Abstract
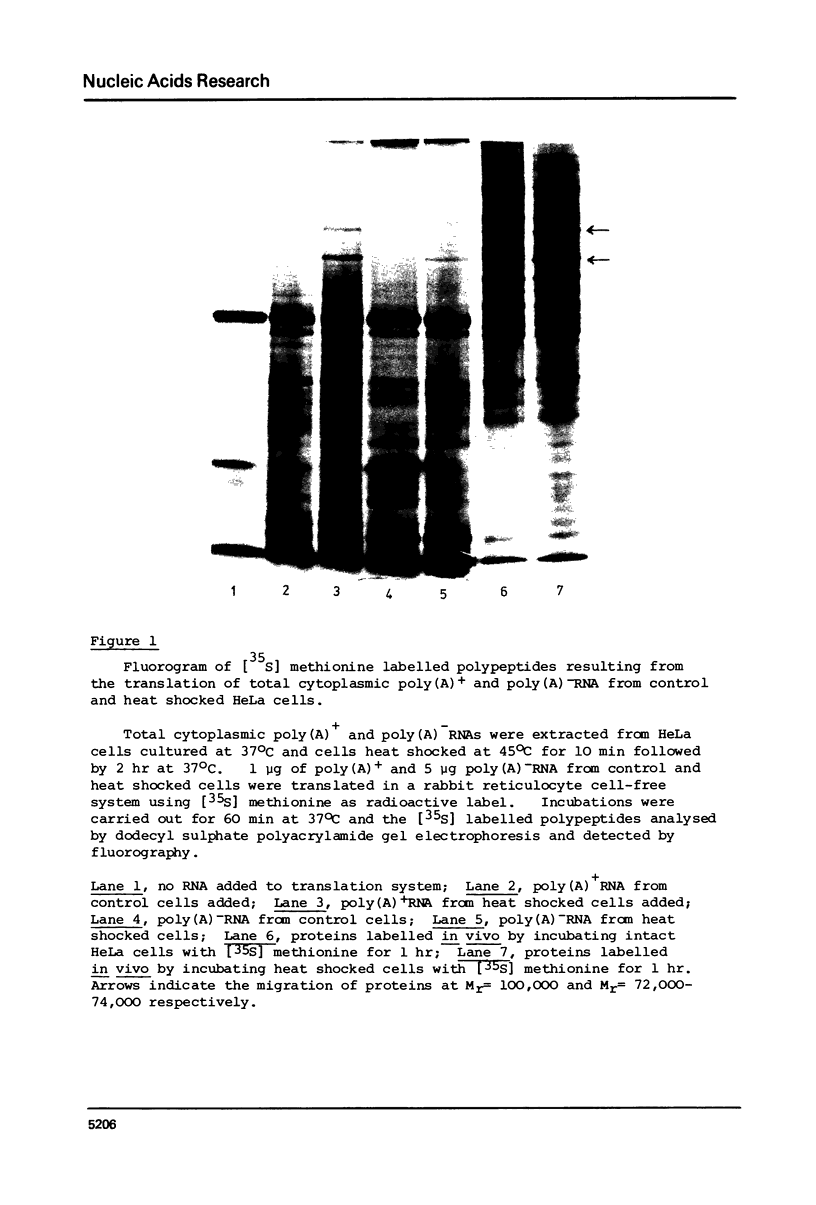
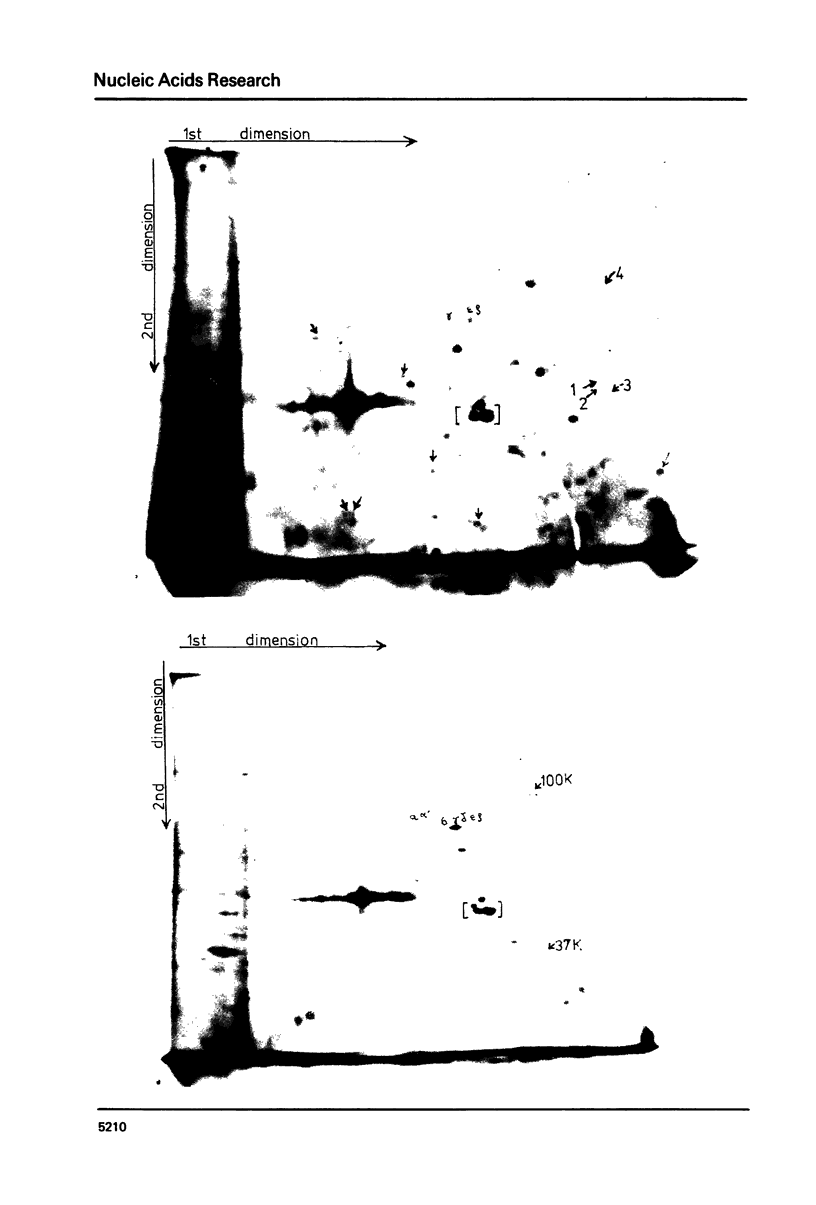
There is an increased synthesis of proteins in the molecular weight region of 100,000 72,000-74,000 and 37,000 two hours after treatment of HeLa cells for 10 min at 45 degrees C. In vitro translation, using a rabbit reticulocyte cell-free protein synthesising system, of HeLa cell cytoplasmic RNA shows that the prominent 72,000-74,000 Mr heat shock protein band comprises seven polypeptide species (namely alpha d beta gamma delta epsilon zeta) and these polypeptides are directly encoded by both polyadenylated and nonpolyadenylated mRNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31(3):356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Most myosin heavy chain mRNA in L6E9 rat myotubes has a short poly(A) tail. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1853–1857. doi: 10.1073/pnas.76.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. The effect of heat shock on RNA synthesis in Drosophila tissues. Cell. 1976 May;8(1):43–50. doi: 10.1016/0092-8674(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. E., Sonenshein G. E., Brawerman G. Characteristics and polyadenylate content of the actin messenger RNA of mouse sarcoma-180 ascites cells. Biochemistry. 1978 Oct 3;17(20):4200–4207. doi: 10.1021/bi00613a014. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Meselson M. Transcription at two heat shock loci in Drosophila. Cell. 1977 Oct;12(2):441–451. doi: 10.1016/0092-8674(77)90120-9. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Katinakis P. K., Slater A., Burdon R. H. Non-polyadenylated mRNAs from eukaryotes. FEBS Lett. 1980 Jul 11;116(1):1–7. doi: 10.1016/0014-5793(80)80515-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Ransom L. J., Graham M. L., Pardue M. L. Transcription and metabolism of RNA from the Drosophila melanogaster heat shock puff site 93D. Chromosoma. 