Abstract
As a part of aging there are known to be numerous alterations which occur in multiple tissues of the body, and the focus of this study was to determine the extent to which oxidative stress and hypoxia occur during adipose tissue aging. In our studies we demonstrate for the first time that aging is associated with both hypoxia (38% reduction in oxygen levels, Po2 21.7 mmHg) and increases reactive oxygen species in visceral fat depots of aging male C57Bl/6 mice. Interestingly, aging visceral fat depots were observed to have significantly less change in the expression of genes involved in redox regulation compared with aging subcutaneous fat tissue. Exposure of 3T3-L1 adipocytes to the levels of hypoxia observed in aging adipose tissue was sufficient to alter multiple aspects of adipose biology inducing increased levels of in insulin-stimulated glucose uptake and decreased lipid content. Taken together, these data demonstrate that hypoxia and increased levels of reactive oxygen species occur in aging adipose tissue, highlighting the potential for these two stressors as potential modulators of adipose dysfunction during aging.
Keywords: adipocyte, antioxidant, obesity, reactive oxygen species, superoxide
during aging, the function, proliferation, size, and number of adipose cells become altered (32–36, 39, 61). Because of the potential for age-related changes in adipose function to modulate overall health and promote metabolic disease, there is a growing interest in studying aging adipose tissue. White adipose tissue is recognized as an important modulator of multiple physiological processes and is strongly linked to the development of multiple morbidities (8, 16, 46, 66, 76). The ability of adipose tissue to exert these effects comes from alterations in the endocrine functions of adipose (22, 52, 71) as well as the contributions of adipose tissue to glucose and lipid homeostasis (10, 26, 37, 75). Understanding aging-adipose interactions is therefore likely important to understanding the basis for metabolic disease in the elderly.
In addition to each of these aforementioned changes in adipose biology, the distribution of adipose tissue in the body is also known to be altered in response to aging (10, 37). Visceral adipose tissue has been suggested to be a more important contributor than subcutaneous fat or total adiposity to the development of metabolic disease (2, 3, 7, 8, 18, 24, 48, 51). Interestingly, studies have also demonstrated that there are significant differences in the biochemistry and function of cells from visceral and subcutaneous fat depots (24, 25, 31, 41). Taken together, these data suggest important adipose depot-specific differences between visceral and subcutaneous fat, although the importance of these differences in the context of aging remain to be defined experimentally.
Oxidative stress and hypoxia are known to occur in a variety of tissues during aging (4, 12, 13, 47, 56, 58, 65, 67), although current studies have not defined whether these events occur in subcutaneous and visceral fat depots during aging. Both oxidative stress and hypoxia are known to occur in adipose tissue in response to diet-induced obesity as well as in genetic models of obesity (53, 62, 63, 70, 72). Both hypoxia and oxidative stress are linked to altered adipose function and metabolic disease in both of these models (53, 62, 73). Taken together, these data highlight the importance of understanding whether oxidative stress and hypoxia occur in aging adipose tissue.
In the present study, we sought to determine for the first time whether hypoxia and oxidative stress occur in aging adipose tissue. Our studies identified that both of these events occur in aging adipose tissue, with visceral depots preferentially affected compared with subcutaneous depots. We then demonstrated that age-related levels of hypoxia increase insulin-stimulated glucose uptake and decrease lipid content in differentiated 3T3-L1 cells. Taken together, these studies raise the potential for increases in hypoxia and oxidative stress modulating adipose function during adipose aging.
MATERIALS AND METHODS
Animal studies.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Six-month-old and 23-mo-old male C57Bl/6 mice (obtained from the contract colony of the National Institute on Aging maintained at Charles River Laboratories) were housed in standard caging with 12:12-h light-dark cycle with food (D12450B from Research Diets, 10% fat, 20% protein, 70% carbohydrate) and water provided ad libitum. Mice were fasted overnight and euthanized by isoflurane anaesthesia followed by rapid decapitation. Subcutaneous (inguinal) and visceral fat pads (epididymal) were manually dissected as described previously (68).
Western blot analysis and hypoxia probe.
Western blotting for hypoxia probe was done using the Hypoxyprobe-1 kit (HPI, Burlington, MA). The Hypoxyprobe-1 was injected (intraperitoneally) at 60 mg/kg body wt 30 min before tissue collection. After tissue collection, the fat tissues were homogenized and sonicated in RIPA buffer (G-Biosciences, St. Louis, MO) containing protease inhibitor (Roche Diagnostics, Indianapolis, IN) and phosphotase inhibitor (Sigma-Aldrich, St. Louis, MO), following manufacturers' instructions. The homogenates were then centrifuged at 15,000 g at 4°C for 15 min. The fat formed a gel-like layer on the top of the tube. The lower, liquid layer containing proteins was carefully extracted and used for protein assay and Western blot analysis as described previously (72, 77).
Briefly, proteins were separated in precast polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose (Bio-Rad). Following transfer, the membrane was stained by MemCode Reversible Stain (Thermo Scientific, Rockford, IL).
The membrane was blocked in 5% milk for 1 h at room temperature. The membranes were incubated with the indicated antibody in 5% milk for 1–2 h at room temperature. Following extensive washes, the results were visualized with 1:5,000 diluted horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch Laboratory, West Grove, PA) and ECL Western Blotting Substrate (Pierce, Rockford, IL).
Cell culture.
Dulbecco's modified Eagle's medium containing high glucose and glutamine (DMEM), bovine and fetal bovine serum (FBS), iron-supplemented bovine calf serum, trypsin, and antibiotics penicillin G-streptomycin were purchased from Fisher Scientifics (Pittsburgh, PA). Insulin, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone were purchased from Sigma-Aldrich.
