Abstract
Menin is the ubiquitously expressed nuclear protein product of the MEN1 gene, which interacts with PKB/Akt in the cytoplasm to inhibit its activity. This study describes a novel insulin-dependent mechanism of menin regulation and interaction with other metabolic proteins. We show that insulin downregulated menin in a time-dependent manner via the human insulin receptor. Inhibition analysis indicated a critical role for the protein kinase Akt in regulation of menin expression and localization. Insulin-mediated decrease in menin expression was abrogated by the PI3K/Akt inhibitor LY-294002 at early time points, from 2 to 7 h. Furthermore, exposure to insulin resulted in the cytoplasmic localization of menin and increased interaction with FOXO1. Fasting followed by refeeding modulates serum insulin levels, which corresponded to an increase in menin interaction with FOXO1 in the liver. Liver-specific hemizygous deletion of menin resulted in increased expression of FOXO1 target genes, namely IGFBP-1, PGC-1α, insulin receptor, Akt, and G-6-Pase. This study provides evidence that menin expression and localization are regulated by insulin signaling and that this regulation triggers an increase in its interaction with FOXO1 via Akt with metabolic consequences.
Keywords: forkhead box O1, Akt, liver, metabolism
menin is the protein product of the multiple endocrine neoplasia type 1 (MEN1) gene, with its loss causing a syndrome characterized by development of neuroendocrine tumors. Menin has been shown to interact with key transcriptional elements such as NF-κB, Smad3, and JunD to regulate expression of their target genes (1, 11, 12). Menin is ubiquitously expressed in all tissues, both endocrine and neuroendocrine (23), and has been described as a tumor suppressor gene in all organs except the liver (21). Previous reports have described a high prevalence of impaired fasting glucose and diabetes mellitus in families with MEN1 inheritance (15). Karnik et al. (13) implicated menin in the development of gestation-induced diabetes and associated the loss of menin in β-cells of pancreatic islets to increased proliferation.
Menin regulates transcription factors downstream of mitogen-activated protein kinase (MAPK) signaling (9) and interacts with cytoplasmic proteins involved in cell cycle regulation (6). A major protein involved in regulation of growth and metabolism is protein kinase B (PKB/Akt). This important serine kinase, found in the cytoplasmic compartment, is inactivated by menin via direct interaction (24). Both the MAPK and Akt pathways are activated downstream of insulin receptor signaling, indicating that menin could be involved in the regulation of this signaling cascade.
Although the physiological role of menin has not yet been elucidated, there have been many proteins identified that interact with menin, alluding to functions related to cell cycle regulation, apoptosis, and transcriptional regulation.
The transcription factor forkhead box O1 (FOXO1) plays a central role in the hepatic regulation of key metabolic genes involved in glucose and fatty acid metabolism as well as cellular growth and differentiation (10, 22). The transcriptional activity of FOXO1 is regulated by insulin via Akt. In the presence of insulin, FOXO1 is inactivated by Akt through phosphorylation, resulting in the translocation of FOXO1 into cytoplasm (14). Deregulated expression and activity of FOXO1 protein results in secondary insulin resistance (8). Fasting and refeeding acutely modulate insulin levels to activate genes involved in glucose production and fatty acid metabolism. In times of feeding and high Akt activity, FOXO1 is exported from the nucleus into the cytoplasm (20). In the liver, FOXO1 mediates the switch from glycolysis and carbohydrate metabolism to gluconeogenesis, with the glucose-6-phosphatase (G-6-Pase) enzyme playing a rate-limiting role (4).
In this study we demonstrate that insulin is a direct regulator of menin expression via the Akt pathway and modulates interaction of menin with FOXO1 in primary hepatocytes and HepG2 cells. We identified that fasting and refeeding regulate the expression of menin in the liver and are also associated with increased interaction of menin with FOXO1 in vivo. We further implicate hepatic menin in glucose metabolism since the hemizygous deletion in the liver induces overproduction of G-6-Pase, IGF-binding protein-1 (IGFBP-1), and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which are FOXO1 target genes involved in hepatic glucose production. Furthermore, liver-specific menin hemizygous mice display reduced glucose levels during glucose tolerance tests, whereas insulin tolerance testing showed no change. These results provide evidence that menin has a metabolic role in the liver, whose interaction with FOXO1 increases during refeeding and is regulated by insulin.
EXPERIMENTAL PROCEDURES
Animal Maintenance
Animals were kept on a 12:12-h dark-light cycle and fed standard chow ad libitum. All procedures were approved by Institutional Animal Care and Utilization Committee at the University of Toledo. Liver-specific menin hemizygous mice on a mix of FVB/129S mice expressing loxP sequences on exons 3–10 of the MEN1 gene and C57/Blk6 mice expressing Albumin-Cre were crossed together, and genotyping was performed with tail lysates. Wild-type (WT) and Flox (loxP) sequences with Cre expression were considered hemizygous for menin in the liver (HETs).
Genotyping.
Primers A and G give the WT allele, and primers F and G give the floxed allele. The primer sequences are as follows: A-Men 1, 5′-TCC AGT CCC TCT TCA GCT TC-3′; F-Men1, 5′-GCC ATT TCA TTA CCT CTT TCT CCG-3′; G-Men1, 5′-TAC CAC TGC AAA GGC CAC GC-3′; Cre forward, 5′-TGA GGT TCG CAA GAA CCT GAT GGA-3′; Cre reverse, 5′-GCC GCA TAA CCA GTG AAA CAG CAT-3′.
