Abstract
Aerobic metabolism requires oxygen and carbon sources brought to tissues via the vasculature. Metabolically active tissues such as skeletal muscle can regulate blood vessel density to match metabolic needs; however, the molecular cues that coordinate these processes remain poorly understood. Here we report that the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1β (PGC-1β), a potent regulator of mitochondrial biology, induces angiogenesis in skeletal muscle. PGC-1β induces the expression of vascular endothelial growth factor (VEGF) in cell culture and in vivo. The induction of VEGF by PGC-1β requires coactivation of the orphan nuclear receptor estrogen-related receptor-α (ERRα) and is independent of the hypoxia-inducible factor (HIF) pathway. In coculture experiments, overexpression of PGC-1β in skeletal myotubes increases the migration of adjacent endothelial cells, and this depends on VEGF signaling. Transgenic expression of PGC-1β in skeletal myocytes dramatically increases muscular vessel density. Taken together, these data indicate that PGC-1β is a potent regulator of angiogenesis, thus providing a novel link between the regulations of oxidative metabolism and vascular density.
Keywords: peroxisome proliferator-activated receptor-γ coactivator-1β
blood vessels provide oxygen and nutrients to tissues to meet their metabolic demands (6). This supply is maintained by the formation of new blood vessels from preexisting vessels in a highly dynamic and tightly regulated process termed angiogenesis. Angiogenesis can occur under both pathological conditions like tumor growth and physiological conditions such as embryonic development, pregnancy, and exercise (5, 7, 30, 33). The process is triggered by the secretion from tissues of numerous soluble factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), angiopoietin (ANGPT), and fibroblast growth factor (FGF) (15). Highly metabolic and dynamic tissues such as skeletal muscle must be able to regulate the supply of nutrients to match the demand needed to perform work. However, the intrinsic signals within tissues that coordinate angiogenesis with the tissue's metabolic needs remain poorly studied.
Hypoxia or low oxygen tension is one potent inducer of angiogenesis via activation of the hypoxia-induced factor (HIF) pathway (16). In the presence of sufficient oxygen, the HIF-1α transcription factor is hydroxylated on key prolines and degraded. However, under conditions of hypoxia, HIF-1α is stabilized and free to dimerize with HIF-1β and activate proangiogenic genes such as VEGF (32). This pathway is active in multiple forms of pathological angiogenesis, but its role in physiological angiogenesis is not clear. Mice that lack HIF-1α in skeletal muscle, for example, have more blood vessels in the deep portion of the muscle rather than fewer, as might be expected (23). Other pathways thus clearly exist.
The peroxisome proliferator-activated receptor (PPAR)γ coactivator 1 (PGC-1) family of transcriptional coactivators regulates metabolic function in various tissues (12, 19, 29). The PGC-1 family consists of three members: PGC-1α, PGC-1β, and PGC-related coactivator (1, 20). These transcriptional regulators have no intrinsic DNA-binding activity but instead are recruited to specific genes by binding to DNA-binding transcription factors. Many transcription factors are targeted by PGC-1s, including PPARs, estrogen-related receptors (ERRs), and nuclear respiratory factors, among many others (25, 40, 43). All three members of the PGC-1 family function in promoting a core program of mitochondrial function, including genes of fatty acid oxidation and transport, electron transport chain, and numerous other mitochondrial structural proteins (19). In addition, each coactivator has unique functions in different tissues, such as control of gluconeogenesis in liver by PGC-1α or macrophage differentiation by PGC-1β (13, 14, 36, 39, 45). Transgenic overexpression of either PGC-1α or -β specifically in skeletal muscle significantly increases mitochondrial content and renders the mice more capable of endurance exercise (3, 8, 21, 41).
Mitochondria require fuel and oxygen, which are transported to the cell via the vasculature. Therefore, mechanisms are likely in place to couple mitochondrial biogenesis to angiogenesis. Recently, we have shown that PGC-1α can increase angiogenesis in skeletal muscle in vivo (2). Conversely, deletion of PGC-1α within skeletal muscle resulted in an impaired angiogenic program in response to hindlimb ischemia and exercise (2, 9, 11, 17). PGC-1α is thus one important integrator of metabolic function and angiogenesis. Here we investigate the role of PGC-1β in angiogenesis in skeletal muscle. Using both cell culture and transgenic models we demonstrate that PGC-1β induces in myocytes a program of angiogenic factors that differs from that activated by PGC-1α and that PGC-1β in myocytes powerfully stimulates endothelial cell migration in cell culture and angiogenesis in vivo.