Murine 3T3-L1 preadipocytes purchased from the American Type Culture Collection (Manassas, VA) were cultured in DMEM-high glucose containing 10% calf serum and antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin). The medium was changed every 48 h. To obtain fully differentiated adipocytes, the 3T3-L1 preadipocytes were plated and grown on 60-mm plates to 1 day postconfluence and induced to differentiate by changing the medium to DMEM containing 10% FBS and 0.5 mM IBMX, 1 μM dexamethasone, and 1.7 μM insulin (MDI) as previously described, with modifications (17, 30). After 48 h, this medium was replaced with DMEM-high glucose medium supplemented with 10% FBS, penicillin-strepomycin, and 0.425 μM insulin. After 48 h, the medium was replaced every 2 days thereafter using DMEM-high glucose and 10% FBS medium. Cells were fully differentiated by 6 days, and they were routinely used at days 7–10 post-MDI treatment.
Hypoxia treatment.
A temperature- and humidity-controlled hypoxic/anaerobic Polymer Glove Box (Coy Laboratory Products, Grass Lake, MI) was used in this study. For hypoxia treatment, the system was set up at 37°C with 38% reduction of atmospheric oxygen (decreasing Po2 to 21.7 mmHg) by replacement with nitrogen. Matured 3T3-L1 adipocytes at 7–8 days post-MDI treatment were transferred to the hypoxic chamber for 6 or 20 h, as indicated in results. At the end of hypoxia treatment, cells were removed from the chamber and collected for future studies.
Quantitative real-time PCR studies.
Total RNA from 3T3-L1 adipocytes was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer's instructions with minor modifications. After cell harvest and homogenization through a blunt 20-gauge needle in the RLT lysis buffer, the lysate was centrifuged at 15,000 g at 4°C for 15 min. The liquid layer beneath the frozen fat layer was carefully extracted and used for total RNA extraction. Total RNA from mouse subcutaneous and visceral fat tissue was isolated using an RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer's instructions. The corresponding cDNA was made from 2 μg of extracted total RNA by M-MuLV transcriptase (New England Biolabs, Ipswich, MA) using 20 μl of the reverse transcription system according to the manufacturer's instructions.
For quantitative real-time PCR (qPCR) analysis, aliquots of cDNA were subjected to qPCR in 20 μl of 1× Brilliant II QPCR and QRT-PCR reagents (Agilent Technologies, Santa Clara, CA), 1× primers and TaqMan probe (6-FAM/ZEN/IBFQ mode), and 10 ng of cDNA. Primers and probes for superoxide dismutase (SOD)1, SOD2, SOD3, catalase, UCP3, and GADPH were ordered from Integrated DNA Technologies (IDT, Coralville, IA) PrimeTime Predesigned qPCR Assays system. Each sample was loaded in triplicate, and negative and positive controls were included. Amplification of GADPH was used as an internal reference gene. PCR amplifications were performed as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles each with 95°C for 15 s and 60°C for 45 s using an ABI PRISM 7000 sequence detector according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). For data analysis, the ΔΔCT method was used. Relative mRNA expression of each gene was expressed as the mean ± SE of 6 independent total RNA extraction and real-time PCR analysis.
qPCR array studies.
PCR array for mouse oxidative stress and antioxidant defense signaling pathway, RT2 First Strand Kit and RT2 Real-Timer SYBR Green/ROX PCR Mix were purchased from SABiosciences (Frederick, MD). QPCR array studies were performed according to the manufacturer's instructions on an ABI Prism 7900HT using 96-well standard block (Applied Biosystems). For data analysis, the ΔΔCT method was used. Each gene's fold changes were calculated as the difference in gene expression between different fat adipose as indicated in the each table. A positive value indicates the fold change of gene upregulation; gene downregulation was calculated as −1/(fold change). Only the statistically significant (P < 0.05) changes that are more than 1.5 or less than −1.5-fold are shown in the Table 1. The result is expressed as the mean of six independent total RNA extraction and real-time PCR analyses. The P value was assessed using the paired t-test.
Table 1.
