Abstract
Syrian hamsters, like many humans, increase food intake and body adiposity in response to stress. We hypothesized that glucocorticoids (cortisol and corticosterone) mediate these stress-induced effects on energy homeostasis. Because Syrian hamsters are dual secretors of cortisol and corticosterone, differential effects of each glucocorticoid on energy homeostasis were investigated. First, adrenal intact hamsters were injected with varying physiological concentrations of cortisol, corticosterone, or vehicle to emulate our previously published defeat regimens (i.e., 1 injection/day for 5 days). Neither food intake nor body weight was altered following glucocorticoid injections. Therefore, we investigated the effect of sustained glucocorticoid exposure on energy homeostasis. This was accomplished by implanting hamsters with supraphysiological steady-state pellets of cortisol, corticosterone, or cholesterol as a control. Cortisol, but not corticosterone, significantly decreased food intake, body mass, and lean and fat tissue compared with controls. Despite decreases in body mass and adiposity, cortisol significantly increased circulating free fatty acids, triglyceride, cholesterol, and hepatic triglyceride concentrations. Although corticosterone did not induce alterations in any of the aforementioned metabolic end points, Syrian hamsters were responsive to the effects of corticosterone since glucocorticoids both induced thymic involution and decreased adrenal mass. These findings indicate that cortisol is the more potent glucocorticoid in energy homeostasis in Syrian hamsters. However, the data suggest that cortisol alone does not mediate stress-induced increases in food intake or body mass in this species.
Keywords: stress, body weight, Cushing's syndrome, obesity, hypothalamic-pituitary-adrenal axis
chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis due to prolonged stress or endocrine disorders (e.g., Cushing's syndrome) is linked to numerous pathological conditions, including cardiovascular disease and obesity (2). Glucocorticoids (cortisol and corticosterone), the final products and active components of the HPA axis, are secreted from the adrenal cortex, and because they are elevated in rodent and human models of obesity (2, 11, 27), they are often considered to be the culprits for the increased risk of developing the metabolic syndrome. In response to mild psychological stressors or glucocorticoid administration, humans often increase food intake and/or body weight (8, 14, 36). Although humans predominately secrete cortisol as their stress hormone, identification of mechanisms involved in stress-induced metabolic alterations is commonly investigated in rat and mouse models, in which corticosterone is the more abundant glucocorticoid. In addition, the characteristic response of most rodents exposed to social, psychological, or physical stressors is to decrease food intake and lose body/adiposity mass (17, 19, 23, 25), an outcome similar to humans following severe stress (13, 22). However, because mild day-to-day stressors are associated with increased body weight gain in some humans, an effect proposed to be attributed to elevated glucocorticoids, there is a critical need for more rodent models that better mimic this phenomenon.
We have reported previously that repeated social stress increases food intake, body mass, and adiposity, primarily in the form of increased visceral obesity (i.e., increased mesenteric and omental fat), in Syrian hamsters (15, 32), consistent with previous findings in Swiss mice (5). We hypothesized that increases in circulating glucocorticoids in response to social defeat facilitates this positive energy balance. Because Syrian hamsters tend to gain weight in response to stress, they may be a particularly apt rodent model for investigating stress-induced body mass gain that is commonly observed in a subset of humans. Of note, Syrian hamsters are dual secretors of cortisol and corticosterone, although cortisol is proposed to be the more liable glucocorticoid (31, 39) and predominant hormone following stress exposure in Syrian hamsters (18, 28). Therefore, we hypothesized that cortisol, but not corticosterone, mediates stress-induced alterations in energy homeostasis in this species. To test this hypothesis, Syrian hamsters were injected with cortisol or corticosterone to mimic the transitory increases in these hormones, as seen following a brief social defeat encounter. We next implanted Syrian hamsters with pellets to determine the effects of prolonged glucocorticoid exposure on energy homeostasis in this species. This approach allowed us to investigate the individual effects of corticosterone and cortisol secretion on food intake, body mass, adiposity, and lipid profiles in this species.
METHODS
Animals and housing conditions.
Adult male Syrian hamsters (∼130 g upon arrival) were individually housed in standard polycarbonate cages (20 × 40 × 20 cm) and acclimated to the housing conditions for ≥1 wk before initiation of the experiment. Hamsters were maintained in a temperature- and humidity-controlled vivarium with a 14:10-h light-dark cycle (lights off at 1300). Hamsters were given free access to pelleted rodent chow (rodent diet, LM485; Harlan Teklad, Madison, WI) and water. All procedures and protocols were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Experiment 1: glucocorticoid injections.
Adrenal-intact hamsters were injected subcutaneously with cortisol (10 or 40 μg/100 g; Steraloids, Wilton, NH), corticosterone (250 μg/kg or 1.25 mg/kg; Steraloids), cocktail (mixed) of the lowest doses of both glucocorticoids (10 μg/100 g cortisol and 250 μg/kg), or vehicle (propylene glycol). Glucocorticoids were dissolved in propylene glycol (n = 10/dose). Animals were injected once/day for 5 days, specifically on days 7, 11, 12, 14, and 17, within the first 2 h of dark onset to mimic the time frame of our previous defeat studies (15, 32).
Experiment 2: pellet implantation.
Hamsters were anesthetized with isoflurane and received control (cholesterol) or cortisol/corticosterone pellets (Steraloids) inserted subcutaneously in the dorsal neck area. Note that all animals had intact adrenals for this study. Hamsters were implanted with 100% (wt/wt) of cortisol, corticosterone, or cholesterol in the pellets (n = 10/group). Hardened pellets were trimmed to weigh 50 mg.
