Abstract
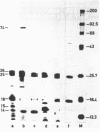
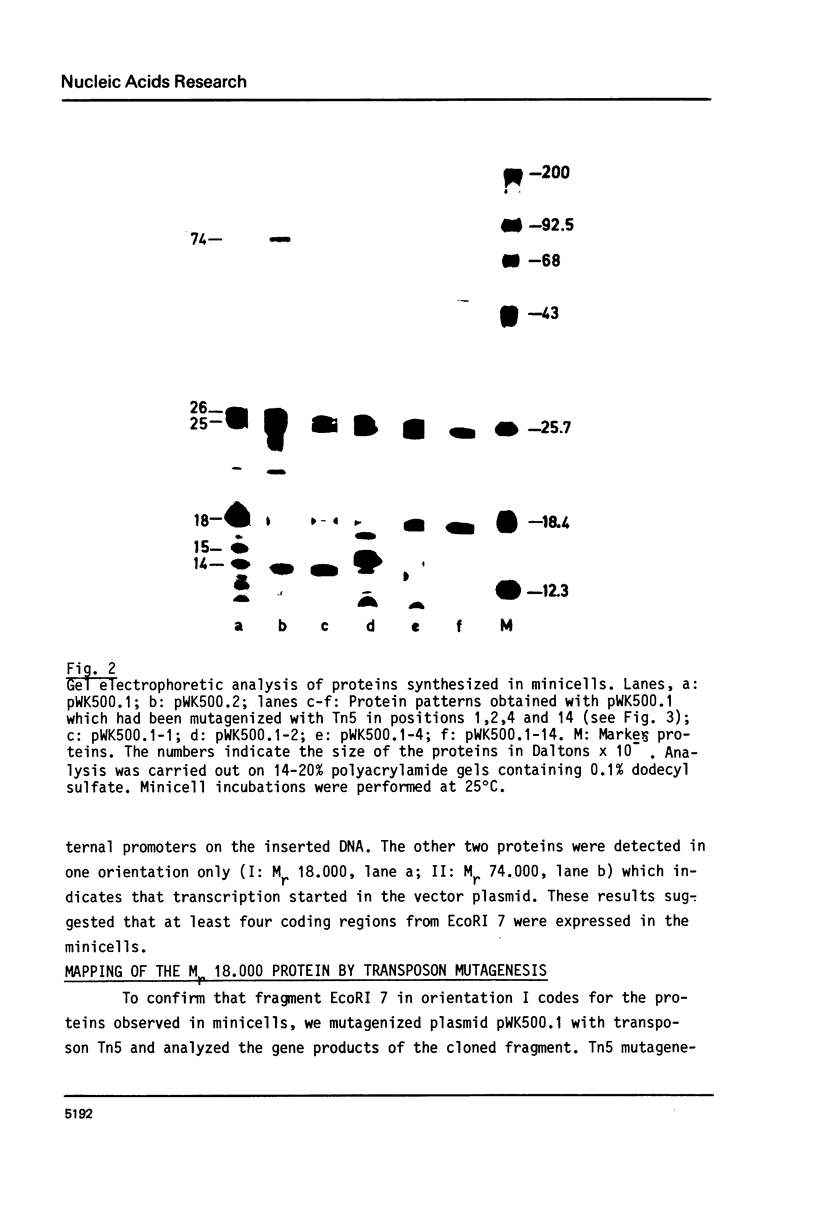
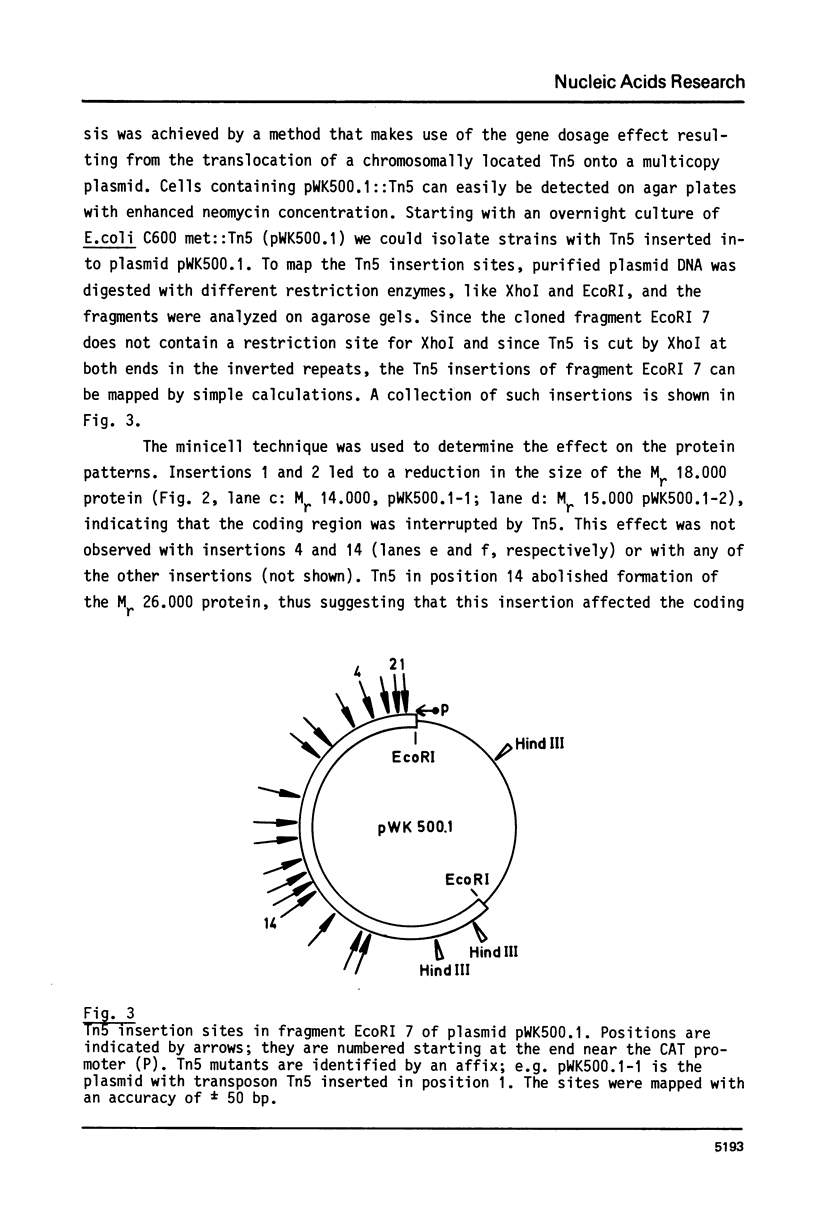
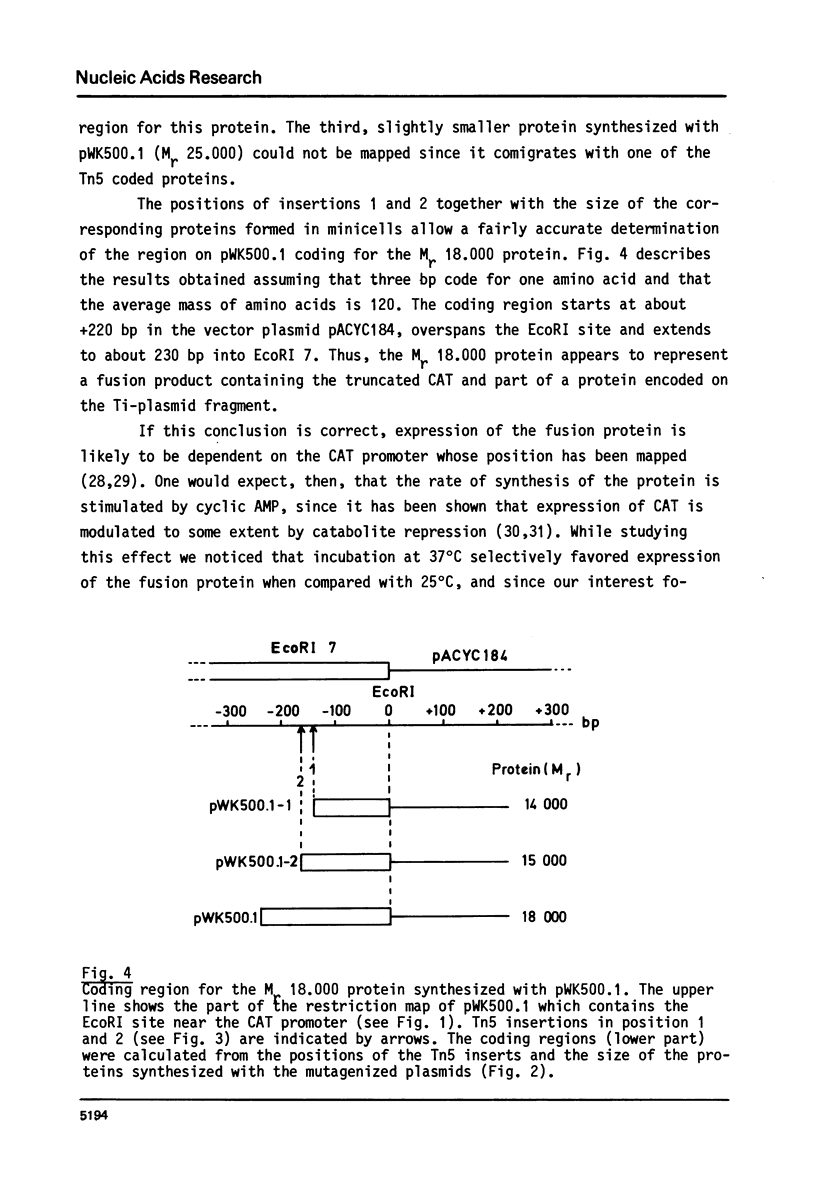
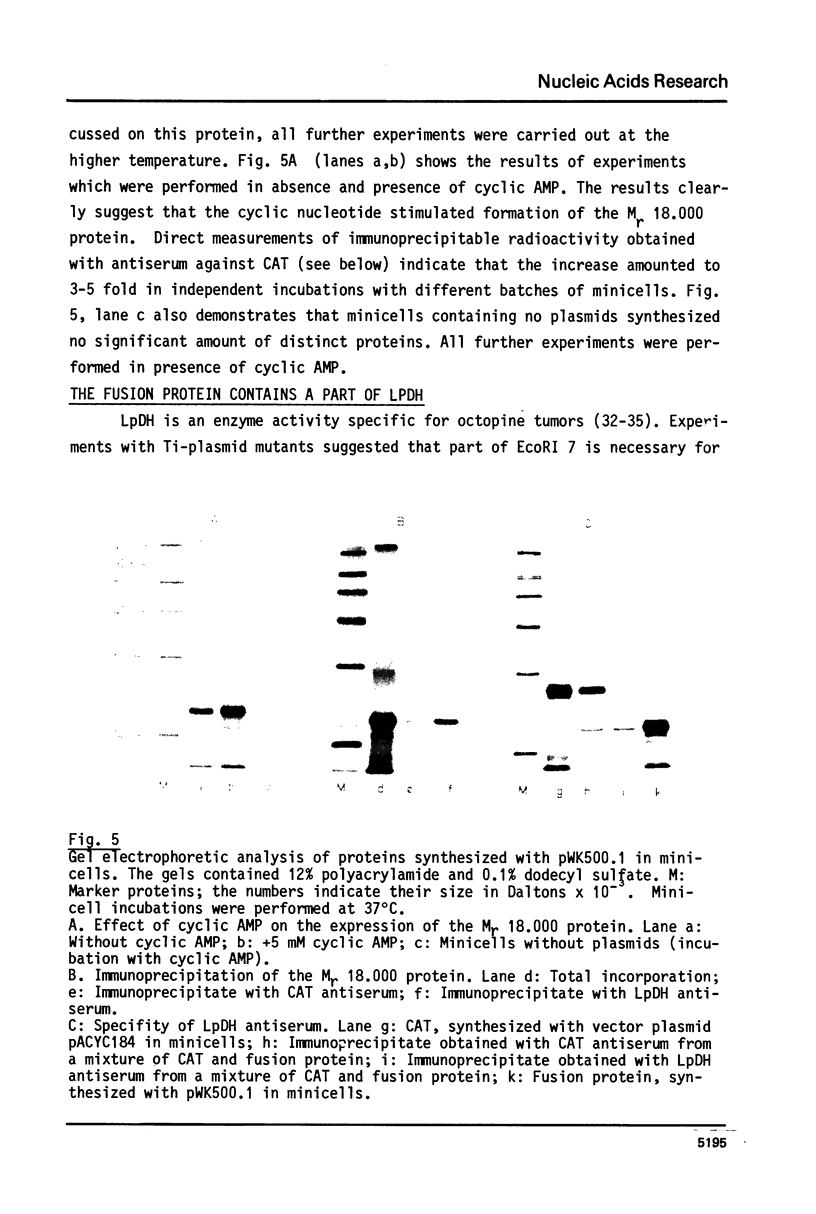
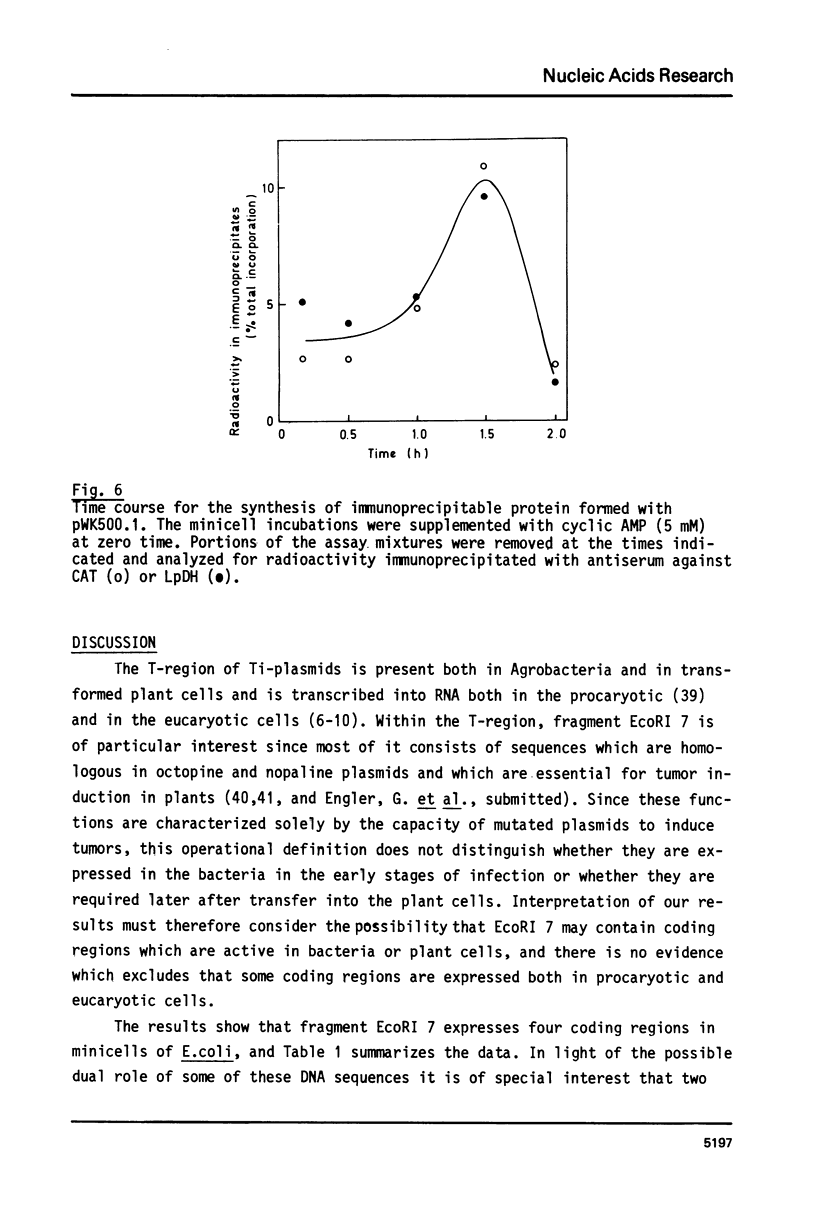
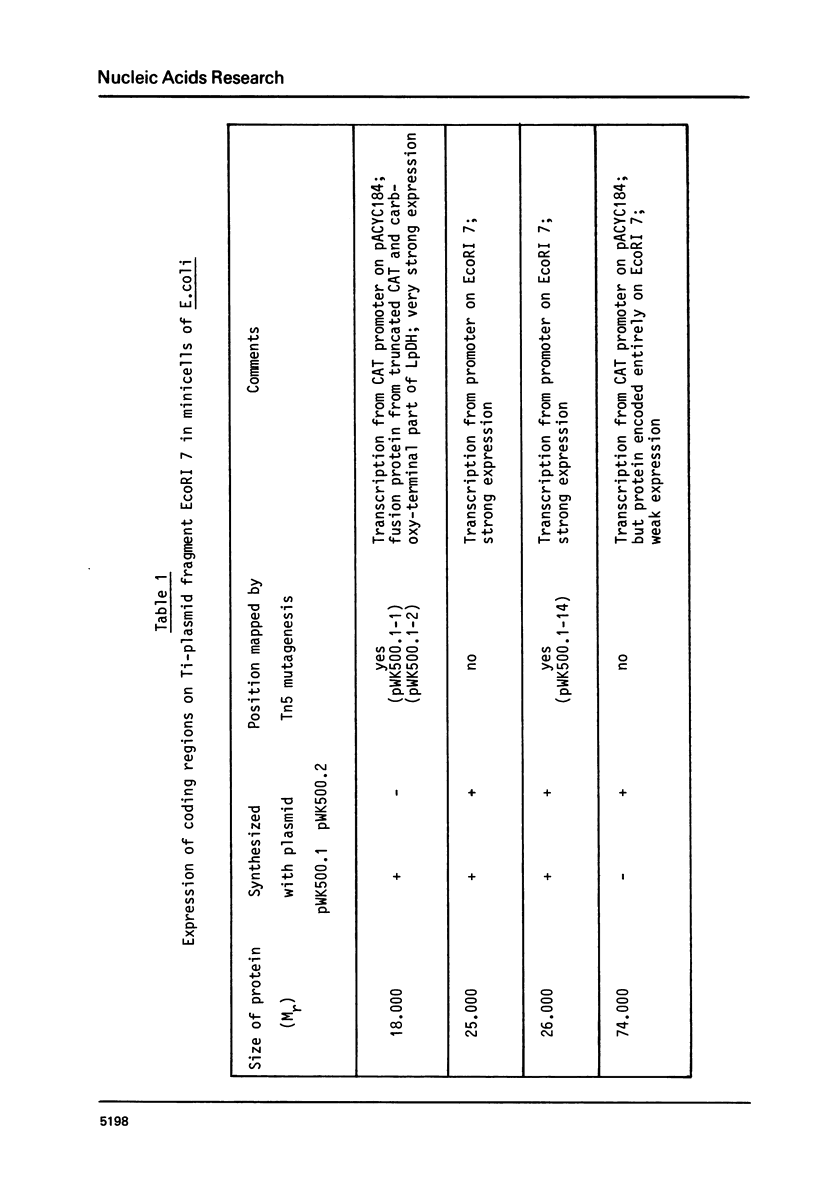
Fragment EcoRI 7 from Ti-plasmid pTi Ach5, a part of the T-DNA in octopine tumors, was cloned in both orientations into pACYC184 and expressed in E. coli minicells. The cells synthesized four proteins from four different coding regions on EcoRI 7. Two of the proteins (Mr 25.000 and 26.000) were expressed with promoters from the Ti-plasmid fragment, while transcription for the two other proteins (Mr 18.000 and 74.000) started with a promoter on pACYC184. The Mr 18.000 protein represented