EXPERIMENTAL PROCEDURES
Animals.
All animal experiments were performed according to procedures approved by the Beth Israel Deaconess Medical Center's Institutional Animal Care and Use Committee. Unless otherwise indicated, 16-wk-old mice were used for all experiments. MCK-PGC-1β transgenic mice (T37) have been described elsewhere (3).
Cells and reagents.
All reagents were procured from Sigma, unless otherwise indicated. Immunostaining was performed using anti-CD31 antibody (BD PharMingen). Human umbilical cord endothelial cells (HUVECs) and C2C12 cells were maintained in endothelial basal medium-2 (EBM-2) and Dulbecco's modified Eagle's medium (DMEM; supplemented with 10% fetal bovine serum), respectively. Isolation and culture of primary skeletal myocytes were performed on entire hindlimb muscle after collagenase/dispase digestion, as described previously (24, 28). Primary myocytes were differentiated in DMEM (5% horse serum), and C2C12 cells were differentiated in DMEM (2% horse serum). Cells were infected with adenovirus at a multiplicity of infection of 10–30, and mRNA expression was measured 48 h later. The adenovirus expressing green fluorescent protein (GFP), PGC-1α, and PGC-1β has been described previously (35). Reporter plasmids containing VEGF enhancer, mutated enhancer, and concatemerized ERRα binding sites have been described (2).
Real-time PCR.
Total RNA was isolated from mouse tissue and cultured cells using the TRIzol (Invitrogen) and Turbocapture (Qiagen) method, respectively. Samples for real-time PCR analyses were reverse transcribed (Applied Biosystems), and quantitative real-time PCRs were performed on the cDNAs in the presence of fluorescent dye (SYBR green; Bio-Rad). Relative expression levels were determined using the comparative cycle threshold method (4).
Endothelial cell migration assay.
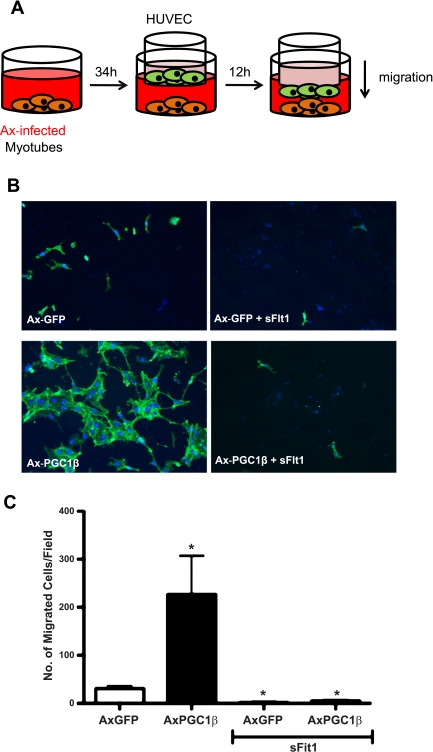
Differentiated C2C12 myotubes in 24-well plates were infected with adenovirus expressing GFP or PGC-1β for 34 h. BSA or soluble Flt1 (100 ng/ml; R & D Systems) was added to the medium for 12 h. Then, 5 × 104 cells of HUVECs were plated on the upper compartment of transwells (8.0 μm pore size) prewarmed with EBM-2 medium for 16 h at 37°C. HUVEC migration to the lower compartment of transwells was measured after 12 h. Migrated HUVECs were fixed with 4% paraformaldehyde in PBS for 20 min at RT, and cells that remained in the upper compartment were removed with cotton swabs. Cells were blocked with 5% BSA in PBS-Tween 20 (PBST; 0.2% Tween 20) and stained with phalloidin-FITC in PBST for 4 h to visualize filamentous actin. Transwell inserts were washed three times in PBST and mounted onto slides with DAPI mounting medium.
Histological analysis.
Quantification of capillaries was performed computationally, using Volocity software (Improvision; PerkinElmer), on three random fields chosen from the midportions of transverse sections from the indicated muscles. All quantifications were performed blindly.