PCR-run conditions.
Tail snips were collected from mice at 21 days after birth and digested in a mixture of direct PCR and tail lysis buffer (Thermo Fisher Scientific, Pittsburgh, PA) plus proteinase K (Roche, Indianapolis, IN) overnight, rotating at 55°C. Lysates were collected and incubated at 85°C for 45 min.
Men1 gene.
The PCR running conditions for the Men-1 gene were an initial step of 95°C for 15 min followed by 35 cycles of denaturing at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 1 min and 30 s with a final step of 1 cycle of 72°C for 10 min and hold at 4°C. The PCR running conditions of the Cre gene were an initial step pf 95°C for 15 min followed by 35 cycles of denaturing at 94°C for 30 s, annealing at 66.6°C for 30 s, and extension at 72°C for 1 minute and 30 s with a final step of 1 cycle of 72°C for 10 min and hold at 4°C. PCR products were run on a 1% agarose gel in TAE for 30 min at 120 V.
Mice were euthanized at 25 wk of age.
Cell Culture
Primary hepatocytes were isolated from livers of WT mice according to the protocol (19), and HepG2 cells were maintained at 37°C and 5% CO2 in DMEM medium containing 10% FBS and penicillin and streptomycin. All experiments were performed on 80% confluent cells.
Transfections
Stable transfection of NIH 3T3 cells with the human insulin receptor has been described previously (7).
Western Blot
The concentration of proteins in tissue and serum lysates was quantitated by BCA protein assay (Pierce, Rockford, IL) prior to analysis by 10 or 4–12% gradient SDS-PAGE (Invitrogen, Carlsbad, CA), respectively, and immunoprobing with specific antibodies. These include polyclonal antibodies against Menin (AbCam, Cambridge, MA, and Santa Cruz Biotechnology, Santa Cruz, CA), FOXO1, (Santa Cruz Biotechnology), p-ERK, and ERK (Cell Signaling Technology, Beverly, MA) in addition to monoclonal antibodies against GAPDH and Lamin B (Sigma-Aldrich, St. Louis, MO). Proteins were detected by Odyssey infrared imaging system using corresponding secondary antibodies conjugated to near infrared dyes.
Cellular Fractionation
Cells were fractionated using the NE-PER nuclear protein extraction kit (Thermo Scientific, Rockford, IL) according to manufacturer's protocol. Briefly, cells were harvested via trypsin-EDTA (Invitrogen) and pelleted at 500 g for 5 min (all centrifuge steps at 4°C). The supernatant was removed, and 200 ml of ice-cold cytoplasmic extraction reagent 1 was added to the pellet (supplemented with protease and phosphatase inhibitors) and vortexed vigorously for 15 s. The mixture was incubated for 10 min prior to 11 μl of cytoplasmic extraction reagent 2 being added and vortexed for 5 s. The mixture was incubated on ice for 1 min, vortexed again, and centrifuged for 5 min at maximum speed. The supernatant (cytoplasmic fraction) was extracted and stored on ice. The pellet was resuspended in 100 μl of nuclear extraction reagent (supplemented with protease and phosphatase inhibitors) and vortexed for 15 s, and steps were repeated every 10 min for a total of 40 min. The mixture was centrifuged at full speed for 10 min, and extracts were subjected to immunoprecipitation and immunoblotting.
Immunoprecipitation
Primary hepatocytes and HepG2 cells were lysed, and proteins (1 mg) were subjected to immunoprecipitation with an anti-menin or anti-FOXO1 polyclonal antibody analyzed on SDS-PAGE, followed by immunoprobing with FOXO1 and, menin.
Quantitative Real-Time PCR
RNA was extracted using the TRIzol method according to the manufacturer's protocol. Following DNAse digestion (DNAfree; Ambion), 100 ng RNA was transcribed into cDNA in a 20-μl reaction using a High Capacity cDNA kit (Applied Biosystems, Carlsbard, CA), analyzed, and amplified (ABI 7900 HT system). PCR was performed in a 10-μl reaction containing 5 μl of cDNA (1/5 diluted), 1× SYBR Green PCR Master Mix (Applied Biosystems), and 300 nM of each primer. The primers are as follows: menin forward, TCATTGCTGCCCTCTATGCC; menin reverse, TCCAGTTTGGTGCCTGTGATG; GAPDH forward, CCACCAGCCCCAGCAAGAGC; GAPDH reverse, GGCAGGGACTCCCCAGCAGT; 18s forward, TTGACGGAAGGGCACCACCAG; 18s reverse, GCACCACCACCCACGGAATCG.
CT values (cycle threshold) were used to calculate the amount of amplified PCR product relative to GAPDH for 18 s. The relative amount of mRNA was calculated as 2−ΔCT. Results were expressed as fold change as means ± SE.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde-PBS and blocked with 20% normal donkey serum-PBS and 0.1% Triton X-100 for 30 min. The slides were incubated for 1 h with a 1:50 dilution of rabbit anti-menin (Bethyl Laboratories, Montgomery, TX), insulin receptor, or FOXO1 antibody. A 1:200 dilution of FITC-conjugated anti-rabbit was used as the secondary antibody.