a fusion product between chloramphenicol acetyltransferase (CAT) on pACYC184 and a part of lysopine dehydrogenase (LpDH), the enzyme synthesizing octopine and lysopine in plant tumor cells. The results suggest that E. coli minicells are a valuable system to study the proteins coded for by the T-region of Ti-plasmids.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Saiki R. K., Yadav N., Gordon M. P., Quetier F. T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4060–4064. doi: 10.1073/pnas.77.7.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Pastan I., Shaw W. V., Rosner J. L. Stimulation by cyclic AMP and ppGpp of chloramphenicol acetyl transferase synthesis. Nat New Biol. 1973 Feb 21;241(112):237–239. doi: 10.1038/newbio241237a0. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Gordon M. P., Nester E. W., Aronson A. I. Transcription of the Agrobacterium Ti plasmid in the bacterium and in crown gall tumors. Plasmid. 1981 Jul;6(1):17–29. doi: 10.1016/0147-619x(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Giphart-Gassler M., Reeve J., van de Putte P. Polypeptides encoded by the early region of bacteriophage Mu synthesized in minicells of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):165–191. doi: 10.1016/0022-2836(81)90339-9. [DOI] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack E., Kemp J. D. Purification and Characterization of the Crown Gall-specific Enzyme, Octopine Synthase. Plant Physiol. 1980 May;65(5):949–955. doi: 10.1104/pp.65.