Statistical analysis.
The data are presented as means ± SE. Statistical analysis was performed with Student's t-test for all in vitro experiments and ANOVAs for all in vivo experiments. P values of <0.05 were considered statistically significant.
RESULTS
PGC1β regulates VEGF both in vitro and in vivo.
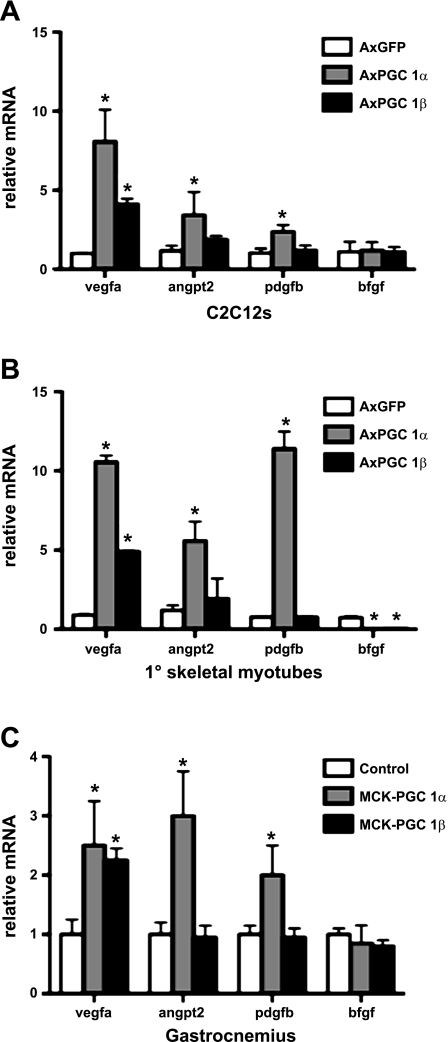
To investigate whether PGC1β induces an angiogenic program in skeletal myocytes, C2C12 myoblasts in cell culture were made to differentiate into myotubes and then infected with adenoviruses expressing PGC-1β vs. GFP control. Forty-eight hours later, RNA was isolated and subjected to reverse transcription, and the relative expressions of angiogenic genes were assessed by quantitative PCR (qPCR). PGC-1β overexpression led to a significant increase in VEGF-A expression (Fig. 1A). However, in contrast to PGC-1α (as a positive control), PGC-1β did not induce the expression of other angiogenic genes (ANGPT2 and PDGFB). C2C12 cells are immortalized cells that have lost many properties of true myoblasts. To investigate the role of PGC-1β in primary cells, skeletal muscle myocytes were isolated from C57Bl6 mice and made to differentiate into myotubes in cell culture. Infection with adenoviruses expressing PGC-1α, PGC-1β, and GFP led to results similar to those observed in C2C12 myotubes (Fig. 1B). In addition, both PGC-1α and -β significantly inhibited the expression of basic FGF (bFGF). To test whether PGC-1β induces VEGF in vivo, levels of VEGF-A message were measured in gastrocnemius muscle from either wild-type or transgenic mice overexpressing PGC-1β in skeletal muscle (MCK-PGC-1β). These mice have been described and induce PGC-1β ∼10-fold specifically in striated muscle (3). Similar to what was observed in cell culture, PGC-1β overexpression induced the expression VEGF-A twofold, whereas PGC-1β did not induce the expression of ANGPT2 or PDGFB (Fig. 1C). Taken together, these data show that PGC-1β can induce a subset of the proangiogenic program activated by PGC-1α.
Fig. 1.
Induction of VEGF-A by peroxisome proliferator-activated receptor-γ coactivator-1β (PGC-1β). A: C2C12 cells were infected with adenovirus (Ax)PGC-1α (as a positive control), AxPGC-1β, and AxGFP as indicated. Forty-eight hours later, RNA was prepared, and expression of the indicated genes was measured by quantitative RT-PCR (qPCR). B: primary muscle myocytes were made to differentiate and were treated as in A. C: RNA was prepared from gastrocnemius muscles of transgenic mice overexpressing PGC-1β in skeletal muscle (MCK-PGC-1α) and MCK-PGC-1β transgenic animals and littermate controls, and expression of the indicated genes was measured by quantitative RT-PCR. Error bars indicate SE; n > 3/group in all parts of the figure. *P < 0.05 compared with control. ANGPT2, angiopoietin 2; bFGF, basic FGF.