Insulin Radioimmunoassay
Whole blood was collected via retroorbital bleeding in anticoagulant-treated capillary tubes at the appropriate time points after an 18-h fast and postrefeeding. Serum was collected after whole blood was spun down at 4°C, and the supernatant was aliquoted to new tubes and stored at −20°C until use. RIA was conducted using Millipore (Billerica, MA) kit no. SRI-13K. Briefly, 10 μl of serum was pipetted into tubes in duplicate and incubated with insulin antibody overnight at 4°C. The next day, 125I-insulin was added to samples, and samples were incubated for 24 h at 4°C. Precipitating reagent was added to tubes, followed by vortexing and centrifugation. All tubes were counted with a γ-counter, and serum insulin was calculated from those values.
Glucose and Insulin Tolerance Tests
Mice were fasted for 18 (glucose) and 6 h (insulin), respectively. Mice were then injected intraperitoneally with either 0.5 U/kg insulin or 1.5 g/kg glucose, and glucose measurements were taken ≤3 h post injection using Accu-Chek glucometers (Roche).
Statistical Analysis
Data were analyzed with SPSS software using one-factor ANOVA analysis or Student's t-test. P values <0.05 were statistically significant.
RESULTS
Insulin Regulates Menin Expression via the Insulin Receptor
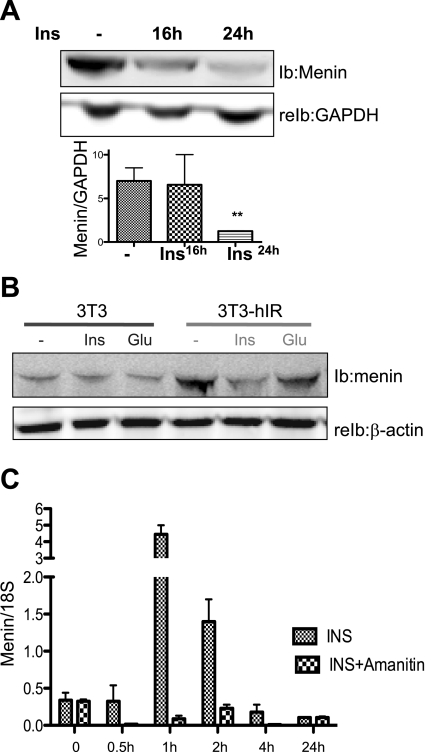
Western analysis reveals that insulin (100 nM) caused a progressive decrease in menin protein levels in mouse primary hepatocytes, with a pronounced effect after 24 h (Fig. 1A).
Fig. 1.
Insulin (Ins) regulates menin expression via the human insulin receptor. A: immunoblot (IB) and reimmunoblot (reIB) analysis of primary hepatocytes exposed to 100 nM insulin for 16 and 24 h shows a time-dependent decrease in menin protein levels. B: NIH 3T3 cells stably transfected with human insulin receptor A (3T3 hIR) were treated with 100 nM insulin and 50 nM glucagon (Glu) for 24 h prior to IB analysis. C: mRNA analysis by RT-PCR of primary hepatocytes extracted from C57BLK/6 mice treated with 100 nM insulin or 1 μg/ml of α-amanitin (Sigma) prior to insulin treatment shows peak expression at 1 and 2 h, inhibited by amanitin at all time points; n > 3 independent experiments in triplicates pooled from livers of >9 C57BLK/6 mice. Values are means ± SE.
To elucidate the mechanism by which insulin regulated menin expression, we treated stably transfected NIH 3T3 cells with human insulin receptor (3T3-hIR) and untransfected cells (3T3) (7) with 100 nM insulin for 24 h prior to evaluating menin levels by Western analysis. As shown in Fig. 1B, insulin failed to modulate menin protein content in the native 3T3 cells. In 3T3-hIR cells, baseline menin protein level was higher than in untransfected cells but was significantly downregulated by insulin. This observation was specific for insulin since glucagon did not change menin expression in either cell group. The data indicate that insulin exerts a downregulatory effect on menin expression level in a manner depending on its receptor.
Effect of Multiple Time Points of Insulin Exposure on Menin mRNA
We investigated the direct effect of insulin on menin in primary hepatocytes isolated from mouse liver. We determined that 100 nM insulin caused a time-dependent biphasic effect on menin expression, increasing menin mRNA at 1 h of exposure and gradually decreasing to below baseline levels by 24 h. The transcription initiation inhibitor, amanitin, inhibited insulin's ability to induce menin mRNA at 1–4 h but not at the 24-h time point, indicating that insulin is an acute transcriptional activator of menin (Fig. 1C). Interestingly, although insulin had no effect on menin mRNA at 24 h, the decrease in menin protein suggests a posttranslational effect on expression.