Experiment 3: pellet implantation.
Following the same procedure as experiment 2, a separate group of hamsters were implanted with control (cholesterol) or a range of cortisol or corticosterone 12.5, 25, and 50% (wt/wt) in the pellets (n = 10/group). Hardened pellets were trimmed to weigh 50 mg.
Food intake and body mass.
Food intake and body mass were measured daily at 1200 for all experiments.
Body composition analysis.
Body composition was analyzed with a quantitative nuclear magnetic resonance (Echo MRI Whole Body Composition Analyzer; Echo Medical Systems, Houston, TX) at the beginning and end of the experiments.
Blood collection, radioimmunoassay, and ELISA.
Animals were euthanized via rapid decapitation between the hours of 0500 and 0900. Trunk blood was collected and centrifuged at 3,000 g for 20 min at 4°C. Plasma samples were stored at −80°C until they were assayed. Kits for cortisol (DiaSorin, Stillwater, MN), corticosterone (MP Biomedicals, Orangeburg, NY), adrenocorticotrophin hormone (ACTH) (DiaSorin, Stillwater, MN), leptin (Crystal Chem, Downers Grove, IL), and insulin (Crystal Chem) were assayed for experiments 2 and 3 according to the manufacturers' instructions. Intra-assay variability was <10% for all assays, and all samples for each hormone were run in a single assay. For the cortisol kit the cross-reactivity with corticosterone is <0.4%, and for corticosterone the cross-reactivity with cortisol is <0.05%. Cortisol, corticosterone, and ACTH were analyzed by radioimmunoassay, and leptin and insulin were analyzed with an ELISA.
Tissue harvesting.
For experiments 2 and 3, white adipose tissue (WAT) depots, including inguinal (IWAT), epididymal (EWAT), retroperitoneal (RWAT), and mesenteric (MWAT) fat pads, were collected and weighed. Thymi and adrenals were collected and cleaned. All tissues were weighed to the nearest 0.01 g. A portion of the liver was also collected and immediately frozen to later determine liver triglycerides.
Lipid profile.
Commercially available kits were used to measure triglycerides (Randox Laboratories, Crumlin, UK), cholesterol (Thermo Fisher Scientific, Middletown, VA), and nonesterified free fatty acids (NEFA; Wako, Richmond, VA) for experiments 2 and 3 as per the manufacturers' instructions. To determine liver triglyceride concentration, 50-mg samples were homogenized in 1 ml of 50 mM Tris·HCl buffer, pH 7.4, containing 150 mM NaCl, 1 mM EDTA, and 1 μM phenylmethylsulfonyl. They were centrifuged at 8,000 g for 20 min, and the supernatant was collected and stored at −80°C until it was assayed.
Statistics.
Data were analyzed using SigmaStat. Body mass data were analyzed using a repeated-measures ANOVA, with drug treatment as the between-subjects factor and time (i.e., day) as the within- or repeating-subjects factor. For the total food intake, NMR, individual fat pad and tissue weights, and hormonal analyses, data were analyzed with one-way ANOVA, with drug treatment as the fixed factor, followed by least significant difference post hoc tests. Differences among groups were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation of the results.
RESULTS
Subcutaneous injections.
In experiment 1, there were no differences in body weight (data not shown), food intake, or body composition, i.e., lean, fat, or water mass, (data not shown), among any of the groups.
Glucocorticoid pellet verification.
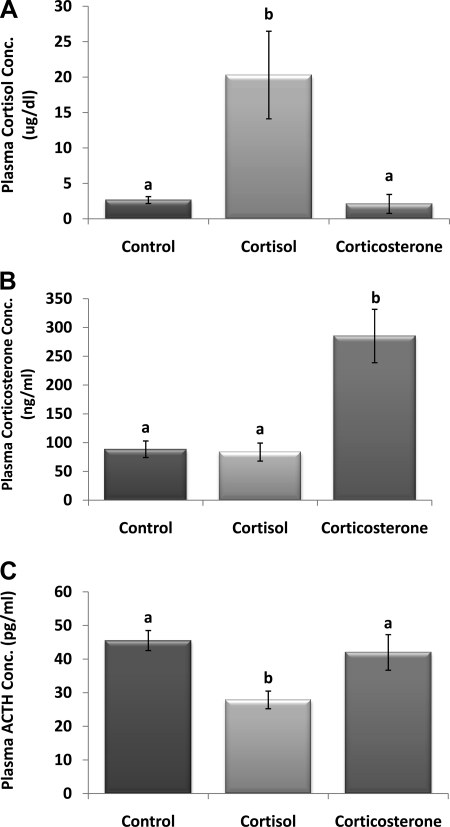
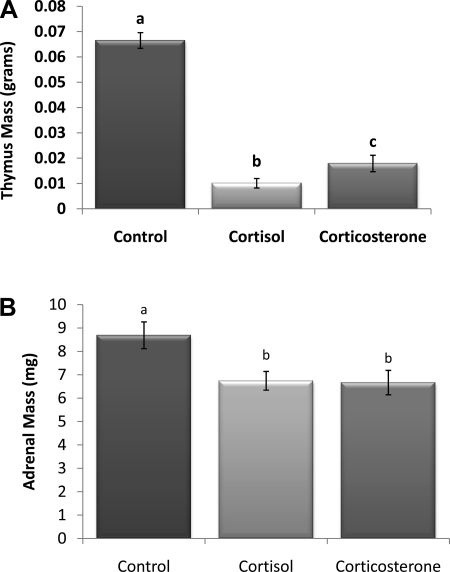
Terminal plasma cortisol concentration in the 100% cortisol pellet group was significantly higher, approximately eight times, than that of controls with vehicle pellets (P < 0.05; Fig. 1A). In contrast, implantation of 100% corticosterone did not alter endogenous cortisol levels. The opposite was true with corticosterone concentration (Fig. 1B), where the 100% corticosterone pellets caused a significant increase (P < 0.05), approximately four times, compared with controls, whereas the 100% cortisol pellets did not alter endogenous corticosterone levels, suggesting little to no cross-reactivity between the kits. A second implication is that neither glucocorticoid significantly suppressed the secretion of the other. ACTH concentration was only significantly decreased in animals with 100% cortisol pellets compared with controls (P < 0.05; Fig. 1C). Thymus mass was significantly decreased in both glucocorticoid groups compared with controls (P < 0.05; Fig. 2A), and the 100% cortisol pellet group had significantly lighter thymi than the 100% corticosterone group (P < 0.05). Adrenal mass was also significantly decreased in both the 100% cortisol and corticosterone groups relative to controls; however, there were no differences between the cortisol and corticosterone groups (P < 0.05; Fig. 2B).