Alterations in redox gene expression in aging adipose tissue
| Gene Symbol | Fold Change | P Value | Gene Full Name |
|---|---|---|---|
| Visceral fat: old/young | |||
| Cat | −1.58 | 0.013372 | Catalase |
| Serpinb1b | 1.74 | 0.010818 | Serine (or cysteine) peptidase inhibitor, clade B, member 1b |
| Ucp3 | −2.27 | 0.0216 | Uncoupling protein-3 (mitochondrial, proton carrier) |
| Subcutaneous fat: old/young | |||
| Aass | 16.49 | 0.000006 | Aminoadipate-semialdehyde synthase |
| Cyba | −2.7 | 0.020605 | Cytochrome b-245, alpha polypeptide |
| Duox1 | 2.61 | 0.01611 | Dual oxidase-1 |
| Ehd2 | 2.66 | 0.005961 | EH-domain-containing 2 |
| Fancc | 1.65 | 0.010463 | Fanconi anemia, complementation group C |
| Fmo2 | 3.77 | 0.000043 | Flavin-containing monooxygenase-2 |
| Gpx3 | 4.46 | 0.000782 | Glutathione peroxidase-3 |
| Gpx4 | 1.86 | 0.030338 | Glutathione peroxidase-4 |
| Gsr | 1.5 | 0.009021 | Glutathione reductase |
| Gstk1 | 2.28 | 0.00333 | Glutathione S-transferase kappa 1 |
| Mb | 4.24 | 0.001073 | Myoglobin |
| Nqo1 | 2.44 | 0.000248 | NAD(P)H dehydrogenase, quinone 1 |
| Park7 | 1.73 | 0.00715 | Parkinson disease (autosomal recessive, early onset) 7 |
| Prdx4 | 1.75 | 0.007949 | Peroxiredoxin 4 |
| Prdx5 | 2.22 | 0.015405 | Peroxiredoxin 5 |
| Prdx6 | 3.18 | 0.000335 | Peroxiredoxin 6 |
| Prdx6-rs1 | 3.01 | 0.000242 | Peroxiredoxin 6, related sequence 1 |
| Ptgs1 | 1.87 | 0.032009 | Prostaglandin-endoperoxide synthase-1 |
| Serpinb1b | −6.01 | 0.039266 | Serine (or cysteine) peptidase inhibitor, clade B, member 1b |
| Slc38a1 | −8.63 | 0.045604 | Solute carrier family 38, member 1 |
| Slc41a3 | 1.61 | 0.041657 | Solute carrier family 41, member 3 |
| Sod1 | 1.82 | 0.047545 | Superoxide dismutase-1, soluble |
| Txnip | 1.66 | 0.028555 | Thioredoxin interacting protein |
| Vim | 1.65 | 0.012064 | Vimentin |
Glucose uptake.
Glucose uptake was carried out as described in Yin et al. (74) with minor modifications. 3T3-L1 adipocytes were first washed twice in PBS and preincubated in serum-free medium for 2 h with 1% BSA and then incubated under normoxic or hypoxic condition for 4 h at 37°C. After that, cells were incubated in PBS containing 200 nM insulin for 30 min before being taken out of the hypoxia chamber and incubated in PBS containing 0.1 mM 2-deoxyglucose and 0.5 μCi/ml 3-O-[methyl-d-14C]glucose (PerkinElmer, Waltham, MA) for 5 min. The cells were washed in ice-cold PBS and solubilized in 0.4 ml of 1% SDS and [14C]glucose uptake was measured using Beckman LS6500 scintillation counter.
Fatty acid uptake.
Fatty acid uptake was carried out as described by Lobo et al. (39), with minor modifications. 3T3-L1 adipocytes were first preincubated for 3 h in Krebs-Ringer-HEPES (KRH) buffer (pH 7.4) containing (in mM) 120 NaCl, 4.7 KCl, 2.2 CaCl2, 10 HEPES, 1.2 KH2PO4, 1.2 MgSO4, and 5.4 glucose. After that, adipocytes were incubated under normoxic or hypoxic condition for 4 h and then incubated with 200 nM insulin for 30 min. Fatty acid uptake was initiated by incubating cells in KRH buffer, pH 7.4, containing 5.4 mM glucose and 0.5 μCi/ml [1-14C]oleic acid, (PerkinElmer, Waltham, MA) along with unlabeled oleic acid, free fatty acid, and BSA. The ratio of fatty acid to BSA used in this assay was adjusted to generate a free fatty acid concentration of 5 nM.
Interstitial Po2.
A fiber-optic oxygen meter (World Precision Instruments, Sarasota, FL) was used to determine interstitial Po2 in the inguinal and epididymal fat pads, as described elsewhere (72). The mice were anesthetized with a rodent cocktail (ketamine 100 mg/kg, xylazine 5 mg/kg, acepromazine 2 mg/kg) and placed on a warm pad. An abdomen incision was made to expose the epididymal and inguinal fat pads. The measurements were made by inserting the optic probe into the fat pads. A mean value was obtained from the both left and right fat pads in each mouse.
Electron paramagnetic resonance.
Reactive oxygen species (ROS) in the subcutaneous and visceral fat tissue were measured using EPR as described previously (14, 43, 44). For ROS, 1-hydroxy-3-methoxycarbony-l,2,2,5,5-tetramethyl-pyrrolidine (CMH) was used to measure tissue ROS production. Briefly, pieces of subcutaneous and visceral tissues were incubated at 37°C with CMH (200 μM) for 30 min for ROS measurement. Aliquots of incubated probe media were then taken in 50-μl disposable glass capillary tubes (Noxygen Science Transfer and Diagnostics) for determination of ROS production in fat tissues. All EPR measurements were performed using an EMX ESR eScan BenchTop spectrometer and super-high-quality factor microwave cavity (Bruker, Germany). For superoxide measurements, tissue pieces were incubated at 37°C with PEG-SOD (50 U/ml) for 30 min, and then the spin probe CMH (200 μM) was added for another 30-min incubation period. This procedure allows for the quantification of the contribution of superoxide to the total ROS levels.
Dihydroethidium staining for ROS.
Cells were incubated for 30 min with 10 μM dihydroethidium dye (solubilized in DMSO) and then washed with cell culture medium. Cells from random ×40 magnification fields were then analyzed for dihydroethidium oxidation by visual microscopy using excitation at 400 nm and emission detection at 585 nm. Approximately 300 cells from six separate experiments were utilized for data analysis.
Oil red O staining.
Differentiated 3T3-L1 adipocytes were washed three times with phosphate-buffered saline and fixed with 10% formalin at room temperature for 1 h. Cells were then washed again with phosphate-buffered saline and stained with fresh stock of Oil red O (3 parts of 0.6% Oil red O in isopropyl alcohol and 2 parts water). Cells were stained for 15 min and washed with twice with phosphate-buffered saline. For plate reader determinations of Oil red O content cells were dissolved with isopropyl alcohol and measured at 520 nm.