Effect of LY-294002 and U-O126 on Menin Expression
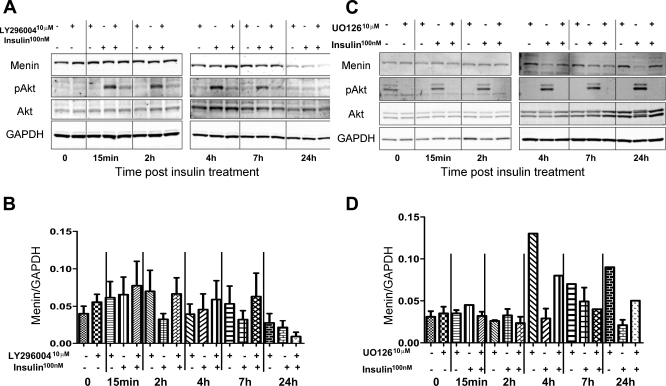
Since insulin activates the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, we investigated the effect of LY-294002 on menin expression with and without insulin. LY-294002 is an inhibitor of PI3K/Akt signaling that regulates cell metabolism and survival (5, 13). In Fig. 2B, we show that LY-294002 abrogated the acute effect of insulin on menin expression between 2 and 7 h, but not at 24 h. Furthermore, the analysis showed that at 24 h both insulin and LY-294002 decreased menin protein levels, but together they had no effect. It is interesting to note that at 24 h Akt phosphorlyation was absent with insulin treatment (Fig. 2A). Figure 2B is a quantification of the near-infrared signal from the conjugated secondary antibodies showing the relative protein expression of menin to GAPDH graphed from three separate experiments.
Fig. 2.
Ins regulates menin expression acutely via the Akt pathway followed by MAPK. A: protein expression of menin, p-Akt, Akt, and GAPDH in HepG2 lysates after overnight serum starvation was determined under the conditions of either 1-h pretreatment of LY-294002 (10 μM), Ins treatment (100 nM), or a combination of both as indicated by the + and − signs at the allotted time points (LY-294002 treatment alone was collected 1 h post-LY-294002 administration). B: quantification of Western blots from A by densitometry showing menin relative to GAPDH. C: protein expression of menin, p-ERK, ERK, and GAPDH in HepG2 lysates after overnight serum starvation was determined under the conditions of either 1-h pretreatment of U-O126 (10 μM), Ins treatment (100 nM), or a combination of both as indicated by + or − signs at the allotted time points (U-O126 treatment alone was collected 1 h post-U-O126administration). D: quantification of Western blots from C by densitometry showing menin relative to GAPDH.
Since insulin activates the MAPK pathway, we performed similar experiments with the MAPK inhibitor U-O126 and determined the effect on menin expression and ERK phosphorylation. In Fig. 2C, we show that U-O126 did not reverse the effect of insulin on menin expression at 2, 4, or 7 h of exposure. Contrary to LY-294002, U-O126 effectively abrogated insulin's effect on menin levels at 24 h. Interestingly, the highest level of ERK phosphorylation is observed with insulin at 24 h (Fig. 2C), the time at which p-Akt was undectectable (Fig. 2A). Figure 2D is a quantification of the near-infrared signal from the conjugated secondary antibodies showing the relative protein expression of menin to GAPDH graphed from three separate experiments.
Insulin Mediates Cellular Localization of Menin Protein
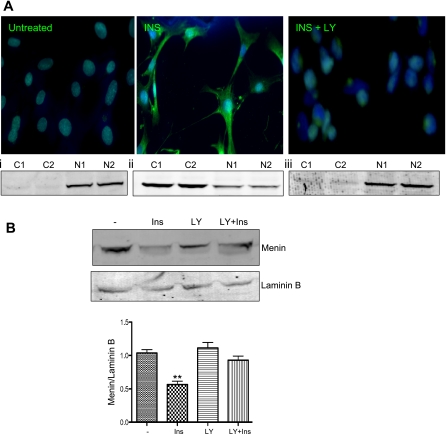
Since Akt is known to mediate the action of insulin to sequester a number of target proteins from the nucleus to the cytoplasm (5), we hypothesized that insulin causes translocation of menin from the nucleus into the cytoplasm. Insulin treatment of primary hepatocytes caused traslocation of menin from the nucleus into the cytoplasm, as demonstrated by increased immunostaining of menin in the cytoplasmic compartment (Fig. 3A, middle, green). In the presence of LY-294002 the effect of insulin was prevented, and menin remained in the nucleus (Fig. 3A, right). These observations were further supported by Western analysis, which revealed a predominant menin expression in the nuclear fractions in the absence of insulin. Insulin treatment, however, caused a reduction in menin nuclear content with a concomitant appearance in the cytoplasmic fractions. LY-294002 prevented the negative effect of insulin on nuclear distribution of menin, consistent with a role for the Akt pathway in mediating cellular localization of the menin protein. Figure 3B shows the decrease in menin expression in nuclear extracts relative to lamin B after treatment with insulin and that which was abrogated with LY-294002 exposure.
Fig. 3.
Ins shuttles menin from the nucleus into the cytoplasm via Akt signaling. A: flourescent images of menin in HepG2 cells after 24-h exposure to 100 nM insulin show cytoplasmic localization inhibited by LY-294002 (INS + LY). A, i–iii: menin expression in cytoplasmic (C) and nuclear (N) fractionation from untreated HepG2 cells (i), HepG2 cells treated with 100 nM insulin (ii), and HepG2 pretreated with LY-294002 for 1 h prior to insulin treatment (iii). B: Western blotting with 30 of μg nuclear lysates from HepG2 cells exposed to 100 nM Ins or 10 μM LY 1 h prior to Ins (LY + Ins). Lamin B used for control of nuclear lysates. Graph shows densitometry of near infrared band intensity from 3 experiments. **P = 0.0014.