Fig. 1.
Pellet verification. A: plasma cortisol concentration. Cortisol concentration was significantly increased in animals with the 100% cortisol pellet. B: plasma corticosterone concentration. Corticosterone concentration was significantly increased in animals with the 100% corticosterone pellet. C: plasma ACTH concentration. ACTH concentration was significantly decreased in animals with 100% cortisol pellet. a,bUnlike letters indicate significance, P < 0.05.
Fig. 2.
A: thymus mass (g). Significant involution occurred in both pellet groups. The cortisol group was also significantly decreased compared with the corticosterone group. B: adrenal mass (mg). Mass was decreased in both glucocorticoid groups. a–cUnlike letters indicate significance, P < 0.05.
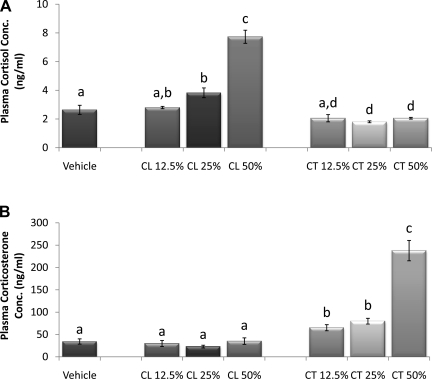
Incremental concentrations of cortisol pellets resulted in a gradual incremental increase in plasma cortisol (Fig. 3A). Plasma cortisol did not increase in hamsters with 12.5% cortisol pellets compared with controls but was significantly increased in those with ≥25% cortisol pellets (P < 0.05). In addition, the 50% cortisol pellet group was significantly higher than the 12.5 and 25% cortisol pellet groups (P < 0.05), and levels in both the 25 and 50% cortisol pellet groups were significantly higher than those in any of the corticosterone pellet groups (P < 0.05). The 25 and 50% corticosterone pellet groups significantly decreased endogenous cortisol levels compared with control, whereas the 25% did not alter cortisol release.
Fig. 3.
Incremental pellet verification. A: plasma cortisol concentration. Cortisol (CL) concentration was significantly increased in those with 25 and 50% cortisol pellets compared with controls and both corticosterone (CT) groups. B: plasma corticosterone concentration. Circulating corticosterone was significantly increased in all corticosterone pellet groups compared with control and all cortisol pellet groups. a–dUnlike letters indicate significance, P < 0.05.
Circulating corticosterone was significantly increased in all corticosterone pellet groups compared with controls and all cortisol pellet groups (P < 0.05; Fig. 3B). Corticosterone levels in animals with 50% corticosterone pellets were significantly higher than those with 12.5 or 25% pellets (P < 0.05).
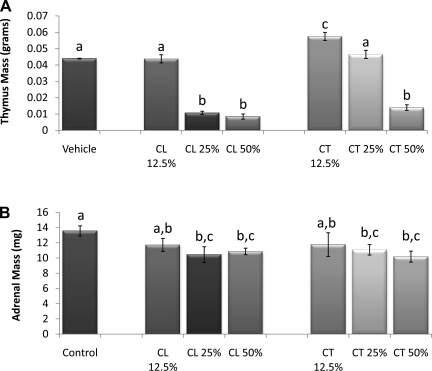
There were no differences in ACTH levels among the incremental glucocorticoid groups (data not shown). Thymus mass was not decreased in either of the 12.5% pellet groups or in the 25% corticosterone group compared with controls (Fig. 4A). Conversely, both the 25 and 50% cortisol pellets significantly reduced thymus mass (P < 0.05) compared with controls, whereas only the 50% corticosterone caused a significant reduction (P < 0.05). The 25 and 50% cortisol and corticosterone groups had significantly smaller adrenals compared with control groups (P < 0.05; Fig. 4B).
Fig. 4.
Incremental pellet verification. A: thymus mass (g). The 25 and 50% CL group had significantly reduced thymus mass compared with controls, whereas only the 50% CT caused a significant reduction. B: adrenal mass (mg); 25% and 50% CL and CT groups had significantly smaller adrenals a–cUnlike letters indicate significance, P < 0.05.
Food intake and body mass, adiposity, and composition.