Statistics.
Statistical significance between two groups was determined using a paired t-test. For all other analyses, statistical significance was determined by ANOVA followed by Fisher's least significant difference post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Age-related changes in adipose tissue.
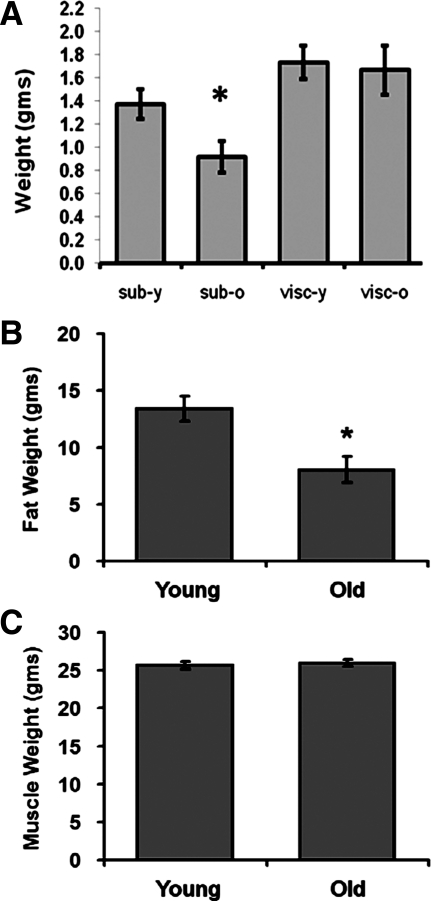
Adult male C57Bl/6 mice (6-mo- and 23-mo-old) were analyzed for changes in visceral (epididymal) and subcutaneous (inguinal) fat pad weight. A significant decrease in subcutaneous adipose tissue was observed in 23-mo-old mice (Fig. 1A), with no significant change in visceral adipose weight observed between the two age groups. NMR analysis demonstrated that the levels of total adipose tissue were decreased in 23-mo-old mice (Fig. 1B), with no significant difference in muscle weight observed between the two age groups (Fig. 1C).
Fig. 1.
Weight of adipose tissues and muscle in adult and aged mice. Adult (6-mo- and 26-mo-old) mice were measured for changes in fat pad weight for visceral (epididymal) and subcutaneous (inguinal) depots (A), total fat weight via NMR (B), and total muscle weight via NMR (C). Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. young (6-mo-old) adult mice.
Age-related changes in hypoxia within adipose tissue.
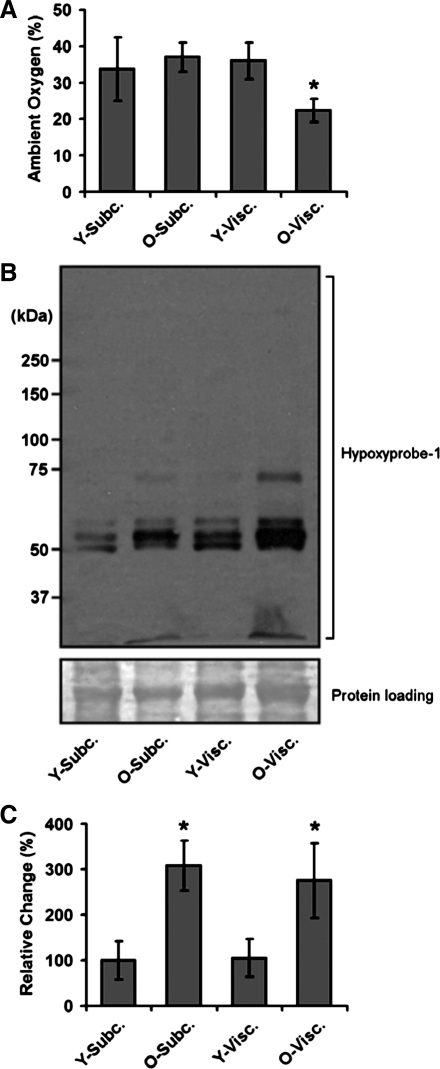
The mice were next analyzed for evidence of hypoxia within visceral and subcutaneous fat depots. In our first experimentation, we conducted direct measurements of oxygen levels within inguinal and epididymal fat depots using an oxygen sensor, whereby oxygen was measured within the tissue of anesthetized mice. In this analysis, we observed that there was no significant difference between the levels of oxygenation in the visceral and subcutaneous depots of 6-mo-old mice (Fig. 2A). In contrast, there was a selective increase in hypoxia (38% reduction in oxygen, po2 21.7 mmHg) within aging visceral fat tissue (Fig. 2). We next utilized an immunohistochemical method to identify the presence of hypoxia. In these studies we observed that aging subcutaneous and visceral fat depots presented evidence for increased hypoxia (Fig. 2). These data, whereby discrepancies between direct oxygen measurements and chemical detection of hypoxia are observed, point to the potential for acute or highly localized episodes of hypoxia occurring in aging adipose tissue. The mechanisms, and potential implications, for these observations are discussed in significant detail below in discussion.
Fig. 2.
Aging induces hypoxia in adipose tissue. A: adult (6-mo- and 26-mo-old) mice were anesthetized, and levels of oxygen measured directly within visceral (epididymal) and subcutaneous (inguinal) fat depots using insertion of an oxygen electrode into the specific depot. B: hypoxia was measured in visceral and subcutaneous fat depots using the Hypoxyprobe method, and results from multiple experiments were quantified (C). See materials and methods for experimental details. Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. young (Y, 6-mo-old) adult mice.