Insulin Enhances the Interaction of Menin with FOXO1
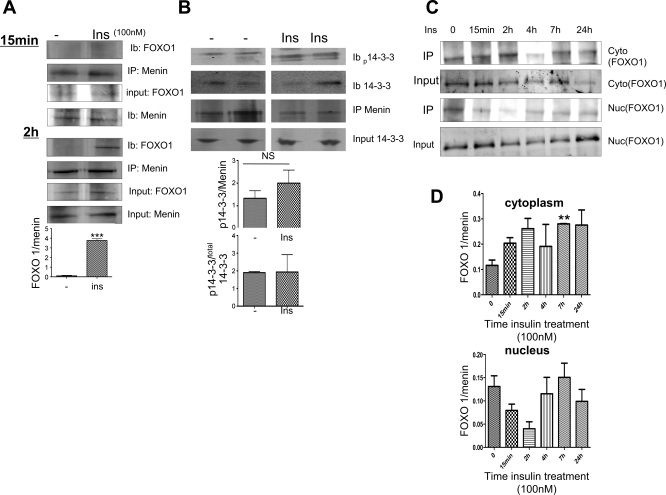
Akt-mediated phosphorylation of FOXO1 induces its cytoplasmic localization in response to insulin (26); thus we hypothesized that insulin induces FOXO1 and menin interaction. Coimmunoprecipitation assays detected FOXO1 in the menin immunopellet (IP) from mouse primary hepatocyte lysates treated with insulin (100 nM) for 2 h but not 15 min (Fig. 4A). The level of interaction is increased by approximately fourfold, as demonstrated by the graph insert depicting FOXO1/menin expression. Coimmunoprecipitation experiments using HepG2 cells further revealed that the complex formation between menin and FOXO1 also involves 14-3-3, which mediates the translocation of FOXO1 from the nucleus into the cytoplasm (Fig. 4B). The insulin-mediated menin-14-3-3-FOXO1 complex was specific since the association of menin with other proteins implicated in insulin metabolism, such as CEACAM1 (16, 17), occurred independently of insulin treatment (data not shown). We further determined the cellular localization using multiple time points at which insulin enhanced the menin FOXO1 interaction. In Fig. 4C, we show that insulin enhanced the cytoplasmic interaction of menin with FOXO1 at 2 h, after which interactions returned back to baseline by 24 h. Furthermore, the highest reduction of menin and FOXO1 interaction in the nucleus with insulin exposure occurred at 2 h. Figure 4D shows the relative expression of FOXO1 to total menin. Note how the interaction trends toward an increase in the cytoplasm, whereas it decreases in the nuclei with early time points.
Fig. 4.
Ins enhances menin interaction with forkhead box O1 (FOXO1) and cellular localization via Akt. A: immunoprecipitation (IP; with menin antibody and probed for FOXO1) with menin antibody, immunoblotted for FOXO1, shows enhanced interaction with 100 nM Ins exposure at 2 h and undetectable FOXO1 with 15 min of Ins treatment. Graph shows IB analysis quantified by near-infrared fluoresence and relative ratio of FOXO1 and menin. Values are means ± SE of >6 experiments. ***P < 0.0005. B: immunoprecipitation with agarose-conjugated menin antibody was immnunoblotted for FOXO1 antibody and phosphorylated and total-14-3-3 antibodies. Graphs below show IB analysis quantified by near-infrared immunofluoresence and expressed as relative ratio of phosphorylated 14-3-3 and menin or phosphorylated 14-3-3 and total 14-3-3. Values are means ± SE of 3 experiments. NS, not significant. C: Western blotting with cytoplasmic and nuclear fractionated lysates from HepG2 cells exposed to 100 nM insulin at designated time points. Input represents total FOXO1 expressed in cytoplasmic and nuclear lysates prior to IP. D: graphs show densitometry of near-infrared band intensity from 3 experiments of relative FOXO1 to menin expression after IP assay (IP). **P = 0.0019.
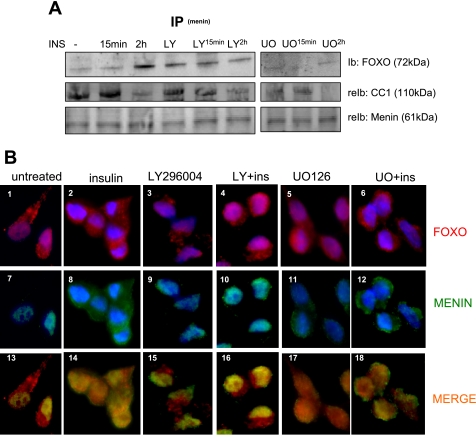
Since Akt is implicated in insulin regulation of FOXO1 cellular distribution, we investigated the effect of Akt inhibitor LY-294002 on the insulin-mediated menin-FOXO1 interaction. As expected, insulin treatment for 2 h but not 15 min elevated the interaction of menin with FOXO1 by approximately threefold in primary hepatocytes (Fig. 5A). Interestingly, the baseline interaction of menin with FOXO1 was elevated with LY-294002 in the absence of insulin but remained unchanged with insulin treatment at 15 min and 2 h (Fig. 5A). Interestingly, the MAPK inhibitor U-O126 inhibited the interaction of menin and FOXO1 independent of insulin.