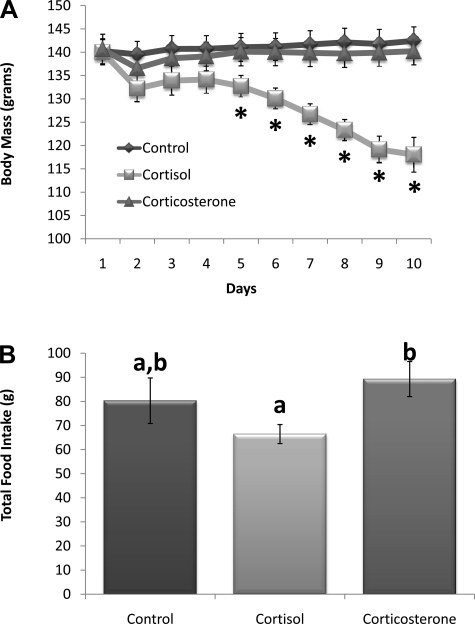
Groups were matched by average body mass on the day of surgery (Fig. 5A). Four days postsurgery, animals with 100% cortisol pellets had a significant decline in body mass (P < 0.05; day 5) that continued throughout the remainder of the study (P < 0.05; days 6–10) compared with controls and animals with 100% corticosterone pellets. Cumulative 10-day food intake (Fig. 5B) was deceased in the 100% cortisol group and was significantly lower than the intake of the 100% corticosterone-treated group (P < 0.05).
Fig. 5.
A: body mass (g). Postsurgery days 5–10, animals with 100% cortisol pellets had a significant decline in body mass (*P ≤ 0.05 vs. control and 100% corticosterone). B: total 10-day food intake. Cumulative food intake was significantly decreased in the cortisol group compared with corticosterone treatment. a,bUnlike letters indicates significance, P < 0.05.
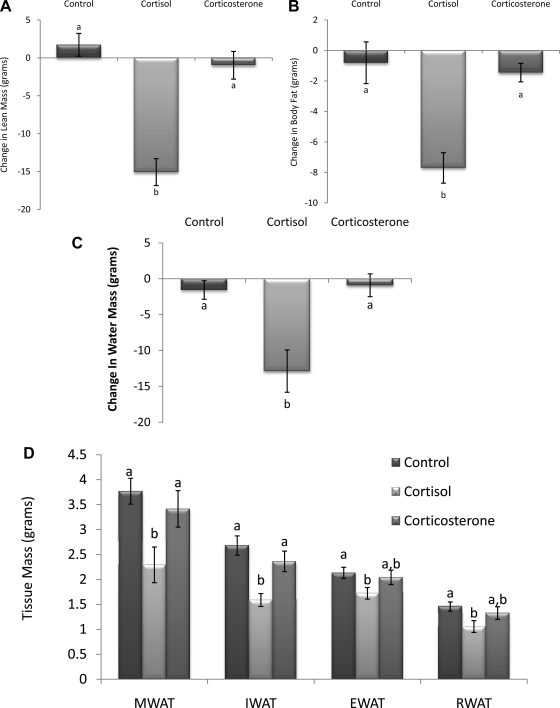
In parallel with changes in body mass, lean, fat, and water mass as measured by NMR were significantly reduced in hamsters with 100% cortisol pellets but unaltered in animals with 100% corticosterone pellets compared with controls (P < 0.05; Fig. 6, A–C). Dissected adipose tissue mass was also significantly decreased in animals with 100% cortisol pellets, with both visceral (MWAT) and subcutaneous (IWAT) adipose depots being significantly decreased compared with the control and 100% corticosterone groups (P < 0.05; Fig. 6D) and with intra-abdominal (EWAT and RWAT) adipose tissue depots being significantly decreased compared with controls only (P < 0.05).
Fig. 6.
Change in lean (A), fat (B), and water mass (C). Lean, fat, and water mass was significantly reduced in animals with cortisol pellets compared with controls. D: total dissected adipose tissue mass (g). Visceral [mesenteric white adipose tissue (MWAT)] and subcutaneous [inguinal white adipose tissue (IWAT)] adipose depots were significantly decreased in the cortisol group compared with control and corticosterone groups. Intra-abdominal [epididymal (EWAT) and retroperitoneal white adipose tissue (RWAT)] depots were significantly decreased in corticol group compared with controls only. a,bUnlike letters indicate significance, P < 0.05.
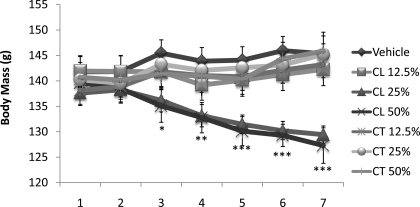
Although there were no differences in cumulative food intake among groups with incremental glucocorticoid pellets (data not shown), body mass was significantly reduced in animals with the 25 or 50% cortisol pellets (P < 0.05; Fig. 7). More specifically, beginning 3 days after surgery the 25 and 50% cortisol groups weighed significantly less than control animals (P < 0.05), and 1 day later those groups also weighed significantly less than all corticosterone pellet groups (P < 0.05). These differences remained through the end of the study.
Fig. 7.
Incremental dosage: body mass (g). Three days after surgery the 25 and 50% cortisol groups weighed significantly less than control animals (*P < 0.05). By day 4, the groups also weighed significantly less than all corticosterone pellet groups (**P < 0.05), followed by significant difference from 12.5% cortisol group on days 5–7 (***P < 0.05).