Age-related increases in ROS and altered expression of redox regulatory gene expression in adipose tissue.
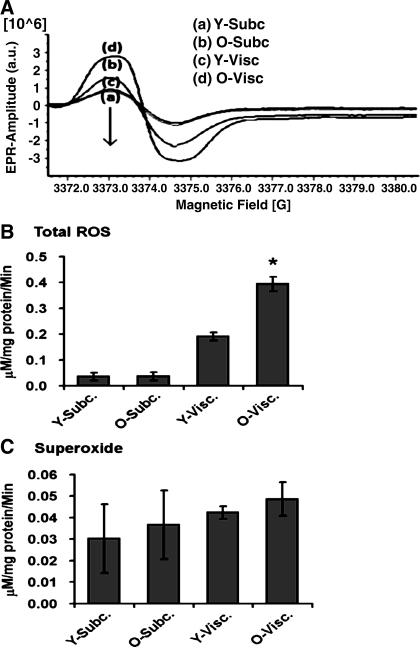
Next, we sought to elucidate whether the levels of ROS within subcutaneous and visceral fat depots were altered during aging. Using electron paramagnetic resonance (EPR), we demonstrated for the first time that the levels of ROS are elevated in aging visceral adipose tissue (Fig. 3), which was not observed in subcutaneous adipose depots. Studies then turned to determining if these age-related changes could be explained, in part, by changes in the expression of genes involved in the generation and detoxification of ROS. Interestingly, in these studies we observed that aging visceral adipose tissue exhibited a markedly low level of altered redox gene expression, for genes involved in the genesis and detoxification of ROS (Table 1), compared with aging subcutaneous fat. In fact, aging subcutaneous fat exhibited an approximately eightfold higher number of genes that exhibited altered levels of expression in response to aging compared with aging visceral adipose tissue (Table 1). Of particular interest was robust elevation in multiple glutathione peroxidises and a specific form of glutathione transferase, which are all involved in detoxification of lipid peroxidation products, raising the potential for subcutaneous adipose tissue being more capable of detoxifying these potentially toxic products of oxidative stress.
Fig. 3.
Aging increases reactive oxygen species (ROS) in adipose tissue. Total levels of ROS were measured in visceral and subcutaneous fat depots from adult (6-mo- and 23-mo-old) mice. Following collection of fat pads, tissue was dissected and incubated with spin label, and amounts of ROS were quantified using electron paramagnetic resonance (EPR). Representative EPR spectra (A) and quantification of total ROS (B) and superoxide (C) are shown; see materials and methods for experimental details. Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. young (6-mo-old)adult mice.
Levels of hypoxia observed in aging adipose tissue promote alterations in ROS and adipose biology.
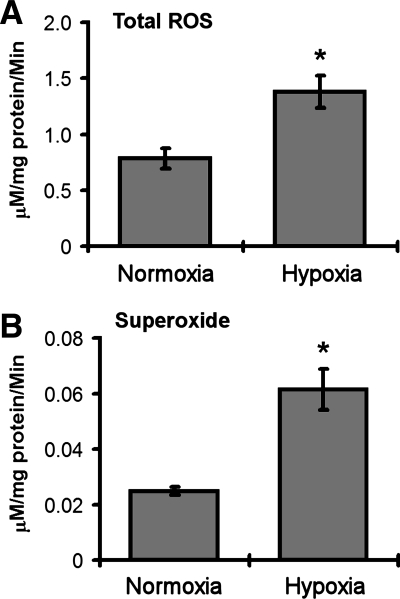
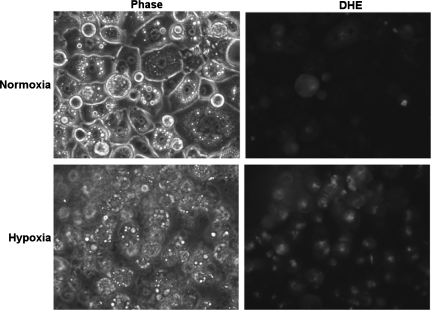
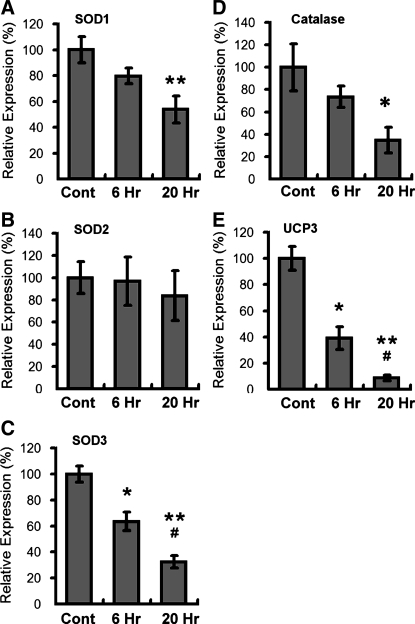
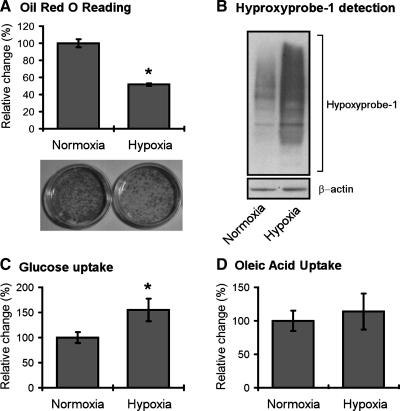
We next conducted studies to determine the effects of age-related hypoxia (∼38% decrease in ambient oxygen), on adipose biology. We observed that this level of hypoxia did not induce cell death in 3T3-L1 adipocytes following 48 h of hypoxia exposure (data not shown). 3T3-L1 adipocytes were then exposed to overnight hypoxia (38% reduction in ambient oxygen) and analyzed for ROS levels using the same EPR technologies outlined above for adipose tissue. In these studies, we observed that hypoxia significantly increased the levels of total ROS (Fig. 4), and increased the levels of superoxide in particular (Fig. 4). Studies with dihydroethidium, a dye preferentially oxidized by superoxide, demonstrated further evidence for hypoxia-induced increases in ROS within adipocytes (Fig. 5). Studies were then conducted to determine whether hypoxia promoted changes in the expression of genes known to be involved in the regulation of ROS. In these studies we saw no change in the levels of SOD2 but saw significant and dramatic decreases in SOD1 and SOD3 following hypoxia (Fig. 6). Similarly, the levels of catalase and UCP3 were decreased following hypoxia (Fig. 6). We then sought to determine whether age-related levels of hypoxia promoted alterations in the lipid content of differentiated 3T3-L1 adipocytes. Within 18 h of hypoxia, there was observed to be a significant reduction in the levels of Oil red O staining, consistent with decreased levels of lipid content following hypoxia (Fig. 7). Cells under these same conditions exhibited significant levels of hypoxyprobe-1 immunostaining consistent with elevated levels of hypoxia (Fig. 7). Additionally, increased levels of insulin-stimulated glucose uptake (Fig. 7) were observed following hypoxia, with no significant alteration in insulin-stimulated free fatty acid uptake observed (Fig. 7).
