 group, we concluded that RGD-IL-24 protein mediated tumor cell killing occurred by both mda-7 and RGD receptors, and the receptor complex of RGD peptide is an excellent target for tumor therapy for its unique expression on solid tumor cell's surface.
Additionally, we found that overexpression of mda-7 induced apoptosis and activated a common caspase cascade including cleavage of caspase-3. And pCDNA3.1/RGD-IL-24 had a larger capacity to activate caspase cascade compared with the unmodified IL-24 gene. No differences were observed in apoptotic pathways downstream of mitochondrial in different tumor cell lines. Further, the differences in response against the apoptotic signal between tumor cells and normal cells are not well known. However, these results imply there are differences in the mechanisms to elicit the apoptotic pathway. At least mda-7 expression mediated by plasmid does not activate the apoptotic pathway in NHLF cells.
In conclusion, the results of our current study showed that the plasmid which expresses adhesion-enhanced RGD-IL-24 protein significantly enhanced therapeutic efficacy in vitro in various tumor cell lines. Since IL-24 protein therapy has showed biological activity in clinical, it is hoped that RGD-IL-24 expressed by pCDNA3.1/RGD-IL-24 will augment the clinical therapeutic efficacy of IL-24 biological therapy for tumor cells.
Acknowledgments
This project is supported by grants from the National Natural Science Foundation of China (No. 30972976, 81071854), the Science and Technology Department of Jiangsu province (No. BK2009091, BK2010177), and the Program for New Century Excellent Talents in University (NCET-08-0700).
Author Disclosure Statement
No competing financial interests exist.
References
- Arap W. Pasqualini R. Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- Brooks PC. Clark RA. Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Buckley CD. Pilling D. Henriquez NV. Parsonage G. Threlfall K. School-Toellner D. Simmons DL. Akbar AN. Lord JM. Salmon M. RGD peptide induces apoptosis by direct caspase-3 activation. Nature. 1999;397:534–539. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- Dumoutier L. Leemans C. Lejeune D. Kotenko SV. Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Gopalkrishnan RV. Chada S. Ramesh R. Grimm EA. Rosenfeld MR. Curiel DT. Dent P. mda-7/IL-24, a novel cancer selective apoptsis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- Hood JD. Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Jiang H. Su ZZ. Lin JJ. Goldstein NI. Young CS. Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV. Su ZZ. Chang Y. Kitada S. Reed JC. Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- Mhashilkar AM. Schrock RD. Hindi M. Liao J. Sieger K. Kourouma F. Zou-Yang XH. Onishi E. Takh O. Vedvick TS. Fanger G. Stewart L. Watson GJ. Snary D. Fisher PB. Saeki T. Roth JA. Ramesh R. Chada S. Melanoma diVerentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- Madireddi MT. Su ZZ. Young CS. Goldstein NI. Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- Nunez G. Benedict MA. Hu Y. Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J. Xu W. Brender T. Yao L. Jones C. West J. Brandt C. Jelinek L. Madden K. McKernan PA. Foster DC. Jaspers S. Chandrasekher YA. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Saeki T. Mhashilkar A. Chada S. Branch C. Roth JA. Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Su ZZ. Lebedeva IV. Sauane M. Gopalkrishnan RV. Valerie K. Dent P. Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M. Gopalkrishnan RV. Lebedeva I. Mei MX. Sarkar D. Su ZZ. Kang DC. Dent P. Pestka S. Fisher PB. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003a;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- Sauane M. Gopalkrishnan RV. Sarkar D. Su ZZ. Lebedeva IV. Dent P. Pestka S. Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003b;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M. Lebedeva IV. Su ZZ. Choo HT. Randolph A. Valerie K. Dent P. Gopalkishnan RV. Fisher PB. Melanoma differentiation associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004;64:2988–2993. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- Sieger KA. Mhashilkar AM. Stewart A. Sutton RB. Strube RW. Chen SY. Pataer A. Swisher SG. Grimm EA. Ramesh R. Chada S. The tumor suppressor activity of MDA-7/IL-24 is mediated by intracellular protein expression in NSCLC cells. Mol Ther. 2004;9:355–367. doi: 10.1016/j.ymthe.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Su Z. Lebedeva IV. Gopalkrishnan RV. Goldstein NI. Stein CA. Reed JC. Dent P. Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ. Madireddi MT. Lin JJ. Young CS. Kitada S. Reed JC. Goldstein NI. Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Tan Z. Thomas EK. Liang P. Conservation of the genomic structure and receptor-mediated signaling between human and rat IL-24. Genes Immun. 2004;5:363–370. doi: 10.1038/sj.gene.6364101. [DOI] [PubMed] [Google Scholar]
- Wang M. Tan Z. Zhang R. Kotenko SV. Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]