Decreases in body mass in the 25 and 50% cortisol groups were a consequence of reduced lean, fat, and water mass as measured by NMR. Although the 50% cortisol group had decreased lean mass relative to all other groups, this was not significant. As such, lean mass did not differ significantly among any of the groups. However, water mass was significantly decreased in the 50% cortisol group compared with all other groups (Table 1). Of note, fat mass as determined by NMR was significantly reduced only in the 25% cortisol group (P < 0.05; Table 1). On the other hand, cortisol dose-dependently decreased total dissected adipose tissue mass, and the highest dose (50%) was significantly different from all other groups (total dissected adipose tissue mass = MWAT + IWAT + EWAT + RWAT; bilateral depots were averaged together before summation). The exact differences in individual adipose tissue mass are summarized in Table 1.
Table 1.
Change in lean and fat mass as measured by NMR and dissected individual and total adipose tissue mass in Syrian hamsters with incremental glucocorticoid concentrations
| NMR Change in Body Composition |
Dissected Adipose Tissue Mass, g |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lean mass | Fat mass | Water mass | MWAT | IWAT | EWAT | RWAT | Total | |
| Vehicle | −2.96 ± 6.83 | −12.80 ± 7.21a,c | 5.645 ± 3.861a | 3.24 ± 0.24a,b | 1.95 ± 0.26a,b | 1.52 ± 0.10 | 1.26 ± 0.16 | 7.97 ± 0.65a,b |
| Cortisol, % | ||||||||
| 12.5 | 1.14 ± 7.35 | −9.02 ± 11.42a,c | 0.043 ± 4.33a | 3.57 ± 0.27a | 2.29 ± 0.20a | 1.87 ± 0.16 | 1.30 ± 0.14 | 9.09 ± 0.73a |
| 25 | −11.84 ± 3.56 | −42.70 ± 6.01b | −5.588 ± 1.85a | 2.79 ± 0.21b | 1.63 ± 0.09b | 1.51 ± 0.09 | 0.10 ± 0.05 | 6.93 ± 0.50b,c |
| 50 | −30.06 ± 12.05 | −33.01 ± 7.68b,c | −21.28 ± 6.25b | 2.62 ± 0.17b | 1.5 ± 0.11b | 1.57 ± 0.09 | 1.11 ± 0.17 | 5.95 ± 0.92c |
| Corticosterone,% | ||||||||
| 12.5 | −1.53 ± 5.24 | −12.97 ± 13.76a,c | 1.337 ± 3.03a | 3.19 ± 0.28a,b | 2.05 ± 0.14a | 1.62 ± 0.12 | 1.12 ± 0.10 | 7.96 ± 0.58a |
| 25 | −4.87 ± 5.68 | 8.36 ± 8.74a | −2.218 ± 3.08a | 3.74 ± 0.30a | 2.16 ± 0.17a | 1.75 ± 0.10 | 1.48 ± 0.18 | 9.11 ± 0.60a |
| 50 | −7.26 ± 7.55 | −2.75 ± 8.79a | −5.382 ± 3.92a | 3.56 ± 0.30a | 2.21 ± 0.24a | 1.77 ± 0.17 | 1.35 ± 0.16 | 8.90 ± 0.84a |
Values are means ± SE. MWAT, mesenteric white adipose tissue; IWAT, inguinal white adipose tissue; EWAT, epididymal white adipose tissue; RWAT, retroperitoneal white adipose tissue. Change in lean body mass: no significant differences among groups. Change in body fat mass: 12.5% cortisol group fat mass was significantly reduced compared with control animals as well as all other experimental groups, except for the 50% cortisol group. Change in water mass: 50% cortisol group; water mass was reduced compared with all other groups. Total dissected fat mass: total dissected adipose tissue mass was significantly decreased in the 25 and 50% cortisol groups compared with 12.5% cortisol and all corticosterone pellet groups; the 50% cortisol group was also significantly decreased compared with controls. Individual dissected adipose tissue mass: MWAT and IWAT were significantly reduced in the 25 and 50% cortisol groups compared with 25% cortisol and corticosterone groups as well as the 50% corticosterone group. a–cUnlike letters indicate significance, P < 0.05.
Lipid profile and insulin and leptin concentration.
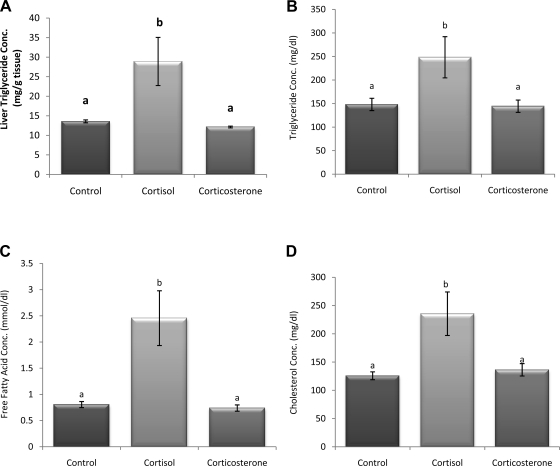
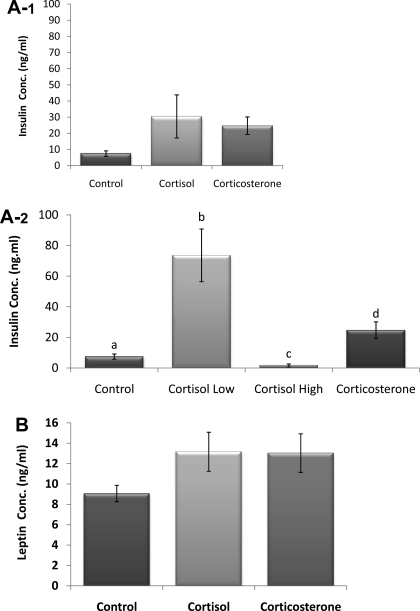
Liver and plasma triglyceride, free fatty acid, and cholesterol levels were all increased significantly in the 100% cortisol pellet group compared with controls and 100% corticosterone (P < 0.05; Fig. 8, A–D). Insulin (Fig. 9A-1) and leptin (Fig. 9B) concentrations were nonsignificantly increased in both groups with glucocorticoid pellets. However, insulin concentrations of the 100% cortisol group (n = 10) contained two subpopulations, those with high insulin values (n = 4) and those with extremely low insulin values (n = 6), hence the large variability in the group. Therefore, with subpopulations considered (Fig. 9A-2), insulin is increased significantly in the cortisol high-insulin and corticosterone groups, whereas the cortisol animals with the low insulin are significantly lower than all groups (P < 0.05). The high-insulin cortisol group is also significantly higher than the corticosterone group.