Fig. 4.
EPR study in differentiated 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were subjected to normoxia or hypoxia (38% reduction of ambient oxygen) as outlined in materials and methods. Cells were analyzed via EPR for total ROS and superoxide levels. See materials and methods for experimental details. Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. normoxia.
Fig. 5.
Hypoxia induces increased ROS as detected by dihydroethidium staining. Differentiated 3T3-L1 adipocytes were subjected to normoxia or hypoxia (38% reduction of ambient oxygen) as outlined in materials and methods. Cells were analyzed for dihydroethidium (DHE) oxidation, an established indicator of ROS, to determine if hypoxia increased ROS levels. Representative image from 6 experiments revealed evidence for increased DHE oxidation following hypoxia consistent with the presence of oxidative stress.
Fig. 6.
Hypoxia induces selective alterations in expression of genes involved in ROS regulation. Differentiated 3T3-L1 adipocytes were subjected to normoxia or hypoxia (38% reduction of ambient oxygen) as outlined in materials and methods. At times indicated, cells were harvested and analyzed via RT-PCR for the level of gene expression. See materials and methods for experimental details. Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. normoxia; **P < 0.05 vs. normoxia; #P < 0.05 vs. 6 h.
Fig. 7.
Hypoxia induces alterations in glucose uptake but not free fatty acid uptake. Differentiated 3T3-L1 adipocytes were subjected to normoxia or hypoxia (38% reduction of ambient oxygen) as outlined in materials and methods. Cells were analyzed for Oil red O (A), Hyproxyprobe-1 levels (B), insulin-stimulated glucose uptake (C), and insulin-stimulated free fatty acid uptake (D) as outlined in materials and methods. Data are expressed as means ± SE and represent results from n = 8 for each experimental end point. *P < 0.05 vs. normoxia.
DISCUSSION
The current study demonstrates for the first time that increases in hypoxia occur in aging fat tissue. Although both of our hypoxia measurements (immunochemical and direct oxygen) point to evidence of increased hypoxia in aging adipose tissue, there is a noted discrepancy between the findings of direct oxygen measurements. Specifically, direct measurements of oxygen via oxygen probe revealed that only aging visceral adipose tissue exhibited hypoxia. In contrast, analysis of hypoxia using the Hyproxyprobe method revealed evidence for hypoxia in both aging subcutaneous and aging visceral depots. The discrepancy between these two methodologies is likely explained by the fact that the direct measurement of oxygen, using the oxygen probe method, produces an acute measure of steady-state oxygen within a tissue at a specific site within the tissue. In our analysis, we observed that there was significant variability in the oxygen level throughout the adipose depot; thus, all studies were required to focus on using measures of oxygen within the same anatomic area of the adipose depot (distal region). In contrast, the Hyproxyprobe method detects the presence of hypoxia via the bioreductive activation of the Hypoxyprobe compound, which occurs within the entire body of adipose tissue and as the result of this reaction generates 2-nitroimidazoles that can then react with proteins throughout the adipose depot. The proteins modified by these reactions can then be detected via immunochemical methodologies. As such, the Hyproxyprobe method is able to measure both acute and chronic hypoxia, unlike the direct oxygen measures used in Fig. 2. Taken together, our hypoxia studies suggest that there is likely very localized and acute hypoxia that occurs in both subcutaneous and visceral adipose (as detected by Hyproxyprobe), that can only sporadically be detected by direct oxygen measures. Studies are underway to identify with Hyproxyprobe immunohistochemistry the specific cell types and tissue regions within these same depots that may be preferentially undergoing age-related hypoxia.