Fig. 5.
Menin interaction with FOXO1 is inhibited by LY. A: IP with menin antibody after exposure to 10 μM LY or 10 μM U-O126 1 h prior to INS for 15 min and 2 h probed with FOXO1-specific antibody and CEACAM1 (CC1). B: images of HepG2 cells stained for menin (green) and FOXO1 (red) after exposure to 100 nM INS, 10 μM LY, LY + Ins, 10 μM U-O126, or U-O126 + Ins (UO + INS). Cells were counterstained with 4,6-diamidino-2-phenylindole (blue) for nuclei. Control is untreated cells.
We also performed immunocytochemical analysis to demonstrate the induced translocation of menin to the cytoplasm by insulin (Fig. 5B, image 8). Consistent with Fig. 5A, the effect of insulin was blocked by LY-294002 (Fig. 5B, image 10). Insulin caused a similar effect on FOXO1 distribution (Fig. 5B, image 4 vs. image 2). Merging menin (Fig. 5B, green) and FOXO1 (Fig. 5B, red) labeling indicated a diffuse interaction between FOXO1 and menin in the presence of insulin in both the nucleus and cytoplasm (Fig. 5B, image 14), and this interaction was localized predominantly to the nucleus with LY-294002 pretreatment (Fig. 5B, image 16). Consistent with the IP data shown in Fig. 5A, U-O126 inhibited the interaction of menin and FOXO1 independent of insulin (Fig. 5B, images 17 and 18). Taken together, the data show that insulin sequesters menin to the cytoplasm and increases its interaction with FOXO1 primarily via activating the Akt signaling pathway.
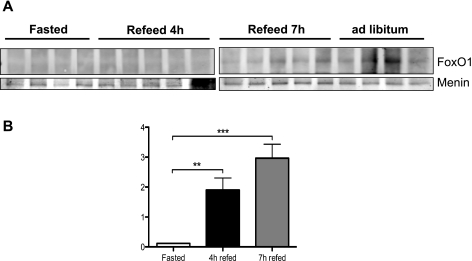
To establish a functional role for our observation in vitro, we first determined the physiological stimuli that will cause the interaction of menin with FOXO1. Because hepatic FOXO1 levels and activity are modulated metabolically in the liver, we subjected C57/BLK6 male mice to a fasting-refeeding paradigm prior to examining menin-FOXO1 interaction in liver lysates. Consistent with the cell data, coimmunoprecipitation analysis detected minimum FOXO1 and menin interaction in the IP derived from fasted animals. However, refeeding by 7 h significantly enhanced the FOXO1 and menin interaction (Fig. 6A), in correlation with the highest plasma insulin levels (Fig. 6B). Furthermore, the menin/FOXO1 interaction in the liver is present at baseline when animals are subjected to ad libitum feeding (Fig. 6A).
Fig. 6.
Menin and FOXO1 interaction in vivo. A: C57/BLK6 mice subjected to 18-h fast followed by 4 h of feeding and 7 h of refeeding. Whole liver lysates from mice were subjected to IP with menin antibody and immunoblotted for FOXO1 and menin by Western blot analysis. B: plasma insulin levels of C57/BLK6 mice subjected to 18-h fast followed by 4 h of feeding and 7 h of refeeding were determined by radioimmunoassay, as described in experimental procedures. Data analyzed using 1-way ANOVA; Turkey post hoc test. ***P < 0.0001; **P < 0.0017.
Partial Loss of Menin in the Liver Increases Akt Signaling and Induces FOXO1 Target Genes
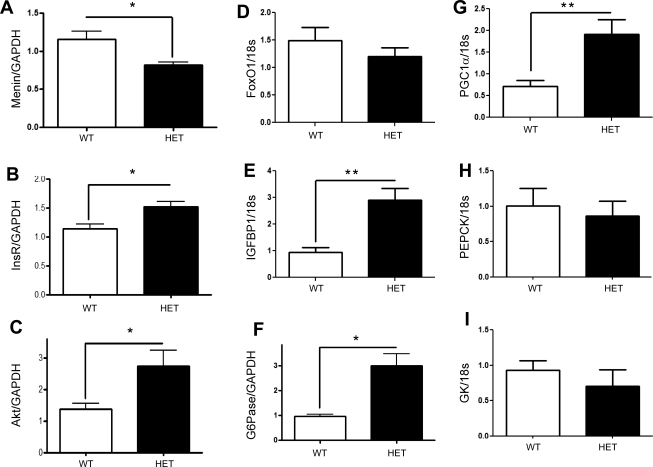
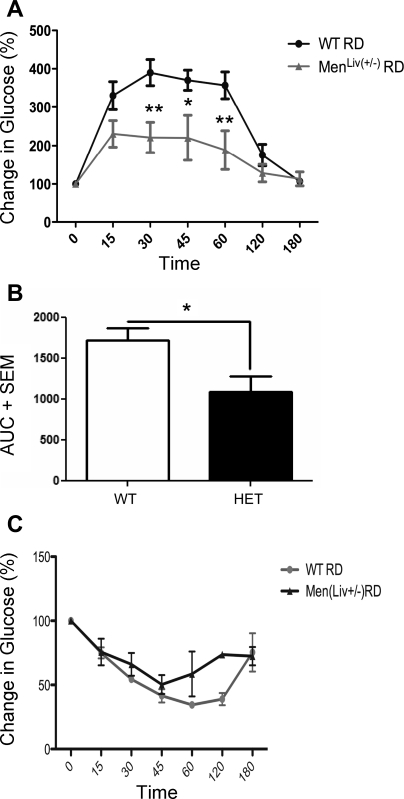
To determine whether menin had a role to play in the metabolic processes of the liver, we developed a hemizygous deletion of menin specifically in hepatocytes using FVB/129S mice expressing loxP sequences on exons 3–10 of the Men1 gene crossed to C57/Blk6 mice expressing Albumin-Cre. At 6 mo of age, we analyzed genes that are direct targets of FOXO1 involved in glucose synthesis. In (Fig. 7A), we show a significant decrease in menin RNA expression in mice with-liver specific hemizygous deletion of menin (HET). Interestingly, whereas the expression of FOXO1 remained unchanged (Fig. 7D), its target genes insulin receptor (Fig. 7B) and Akt (Fig. 7C), as well as IGFBP-1 (Fig. 7E), G-6-Pase (Fig. 7F), and PGC-1α (Fig. 7G), were all significantly upregulated, consistent with enhanced FOXO1 activity. In contrast, the FOXO1-independent metabolic gene glucokinase was unchanged, and interestingly, the gluconeogenic marker PEPCK also remained unchanged. Furthermore, the menin hemizygote mice displayed reduced glucose excursions during glucose tolerance test compared with control mice, whereas the insulin tolerance test showed no change (Fig. 8).
Fig. 7.