Fig. 8.
Liver triglyceride concentration (A), triglyceride (B), free fatty acid (C), and cholesterol (D). Liver and plasma triglyceride, free fatty acid, and cholesterol were all significantly increased in the 100% cortisol pellet group compared with control and 100% corticosterone. a,bUnlike letters indicate significance, P < 0.05.
Fig. 9.
Insulin (A-1), insulin subpopulation (A-2), and leptin concentration (B). With subpopulations considered, insulin concentration was significantly increased in one-half of the cortisol group, whereas it was significantly decreased in the other compared with control and 100% corticosterone groups. The 100% corticosterone was also significantly different from the control animals. No significant differences in leptin concentration. a–dUnlike letters indicate significance, P < 0.05.
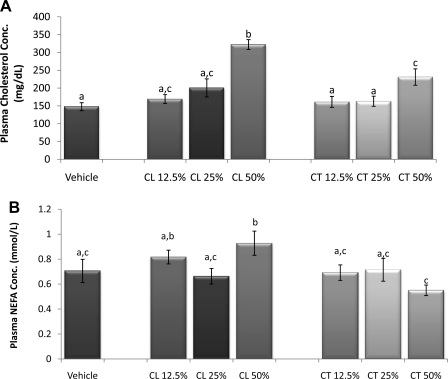
Liver and plasma triglyceride concentrations did not differ among incremental glucocorticoid groups (data not shown). However, hamsters receiving 50% cortisol or corticosterone pellets had a significant increase in plasma cholesterol compared with controls as well as compared with the 12.5 and 25% cortisol and corticosterone pellet groups (P < 0.05; Fig. 10A), and the cholesterol in the 50% cortisol group was higher than in the 50% corticosterone group. NEFA concentrations were increased only in the 50% cortisol group (P < 0.05; Fig. 10B).
Fig. 10.
Incremental dosage. A: cholesterol concentration. The 50% cortisol or corticosterone pellets caused a significant increase in cholesterol concentration compared with controls and the 12.5 and 25% cortisol and corticosterone pellet groups. B: free fatty acid concentration. Compared with control, nonesterified fatty acid (NEFA) concentration was significantly increased in the 50% cortisol group. a–cUnlike letters indicate significance, P < 0.05.
DISCUSSION
The goal of the present study was to determine whether increases in glucocorticoids (as occurs following social defeat) facilitate stress-induced body weight and adiposity gain. To accomplish this, we injected Syrian hamsters with varying concentrations of cortisol and corticosterone to emulate our previously published defeat regimens (15, 32). The selected doses were based upon data that indicated significant changes in stress-related behavior and/or energy homeostasis (7, 41). Food intake, body weight, and composition were not altered following glucocorticoid injections. As such, we moved toward examining the effect of sustained glucocorticoid exposure on energy homeostasis with pellets. The primary finding is that cortisol, but not corticosterone, dose-dependently decreases food intake, body mass, lean tissue, and adiposity, whereas plasma free fatty acids, cholesterol and triglyceride, and hepatic triglyceride content simultaneously increase. Although corticosterone did not impact any of the metabolic end points in the study, both glucocorticoids induced thymic involution and decreased adrenal mass, thus confirming that both exogenous glucocorticoids were active. To our knowledge, these data are the first to demonstrate differential metabolic effects of cortisol and corticosterone in Syrian hamsters. However, it appears as though neither glucocorticoid exclusively mediates stress-induced increases in food intake/body mass.
In humans, excess cortisol secretion is associated with obesity and many obesity-related diseases (i.e., diabetes) (6, 37). However, in rats, depending upon the dosage, glucocorticoids (corticosterone) can have both lipolytic as well as adipogenic effects (9). In the present study, high doses of cortisol in Syrian hamsters cause a significant loss of body mass via lean, fat, and water mass. These decreases are specific to cortisol because they do not occur with corticosterone. Analogous to these cortisol-mediated effects on energy homeostasis in Syrian hamsters, high doses of corticosterone blunt increases in body mass and significantly decrease lean tissue in rats (1), although unlike hamsters, rats do not have an overall loss of body or adipose tissue mass. In rats, corticosterone dose-dependently breaks down muscle protein and at chronic high doses causes significant decreases of lean tissue mass (35), which is consistent with what occurred with the high doses of cortisol (50 and 100%) in Syrian hamsters. Although glucocorticoids decrease adipose mass in part by increasing lipolysis, studies in rats demonstrate that high (100%) chronic levels of corticosterone do not sufficiently induce loss of adiposity (9, 20, 38), and this is in contrast to our findings. These inconsistencies between the current and previous studies may be explained by the fact that many previous studies were conducted in rats with the adrenals removed, which in and of itself can drastically alter metabolism. In addition, different metabolic outcomes of the two glucocorticoids in our model may reflect differential affinities of glucocorticoids for specific receptors or for cortisol/corticosterone-binding globulin capacity.