Numerous studies have demonstrated that the effects of aging on adipose tissue can be very anatomically and cell type specific. For example, preadipocytes are known to be particularly vulnerable to age-related changes in gene expression compared with mature adipocytes (1, 9, 10, 28, 60). Our studies indicate that the microenvironment where vulnerable cells (preadipocytes) are located, within the aging adipose tissue, may have dramatic effects on the ultimate long-term function of individual cells. These highly focal periods, or focused sites, of hypoxia during aging may arise as the result of decreased vascularization of the adipose tissue due to increased adipose cell size or as the result of adipose remodeling. However, the fact that the age-related hypoxia observed in the present study appeared to be very mild and potentially transitory, it is possible that alternative mechanisms may be responsible for mediating hypoxia in aging adipose tissue. These mechanisms are likely to be much more rapidly reversible than hypoxia induced by either increases in adipose size or tissue remodeling. For example, events such as localized elevations in inflammation may promote changes in regional blood flow and thereby induce hypoxia. Studies are currently underway to begin to identify the basis for age-related increases in hypoxia within adipose tissue.
The majority of studies to date have studied the effects of anoxia (≥99% reduction in oxygen) on adipose cells (40, 42, 49, 54, 72). This level of hypoxia is likely to occur in adipose tissue in conditions such as diet-induced obesity and/or type 2 diabetes, where rapid tissue expansion or severe tissue remodeling is known to occur (62, 69, 72). Studies of anoxia are therefore very informative of what is occurring in adipose tissue in those experimental and clinical paradigms. However, it is less clear that such high levels and durations of anoxia occur in aging fat tissue per se; therefore, there is a heightened need to understand the effects of less severe levels of hypoxia on adipose biology. In the current study, we demonstrate for the first time that a 38% reduction in oxygen, similar to what is observed in aging visceral adipose tissue, is sufficient to alter glucose uptake, redox gene expression, and ROS levels. Understanding the similarities and differences between the effects of hypoxia and anoxia on adipose cells, particularly in primary adipocytes, is going to be a critical next step in elucidating the contribution of hypoxia to adipose aging. Previous studies have shown that events such as HIF-1 induction and HIF-1 DNA binding can vary severalfold when moving from 0.5% to 2% oxygen (27, 45). Second, it is well established that the ultimate consequences of hypoxia on cell biology are modulated by numerous stimuli relevant to adipocytes, including MAPK (38), IGF-I (64), and nitric oxide (6). In this scenario, the ultimate effects of hypoxia on adipocytes are regulated not only by the amount and duration of hypoxia but also by the summation of cell signaling and autocrine pathways that regulate hypoxia-associated signal transduction.
These studies are the first to show increases in the levels of ROS in aging adipose tissue. ROS are known to be essential to many cell signaling pathways and likely play a key physiological role in adipose biology (5, 19, 20, 23, 57). In contrast to physiological redox signaling, deleterious oxidative stress is known to occur in many tissues with aging and is known to contribute to the deleterious changes in tissue function that are observed in many aging tissues (15, 21, 55, 59). Our studies point to the potential for deleterious oxidative stress to occur in response to age-related increases in hypoxia. This is based on the presence of both hypoxia and increased ROS in aging adipose tissue, and on our findings that age-related levels of hypoxia are sufficient to increase ROS and decrease the levels of key antioxidant enzymes. Such data point to the potential for even low levels of hypoxia interacting in a synergistic manner with elevations in ROS to promote oxidative stress in aging adipose tissue. Interestingly, our current study suggests that subcutaneous fat undergoes much more robust elevations in redox regulatory genes during aging compared with visceral adipose tissue. Such data raise the specter of visceral adipose tissue potentially being more vulnerable during aging to the deleterious effects of hypoxia and ROS due to a decreased ability to increase vital antioxidant pathways compared with subcutaneous fat tissue. Clearly, although more investigation is needed, these data point to the potential of age-related increases in oxidative stress within adipose tissue being somewhat fat depot specific.
Age-related levels of hypoxia were sufficient to increase insulin-stimulated glucose uptake, with both acute (not shown) and chronic hypoxia observed to increase insulin-stimulated uptake. Previous studies have demonstrated that short-term (4 h) anoxia increases insulin-stimulated glucose uptake (70, 74), whereas chronic anoxia decreases insulin-stimulated glucose uptake. Interestingly, the levels of hypoxia in the present study did not significantly alter free fatty acid uptake but did significantly reduce the levels of lipid droplets as measured by decreased Oil red O content. Decreased adipose size, presumably due at least in part to decreased lipid content, are known to occur during adipose aging (33–36). Because we did not see a decrease in free fatty acid uptake in response to age-related levels of hypoxia, our data suggest a role for hypoxia potentially increasing lypolysis and thereby contributing to established age-related alterations in lipid homeostasis in adipocytes.
It is important to note that the present study used animals that were not placed on high-fat diets (HFDs), and therefore do not represent commonly studied models of diet-induced obesity. Studies from our laboratory and others' have demonstrated that HFDs exacerbate tissue dysfunction in aging animals (11, 29, 46, 68, 76). Additionally, studies from our laboratory and others' have shown that HFDs promote much greater amounts of adipose gain, and presumably adipose tissue dysfunction, compared with younger animals (46, 50, 66). These data point to important, and as yet unknown, interactions between obesity and aging. We anticipate that clinically relevant alterations in adipose tissue occur in aging adipose tissue following HFD exposure, which do not occur or occur to a lesser degree, than is observed in young adults exposed to a HFD. While the basis for these alterations is likely to be complex, it is likely that a role for both hypoxia and oxidative stress will be identified. Understanding the basis for the genesis of adipose pathology during aging, particularly in response to HFD exposure, will have important clinical relevance to understanding the basis for metabolic disease and its associated morbidities in the elderly.