Induction of VEGF by PGC-1β is HIF independent.
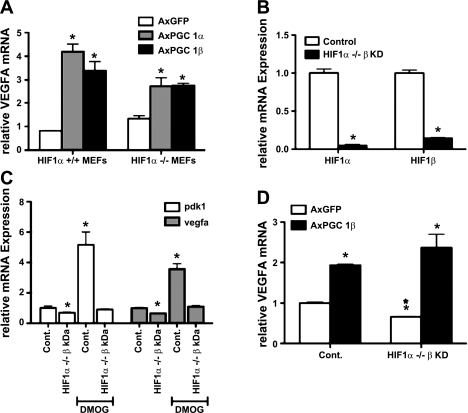
Induction of VEGF and angiogenesis has been studied most extensively in the context of hypoxia and the activation of the HIF-1 transcription factor pathway (32). Previously, we argued that PGC-1α induces its angiogenic program independently of HIF-1α activity (2), although others have suggested otherwise (27). Although PGC-1β shares moderate homology with PGC-1α, the repertoires of transcription factors coactivated by PGC-1α and -β differ significantly (19, 29). Therefore, we sought to test whether PGC-1β requires the HIF pathways to induce the expression of VEGF-A. Mouse embryonic fibroblasts (MEFs) isolated from either wild-type or HIF-1α−/− embryos were infected with adenoviruses expressing PGC-1β or GFP control. PGC-1β induced VEGF-A expression in these cells two- to threefold in both the absence and presence of HIF-1α (Fig. 2A), suggesting that PGC-1β induces VEGF independently of HIF activity. It is possible, however, that some HIF activity remains in these cells due to the presence of HIF-2α. Moreover, MEFs are heterogeneous cells that poorly model intact tissue. To address both of these issues, primary satellite cells were isolated from mice homozygous for HIF-1α floxed alleles and differentiated into myotubes in cell culture. The myotubes were then infected with adenovirus encoding Cre recombinase to delete the HIF-1α alleles, as well as adenovirus encoding short hairpin against HIF-1β (also known as ARNT), vs. GFP control (Fig. 2B). HIF-1β is the obligate heterodimer to both HIF-1α and HIF-2α, and thus no HIF activity should be possible in the double-infected myotubes. To confirm the absence of HIF activity, the cells were treated with dimethyloxaloylglycine (DMOG; a hypoxia mimetic) for 24 h. As shown in Fig. 2C, induction by DMOG of the known HIF targets pyruvate dehydrogenase kinase 1 (PDK1) and VEGF was completely abrogated in the double-infected cells. Notably, expression of PDK1 and VEGF-A was also reduced at baseline in the double-infected cells. These cells were then infected with PGC-1β or control adenovirus for 48 h, and the expression of VEGF-A was then assessed by qPCR. As shown in Fig. 2D, PGC-1β induced VEGF-A two- to threefold in these cells whether they contained HIF activity or not. Therefore, PGC-1β induction of VEGF is HIF independent.
Fig. 2.
Hypoxia-inducible factor (HIF)-independent induction of VEGF-A. A: HIF-1α-null and control mouse embryonic fibroblasts (MEFs) were infected with AxPGC-1α, AxPGC-1β, and AxGFP. After 48 h, RNA was prepared and expression of indicated genes measured by qPCR. B: differentiated primary muscle myocytes bearing floxed alleles of HIF-1α were infected with AxCre and with AxshHIF-1β (HIF-1α−/− β-KD). Expression of HIF-1α and -β were measured by qPCR. C: HIF-1α−/− β-KD-differentiated primary muscle myocytes cells were treated with 1 mM dimethyloxaloylglycine (DMOG; hypoxia mimetic) for 24 h, and pyruvate dehydrogenase kinase 1 (PDK1) and VEGF-A expression were determined by qPCR. D: HIF-1α−/− β-KD-differentiated primary muscle myocyte cells were infected with AxPGC-1α, AxPGC-1β, and AxGFP. VEGF-A expression was measured after 48 h. Error bars indicate SE; n > 3/group in all parts of the figure. *P < 0.05 compared with control.
ERRα-dependent induction of VEGF by PGC-1β.