Decreases in body, lean, and adipose tissue mass could to some extent be attributed to decreases in food intake, but it is likely that other metabolic effects of glucocorticoids played a role. For example, the only cortisol pellet concentration to elicit significant decreases in food intake was 100%, although 25, 50, and 100% cortisol pellets significantly decreased body mass. This implies that decreased food intake in the cortisol-treated groups is accompanied by a change in metabolism favoring weight loss. Indeed, chronic glucocorticoid release is reported to increase core body temperature (12) and overall energy expenditure (42) and to decrease sleep/increase hours of arousal (16), all of which will promote decreases in body mass with normal food consumption. However, these parameters were not assessed in the present experiment.
Humans with chronically elevated glucocorticoids are characterized by increased insulin and leptin concentrations. More specifically, excessive glucocorticoid release increases gluconeogenesis in the liver, which subsequently inhibits insulin sensitivity in the liver and skeletal muscle and ultimately elevates circulating insulin (29). Initial evaluation revealed that neither 100% cortisol nor corticosterone significantly increased circulating insulin, but subsequent evaluation revealed two divergent subpopulations in the 100% cortisol group, those with extremely high or low circulating insulin. The deviation within this group may represent a dynamic time point where hamsters are transitioning from the consequences of acute (soaring insulin levels) to chronic (severe decline in insulin levels) excessive glucocorticoid release. Indeed, research demonstrates that high concentrations of glucocorticoids first elevate insulin (29), but if unrelenting, pancreatic β-cells undergo apoptosis and dysfunction, which reduces insulin synthesis and secretion (30). In humans, excessive glucocorticoid-induced increases in leptin result following enhanced leptin synthesis and secretion (24); however, this was not significantly increased in the 100% cortisol or corticosterone groups.
It is established that insulin and leptin are adiposity signals that are secreted in proportion to the quantity of stored fat. However, neither of the glucocorticoid groups gained body/adiposity mass; in fact, the 100% cortisol group lost significant fat mass despite moderate increases in insulin and leptin compared with controls. Therefore, unlike Cushing's in humans (3), excessive glucocorticoid release did not increase visceral adipose tissue mass in Syrian hamsters. This may be due to the fact that the cortisol concentrations used in the present study are exponentially higher than those typically observed to increase adiposity by pituitary/adrenal Cushing's or multiple glucocorticoid injections. However, excessive high cortisol release in hamsters increases circulating triglycerides, cholesterol, and free fatty acids, as demonstrated in humans (4). Furthermore, like humans (10), but unlike rats (38), high doses of cortisol in hamsters significantly increase liver triglyceride storage, which is likely a result of cortisol-enhanced release of free fatty acids (4) and liver activity of lipid-synthetic enzymes (21).
Standard indices of increased HPA axis drive, due to either chronic stress or excessive exogenous glucocorticoid administration, are thymus (33) and adrenal (40) mass reduction. Both cortisol and corticosterone caused adrenal hypotrophy and dose-dependently induced thymic involution in Syrian hamsters, although reductions in thymus mass were evident at lower concentrations of cortisol. This finding indicates that Syrian hamsters are responsive to corticosterone, although cortisol appears to be generally more potent. Conversely, high doses of cortisol, but not corticosterone, reduced ACTH levels, a finding that is consistent with corticosterone in rats and mice (26). This implies that the pituitary also has preferential affinity for cortisol in the hamster model. To our knowledge, there are no data regarding differential effects of the two glucocorticoids on ACTH secretion in Syrian hamsters.
The data presented here indicate tissue-specific effects of glucocorticoids in a dual secreting model, with cortisol having marked effects on thymus and adrenal gland growth as well metabolic end points. In contrast, corticosterone modulated only thymus and adrenal mass growth. One may argue that the differential effects of the glucocorticoids may be due to differential increases in blood concentrations of each hormone. For example, the highest dose of cortisol (100%) increased circulating cortisol concentrations approximately eightfold relative to vehicle-treated hamsters, whereas the same dosage of corticosterone increased corticosterone concentrations only fourfold. We do not believe that this is the reason for the more potent effects of cortisol vs. corticosterone on metabolic end points. As a case in point, the 50% cortisol dose that elicited a fourfold increase in blood cortisol also modulated many of the metabolic end points in the study. Although cortisol is the more biologically active hormone in Syrian hamsters, previous studies indicate that cortisol and corticosterone bind the type II glucorticoid receptor with similar affinity in the brain (34). At present, we do not know the underlying mechanisms that may account for the differential effects of these hormones on certain target tissues. The most parsimonious explanation is differing binding affinities of the glucocorticoids depending upon the target tissue. For example, perhaps in this species, adipose depots are more responsive to cortisol vs. corticosterone, whereas the thymus and adrenal glands are equally responsive to both glucocorticoids.
The goal of the study was to determine the underlying hormonal mechanisms that may account for the social stress-induced increases in body mass and adiposity in Syrian hamsters. We hypothesized that the social stress-induced body mass and adiposity gain (15, 32) might be due to increases in circulating glucocorticoids. However, in the present study, neither glucocorticoid regimen (i.e., acute injections or chronic pellets) elicited an increase in body mass or adiposity, suggesting that glucocorticoids (at least the doses in this study) alone are not sufficient to favor increased body mass and adipose growth. Certainly, stress is not characterized solely by excess glucocorticoids, since there are other factors that are released in response to stress. Perhaps it is not increased glucocorticoids that are responsible for stress-induced increases in body mass but rather metabolic alterations that occur during the recovery from stress that promote adiposity, as seen in our social stress model.