DISCLOSURES
No conflicts of interest are reported by the author(s).
REFERENCES
- 1. Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano GM, Fabbri A, Caprio M. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem 110: 564–572, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bahr J, Klöting N, Klöting I, Follak N. Transplantation of adipose tissue protects BB/OK rats from type 1 diabetes development. Transpl Immunol 24: 238–240, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 48: 94–98, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgo M. p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem 283: 34283–34293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17: 446–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signalling. Cardiovasc Res 75: 275–282, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant Protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 90: 2282–2289, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53: 2087–2094, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci 65: 242–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol 42: 463–471, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dasuri K, Ebenezer PJ, Zhang L, Fernandez-Kim SO, Uranga RM, Gavilán E, Di Blasio A, Keller JN. Selective vulnerability of neurons to acute toxicity after proteasome inhibitor treatment: implications for oxidative stress and insolubility of newly synthesized proteins. Free Radic Biol Med 49: 1290–1297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal 8: 163–172, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Douglas RM, Haddad GG. Can O2 dysregulation induce premature aging? Physiology (Bethesda) 23: 333–349, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-κB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol 296: F298–F305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev 3: 168–177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology 149: 2628–2636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem 277: 4062–4068, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine mediated process? Diabetes 51: 2951–2958, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 7: 1021–1031, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomes P, Simão S, Silva E, Pinto V, Amaral JS, Afonso J, Serrão MP, Pinho MJ, Soares-da-Silva P. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev 2: 138–145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol 304: 19–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35: 505–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta 1790: 1117–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11: 11–18, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S57–S63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang BH, Smenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol 271: C1172–C1180, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatore K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol 280: R1772–R1780, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Keller JN. Reciprocal interactions between diet, metabolism, and the nervous system. Biochim Biophys Acta 1792: 393–394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kilroy G, Burk DH, Floyd ZE. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One 9: e6940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirkland JL, Dax EM. Adipose hormone responsiveness and aging in the rat. J Am Geriatr Soc 32: 219–228, 1984 [DOI] [PubMed] [Google Scholar]
- 33. Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat cell function. J Amer Geriatr Soc 45: 959–967, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol Cell Physiol 258: C206–C210, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Kirkland JL, Hollenberg CH, Gillon WS. Effects of fat depot size on differentiation-dependent gene expression in rat preadipocytes. Int J Obes 20, Suppl 3: S102–S107, 1996 [PubMed] [Google Scholar]
- 36. Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 37: 757–767, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Kuk JL, Saunders TJ, Davidson L, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 8: 339–348, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Ye D. Cancer therapy by targeting hypoxia-inducible factor-1. Curr Cancer Drug Targets 10: 782–796, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DJ. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. Lipid Res 48: 609–620, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Mack I, BelAiba RS, Djordjevic T, Görlach A, Hauner H, Bader BL. Functional analyses reveal the greater potency of preadipocytes compared with adipocytes as endothelial cell activator under normoxia, hypoxia, and TNFα exposure. Am J Physiol Endocrinol Metab 297: E735–E748, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58: 803–812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maenhaut N, Boydens C, Van de Voorde J. Hypoxia enhances the relaxing influence of perivascular adipose tissue in isolated mice aorta. Eur J Pharmacol 641: 207–212, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med 46: 462–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-(kappa)B-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85: 473–483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyata T, Takizawa S, van Ypersele de Strihou Hypoxia C. 1. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. Am J Physiol Cell Physiol 300: C226–C231, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114: 1581–1589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogunshola OO, Antoniou X. Contribution of hypoxia to Alzheimer's disease: is HIF-1alpha a mediator of neurodegeneration? Cell Mol Life Sci 66: 3555–3563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 117: 798–805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pérez de Heredia F, Wood IS, Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflügers Arch 459 509–518, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219: 25–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pitombo C, Araujo EP, De Souza CT, Pareja JC, Geloneze B, Velloso LA. Amelioration of diet-induced diabetes mellitus by removal of visceral fat. J Endocrinol 191: 699–706, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol 323: 20–34, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58: 95–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol 15: S29–S36, 2010 [PubMed] [Google Scholar]
- 56. Rossi P, Marzani B, Giardina S, Negro M, Marzatico F. Human skeletal muscle aging and the oxidative system: cellular events. Curr Aging Sci 1: 182–191, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17: 422–427, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol 30: 648–652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sesti F, Liu S, Cai SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration? Trends Cell Biol 20: 45–51, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell 9: 667–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60: 329–339, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Trayhurn P, Wood IS. Adipokines: inflammation and pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Triens C, Giorgetti-Peraldi S, Murdaca J, Montheouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor 1. Mol Endocrinol 19: 1304–1317, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal 13: 539–549, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller JN. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem 114: 344–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl 114: S3–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 68. White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis 35: 3–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 68: 370–377, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Wood IS, Wang B, Lorente-Cebrian S, Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-glucose uptake in human adipocytes. Biochem Biophys Res Commun 361: 468–473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? Dig Dis Sci 54: 1847–1856, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296: E333–E342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res 96: 1042–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Zhang L, Bruce-Keller AJ, Dasuri K, Nguyen AT, Liu Y, Keller JN. Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim Biophys Acta 1792: 417–422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang L, Ebenezer PJ, Dasuri K, Bruce-Keller AJ, Fernandez-Kim SO, Liu Y, Keller JN. Activation of PERK kinase in neural cells by proteasome inhibitor treatment. J Neurochem 112: 238–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]