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J., Smith D. H. Catabolite repression of chloramphenicol acetyl transferase synthesis in E. coli K12. Biochem Biophys Res Commun. 1971 Jan 8;42(1):57–62. doi: 10.1016/0006-291x(71)90361-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kemp J. D. Octopine as a marker for the induction of tumorous growth by agrobacterium tumefaciens strain B6. Biochem Biophys Res Commun. 1976 Apr 5;69(3):816–822. doi: 10.1016/0006-291x(76)90948-7. [DOI] [PubMed] [Google Scholar]
- Koekman B. P., Ooms G., Klapwijk P. M., Schilperoort R. A. Genetic map of an octopine TI-plasmid. Plasmid. 1979 Jul;2(3):347–357. doi: 10.1016/0147-619x(79)90018-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Localisation of the transcription initiation site of the chloramphenicol resistance gene on plasmid pAC184. FEBS Lett. 1980 Apr 21;113(1):42–46. doi: 10.1016/0014-5793(80)80490-x. [DOI] [PubMed] [Google Scholar]
- McPherson J. C., Nester E. W., Gordon M. P. Proteins encoded by Agrobacterium tumefaciens Ti plasmid DNA (T-DNA) in crown gall tumors. Proc Natl Acad Sci U S A. 1980 May;77(5):2666–2670. doi: 10.1073/pnas.77.5.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Vreugdenhil D., Schilperoort R. A. Properties of D(+)-lysopine dehydrogenase from crown gall tumour tissue. Biochim Biophys Acta. 1977 Dec 8;485(2):268–277. doi: 10.1016/0005-2744(77)90163-2. [DOI] [PubMed] [Google Scholar]
- Pühler A., Burkardt H. J., Klipp W. Cloning of the entire region for nitrogen fixation from Klebsiella pneumoniae on a multicopy plasmid vehicle in Escherichia coli. Mol Gen Genet. 1979 Oct 2;176(1):17–24. doi: 10.1007/BF00334290. [DOI] [PubMed] [Google Scholar]
- Reeve J. Use of minicells for bacteriophage-directed polypeptide synthesis. Methods Enzymol. 1979;68:493–503. doi: 10.1016/0076-6879(79)68038-2. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Otten L., Simons G., Schmalenbach W., Schröder J., Schröder G., Van Montagu M., de Vos G., Schell J. Nuclear and polysomal transcripts of T-DNA in octopine crown gall suspension and callus cultures. Mol Gen Genet. 1981;182(2):255–262. doi: 10.1007/BF00269667. [DOI] [PubMed] [Google Scholar]
- Yang F., McPherson J. C., Gordon M. P., Nester E. W. Extensive transcription of foreign DNA in a crown gall teratoma. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1273–1277. doi: 10.1016/0006-291x(80)90424-6. [DOI] [PubMed] [Google Scholar]