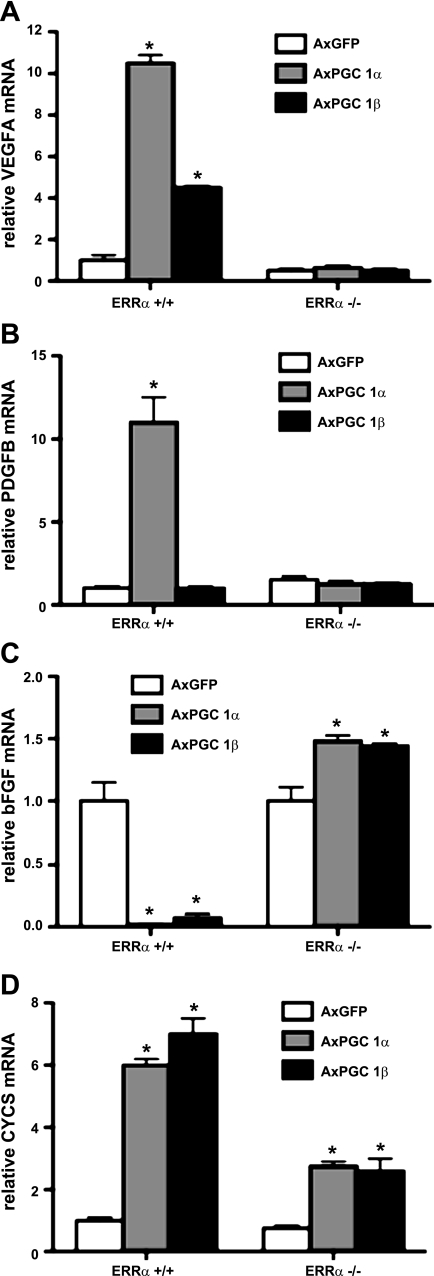
Previously, we have shown that the induction of VEGF-A by PGC-1α required coactivation of ERRα (2). To test whether PGC-1β induction of VEGF was also an ERRα-dependent process, primary differentiated myotubes from wild-type or ERRα−/− cells were infected with adenoviruses encoding for PGC1-β vs. GFP control. Whereas PGC-1β induced VEGF-A fourfold in wild-type cells, PGC-1β failed to induce the expression of VEGF in the absence of ERRα (Fig. 3A). The repression of bFGF by PGC-1β was also blocked in ERRα−/− cells (Fig. 3C). The induction of other ERRα-dependent genes (cytochrome c, somatic) was also impaired, although interestingly, it was not completely blocked (Fig. 3D). Therefore, the induction of VEGF-A by PGC-1β is ERRα dependent.
Fig. 3.
Estrogen-related receptor-α (ERRα)-dependent induction of VEGF-A. A–D: differentiated primary muscle myocytes from either ERRα-null or control mice were infected with AxPGC-1α and AxPGC1-β (AxGFP as a control) for 48 h. RNA was prepared and expression of the indicated genes measured by qPCR. Error bars indicate SE; n > 3/group in all parts of the figure. *P < 0.05 compared with control.
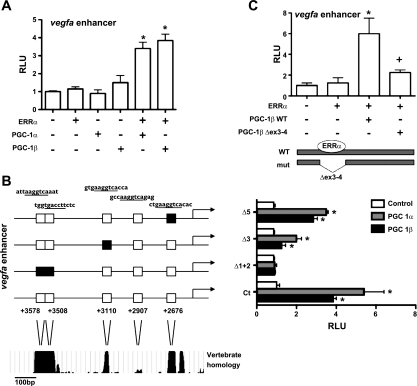
Previously, we described a novel enhancer in the first intron of the VEGF-A gene that is responsive to PGC-1α (2). To test whether PGC-1β could activate this enhancer, a luciferase reporter plasmid containing the enhancer upstream of the SV40 promoter was cotransfected with plasmids expressing ERRα, PGC-1α or -β, or empty control vectors. Neither ERRα nor PGC-1β alone was sufficient to induce activity of the enhancer, but addition of both PGC-1β and ERRα synergized to activate the VEGF enhancer fourfold (Fig. 4A). Adding PGC-1α and -β together had no synergistic effect (data not shown). Sequence analysis of the VEGF enhancer revealed five putative ERRα binding sites (Fig. 4B); four of the five sites are highly conserved across vertebrate species. Systematic mutations of these conserved ERRα binding sites within the VEGF enhancer reporter construct resulted in a significant decrease in the ability of PGC-1β to activate the VEGF-A enhancer (Fig. 4B). Most notably, deletion of nucleotides 3,578–3,508 completely blocked the induction by PGC-1β (Fig. 4B). PGC-1β is known to interact with ERRα via amino acids located in exons 3–4. A mutant version of PGC-1β lacking these amino acids fails to interact with ERRα (37). This mutant had a significantly reduced ability to activate the VEGF-A enhancer (Fig. 4C). Taken together, these data indicate that PGC-1β activates expression of VEGF-A via coactivation of ERRα on conserved ERRα binding sites within the VEGF-A enhancer.