GRANTS
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases: DK-087816 (M. T. Foster), DK-017844 (S. C. Woods), DK-078201 (S. C. Woods), and DK-066596 (R. R. Sakai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. James P. Herman for thoughtful comments concerning this project.
REFERENCES
- 1. Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology 131: 585–594, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94: 2692–2701, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 88: 5593–5602, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing's syndrome. Neuroendocrinology 92, Suppl 1: 86–90, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29: 899–910, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev 2: 73–86, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Brinks V, de Kloet ER, Oitzl MS. Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol 216: 375–382, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol 165: 828–837, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Campbell JE, Peckett AJ, D'souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol 300: C198–C209, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Christian CD, Jr, Schneider RP. Fatty tumor of the liver in a patient with Cushing's syndrome. Arch Intern Med 143: 1605–1606, 1983 [PubMed] [Google Scholar]
- 11. Cunningham JJ, Calles-Escandon J, Garrido F, Carr DB, Bode HH. Hypercorticosteronuria and diminished pituitary responsiveness to corticotropin-releasing factor in obese Zucker rats. Endocrinology 118: 98–101, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Devenport L, Knehans A, Sundstrom A, Thomas T. Corticosterone's dual metabolic actions. Life Sci 45: 1389–1396, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Donohoe TP. Stress-induced anorexia: implications for anorexia nervosa. Life Sci 34: 203–218, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26: 37–49, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 290: R1284–R1293, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Grønli J, Murison R, Bjorvatn B, Sørensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res 150: 139–147, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 282: R77–R88, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiol Behav 47: 949–956, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol 10: 753–764, 1999 [DOI] [PubMed] [Google Scholar]
- 20. la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology 145: 2174–2185, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lau DC, Roncari DA. Effects of glucocorticoid hormones on lipid-synthetic enzymes from different adipose tissue regions and from liver. Can J Biochem Cell Biol 61: 1245–1250, 1983 [DOI] [PubMed] [Google Scholar]
- 22. Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology 57: 95–115, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 55: 747–753, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Masuzaki H, Ogawa Y, Hosoda K, Miyawaki T, Hanaoka I, Hiraoka J, Yasuno A, Nishimura H, Yoshimasa Y, Nishi S, Nakao K. Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing's syndrome. J Clin Endocrinol Metab 82: 2542–2547, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM. Changes in Behaviour and Body Weight Following a Single or Double Social Defeat in Rats. Stress 1: 21–32, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Murakami K, Akana SF, Dallman MF. Dopamine-beta-hydroxylase activity is necessary for hypothalamo-pituitary-adrenal (HPA) responses to ether, and stress-induced facilitation of subsequent HPA responses to acute ether emerges as HPA responses are inhibited by increasing corticosterone (B). J Neuroendocrinol 9: 601–608, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Naeser P. Effects of adrenalectomy on the obese-hyperglycemic syndrome in mice (gene symbol ob). Diabetologia 9: 376–379, 1973 [DOI] [PubMed] [Google Scholar]
- 28. Ottenweller JE, Tapp WN, Burke JM, Natelson BH. Plasma cortisol and corticosterone concentrations in the golden hamster, (Mesocricetus auratus). Life Sci 37: 1551–1558, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Page R, Boolell M, Kalfas A, Sawyer S, Pestell R, Ward G, Alford F. Insulin secretion, insulin sensitivity and glucose-mediated glucose disposal in Cushing's disease: a minimal model analysis. Clin Endocrinol (Oxf) 35: 509–517, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol 46: 85–95, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Schindler WJ, Knigge KM. Adrenal cortical secretion by the golden hamster. Endocrinology 65: 739–747, 1959 [DOI] [PubMed] [Google Scholar]
- 32. Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 292: R283–R290, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol 22: 13–23, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Sutanto W, De Kloet ER. Species-specificity of corticosteroid receptors in hamster and rat brains. Endocrinology 121: 1405–1411, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Tomas FM, Munro HN, Young VR. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J 178: 139–146, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uddén J, Björntorp P, Arner P, Barkeling B, Meurling L, Rössner S. Effects of glucocorticoids on leptin levels and eating behaviour in women. J Intern Med 253: 225–231, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest 26: 616–619, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Warne JP, Akana SF, Ginsberg AB, Horneman HF, Pecoraro NC, Dallman MF. Disengaging insulin from corticosterone: roles of each on energy intake and disposition. Am J Physiol Regul Integr Comp Physiol 296: R1366–R1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whitehouse BJ, Vinson GP. Species variations in steroid biosynthetic pathways: the formation of cortisol in hamster adrenal tissue in vitro. J Steroid Biochem 12: 307–312, 1971. [Google Scholar]
- 40. Wilkinson CW, Engeland WC, Shinsako J, Dallman MF. Nonsteroidal adrenal feedback demarcates two types of pathways to CRF-ACTH release. Am J Physiol Endocrinol Metab 240: E136–E145, 1981 [DOI] [PubMed] [Google Scholar]
- 41. Wommack JC, Delville Y. Cortisol controls the pubertal development of agonistic behavior in male golden hamsters via type II corticosteroid receptors. Horm Behav 51: 306–312, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Woodward CJ, Emery PW. Energy balance in rats given chronic hormone treatment. 2. Effects of corticosterone. Br J Nutr 61: 445–452, 1989 [DOI] [PubMed] [Google Scholar]