Effect of hepatic menin on FOXO1 target genes. C57/BLK6/FVB(129S) mice hemizygous for multiple endocrine neoplasia type 1 (MEN1) in the liver were subjected to an 18-h fast, and livers were extracted and analyzed. Genes that are direct targets of FOXO1 and directly involved in hepatic glucose production shown to be differentially expressed in the liver-specific hemizygous mice (HETs) vs. wild type (WT) based on results from quantitative real-time PCR. A: menin mRNA is decreased in the HETs; *P < 0.0145. B: insulin receptor (InsR); *P < 0.0172. C: total Akt; *P < 0.0249. D: total FOXO1. E: IGFBP-1; **P < 0.0026. F: glucose-6-phosphatase (G-6-Pase); *P < 0.0030. G: PGC-1α; *P < 0.0054. H: PEPCK. I: glucokinase (GK).
Fig. 8.
Glucose metabolism in mice with liver-specific hemizygote expression of menin. A: glucose tolerance tests (n = 5/group) in liver-specific hemizygote mice [MenLiv(+/−)] and control litter mates (WT). B: area under the curve of glucose tolerance test for liver-specific HET relative to same-strain WT. C: insulin tolerance tests (n = 5/group) in liver-specific hemizygote mice [MenLiv(+/−)] and WT. **P < 0.01; *P < 0.05.
The implications for these findings are under investigation in our laboratory; however, we hypothesize that the interactions between menin and FOXO1 play a role in induction of FOXO1-specific target genes described in Fig. 7 and may partially explain the observed phenotype of reduced glucose excursion in mice with partial deletion of the MEN1 gene in the liver after intraperitoneal glucose tolerance test but not intraperitoneal insulin tolerance test shown in Fig. 8.
DISCUSSION
Hepatic glucose production (HGP) plays an important role in maintaining glucose homeostasis and is mediated by FOXO1 and activated by fasting to induce gluconeogenic and glycogenolytic genes involved in HGP. Menin is a tumor suppressor protein that interacts and negatively regulates the activities of several transcription factors (2). Although loss of the MEN1 gene is associated with insulinomas and increased serum insulin, the reciprocal relationship between menin and insulin has not been well delineated. We show for the first time that insulin is a direct regulator of menin expression at both the transcriptional and translational levels. Furthermore, insulin effect is biphasic, with an initial early response mediated by Akt (Fig. 2A) and by 24 h predominantly by MAPK signaling (Fig. 2B).
Menin has never previously been described to be involved in FOXO1-mediated HGP, and no glucose metabolism defects have been described in liver-specific menin-deficient mice. The most intriguing interpretation of our data is in support of the hypothesis that menin plays an important role in metabolism in the liver. FOXO1 has an established role in maintaining the balance between the gluconeogenesis and glycolysis pathways in times of fasting and feeding. FOXO1 localization is controlled by PKB/Akt signaling downstream of the insulin receptor. In times of feeding, insulin signaling is high, along with activation of Akt, which causes phosphorylation of FOXO1 and its chaperone 14-3-3. This phosphorylation causes FOXO1 to be sequestered to the cytoplasm, downregulating its transcriptional effects. In times of reduced Akt signaling (i.e., fasting), FOXO1 is nuclear and exerts its effects on various target genes such as G-6-Pase, which catalyzes the rate-limiting step in gluconeogenesis. Our data shows that insulin enhances the interaction of menin with FOXO1 within the cytoplasm (Figs. 4C and 5B, image 14), and loss of menin is associated with upregulation of FOXO1 target genes (Fig. 7). These observations suggest that menin may repress FOXO1 transcriptional activity by recruiting the FOXO1–14-3-3 complex into the cytoplasm. We propose that insulin binding to its receptor activates the Akt signaling pathway and downregulates menin levels at early time points (acute phase), whereas MAPK regulation of menin sets in at later time points (chronic phase). Interestingly, the reduction in menin expression occurs at a later time point (∼24 h), really suggesting a MAPK-mediated effect, whereas interaction with FOXO1 is an early time event mediated by Akt. Since menin is a rate-limiting protein required to inhibit Akt activation, its interaction directly with FOXO1 may be required to repress FOXO1 transcriptional activity by causing its translocation into the nucleus.