1980;80(3):237–252. doi: 10.1007/BF00292683. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Gros F. Coding potential of non-polyadenylated messenger RNA in mouse Friend cells. J Mol Biol. 1980 May 5;139(1):61–83. doi: 10.1016/0022-2836(80)90116-3. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Benicourt C. Post-Translational Proteolytic Cleavage of In Vitro-Synthesized Turnip Yellow Mosaic Virus RNA-Coded High-Molecular-Weight Proteins. J Virol. 1980 Apr;34(1):85–94. doi: 10.1128/jvi.34.1.85-94.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Fractionation and functional analysis of newly synthesized and decaying messenger RNAs from vegetative cells of Dictyostelium discoideum. J Mol Biol. 1979 Mar 5;128(3):371–395. doi: 10.1016/0022-2836(79)90093-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Ruderman J. V., Woodland H. R., Sturgess E. A. Modulations of histone messenger RNA during the early development of Xenopus laevis. Dev Biol. 1979 Jul;71(1):71–82. doi: 10.1016/0012-1606(79)90083-6. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Slater A., Cato A. C., Sillar G. M., Kioussis J., Burdon R. H. The pattern of protein synthesis induced by heat shock of HeLa cells. Eur J Biochem. 1981 Jul;117(2):341–346. doi: 10.1111/j.1432-1033.1981.tb06343.x. [DOI] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Gros F. The majority of calf muscle cell messenger RNAs contain poly(A). Biochim Biophys Acta. 1978 Jul 24;519(2):372–382. doi: 10.1016/0005-2787(78)90090-4. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]