We further observed that partial deletion of menin does not affect the expression of FOXO1 but induces the expression of FOXO1-specific genes in support of the hypothesis that menin negatively regulates FOXO1 activity at the posttranscriptional level via direct interaction. Indeed, the finding that insulin increases the interaction between menin and FOXO1 via an Akt-dependent pathway is entirely novel and implicates menin in FOXO1-mediated effects of insulin on glucose homeostasis and production (5, 18). FOXO1 is expressed in tissues involved in energy metabolism such as the liver (25), pancreas, and gut (3), and inhibition of its function by insulin (5) may require menin.
Thus our current studies suggest that although menin may be mediating the mitogenic functions of insulin, an important effect may be to modulate the insulin-mediated FOXO1-regulated metabolic pathways in the liver. Furthermore, since menin is an inhibitor of Akt activation, its expression may be the rate-limiting step in maintaining the metabolic homeostasis within the liver regulated by insulin.
In summary, insulin regulates menin expression, subcellular localization, and interaction with FOXO1 via nuclear cytoplasmic shuttling. Loss of menin leads to enhanced FOXO1 activity, as evidenced by a specific increase in FOXO1 target genes. Although the physiological significance of insulin's regulation on the menin-FOXO1 interaction in the liver needs to be further elucidated, the current study indicates that insulin regulation of menin expression and interaction with FOXO1 may be essential for the regulation of hepatic glucose production in the liver. We propose that menin is required for the optimal repression of FOXO1 transcriptional activity, a protein that plays crucial roles in positive and negative regulation of cell proliferation, gluconeogenesis, and glycolysis.
GRANTS
The work has been supported by institutional funds to E. J. Mensah-Osman and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-054254 and DK-083850) and the US Department of Agriculture (USDA 38903-02315) to S. M. Najjar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, Burns AL. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96: 143–152, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal SK, Kennedy PA, Scacheri PC, Novotny EA, Hickman AB, Cerrato A, Rice TS, Moore JB, Rao S, Ji Y, Mateo C, Libutti SK, Oliver B, Chandrasekharappa SC, Burns AL, Collins FS, Spiegel AM, Marx SJ. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res 37: 369–374, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 114: 1493–1503, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab 285: E718–E728, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Liu R, Jiang X, Lu J, Jiang J, Zhang C, Li X, Ning G. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Mol Cell Biol 29: 5477–5487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choice CV, Howard MJ, Poy MN, Hankin MH, Najjar SM. Insulin stimulates pp120 endocytosis in cells co-expressing insulin receptors. J Biol Chem 273: 22194–22200, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 8: 65–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallo A, Cuozzo C, Esposito I, Maggiolini M, Bonofiglio D, Vivacqua A, Garramone M, Weiss C, Bohmann D, Musti AM. Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation. Oncogene 21: 6434–6445, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O'Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem 275: 30169–30175, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, Burns AL. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene 20: 4917–4925, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci USA 98: 3837–3842, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318: 806–809, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100: 11285–11290, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCallum RW, Parameswaran V, Burgess JR. Multiple endocrine neoplasia type 1 (MEN 1) is associated with an increased prevalence of diabetes mellitus and impaired fasting glucose. Clin Endocrinol (Oxf) 65: 163–168, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Najjar SM. Regulation of insulin action by CEACAM1. Trends Endocrinol Metab 13: 240–245, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Najjar SM, Yang Y, Fernstrom MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab 2: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108: 1359–1367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Powers AC, Efrat S, Mojsov S, Spector D, Habener JF, Hanahan D. Proglucagon processing similar to normal islets in pancreatic alpha-like cell line derived from transgenic mouse tumor. Diabetes 39: 406–414, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423: 550–555, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Scacheri PC, Crabtree JS, Kennedy AL, Swain GP, Ward JM, Marx SJ, Spiegel AM, Collins FS. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mamm Genome 15: 872–877, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem 275: 36324–36333, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Trump D, Farren B, Wooding C, Pang JT, Besser GM, Buchanan KD, Edwards CR, Heath DA, Jackson CE, Jansen S, Lips K, Monson JP, O'Halloran D, Sampson J, Shalet SM, Wheeler MH, Zink A, Thakker RV. Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM 89: 653–669, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK, Marx SJ. The tumor suppressor protein menin inhibits akt activation by regulating its cellular localization. Cancer Res 71: 371–382, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281: 10105–10117, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 378: 839–849, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]