Fig. 4.
PGC-1β coactivates ERRα on VEGF enhancer. A: C2C12 cells were transfected with VEGF enhancer luciferase construct ± ERRα in the presence or absence of PGC-1α and PGC-1β. Relative luciferase activity was assessed after 24 h. B: C2C12 cells were transfected with either wild-type VEGF enhancer or VEGF enhancer with mutated ERRα binding sites (Δ1 + 2, Δ3, Δ5) plus ERRα and PGC-1α or PGC-1β. C: C2C12 cells were transfected with VEGF enhancer plus ERRα and either wild-type PGC-1β or mutant PGC-1β (lacking exons 3–4, 112 amino acids). Relative luciferase activity was assessed after 24 h. Error bars indicate SE; n > 3/group in all parts of the figure. *P < 0.05 compared with enhancer alone.
PGC-1β in myocytes promotes endothelial cell migration.
The generation of new blood vessels requires the activation, proliferation, and migration of endothelial cells. Therefore, we tested whether PGC-1β expression in myocytes can stimulate the migration of adjacent endothelial cells. C2C12 cells were made to differentiate into myotubes in the bottom wells of modified Boyden chambers (Transwell system). The cells were then infected with PGC-1β or control virus. Thirty-four hours later, HUVECs were seeded into the top chamber of the Transwell system without the underlying cultured medium being changed. Twelve hours later, the endothelial cells that migrated to the bottom chamber were counted (Fig. 5A). As shown in Fig. 5, B and C, PGC-1β-infected C2C12 cells markedly stimulated the migration of the endothelial cells compared with control-infected myotubes. Preincubation with soluble VEGF receptor (sFlt1), which binds to and inhibits VEGF function, completely abolished the induced migration. Overexpression of PGC-1α in C2C12 cells yielded similar results (data not shown). Taken together, these data show that PGC-1β in myocytes can increase the migration of adjacent endothelial cells at least in part via increased VEGF expression and secretion.
Fig. 5.
PGC-1β induces endothelial migration. A: schematic of endothelial migration assay. B: differentiated C2C12 myotubes were infected with either AxGFP or AxPGC-1β adenovirus for ∼34 h. BSA or soluble Flt1 (100 ng/ml) was added to the medium for 12 h. Human umbilical cord endothelial cells (HUVECs) plated on Transwell inserts were then added and allowed to migrate thru Transwell filter for 12 h. Migrated HUVECs were detected by phalloidin-FITC and 4,6-diamidino-2-phenylindole (DAPI) staining. C: quantification of B. Error bars indicate SE; n > 3/group in all parts of the figure. *P <0.05 compared with AxGFP control.
Increased angiogenesis in PGC-1β transgenic mice.
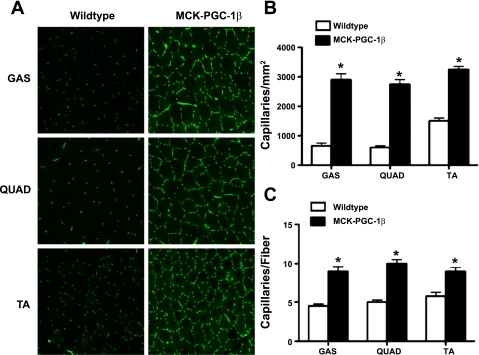
To test whether PGC-1β can induce angiogenesis in intact organisms, the MCK-PGC-1β transgenic mice described above were used. Various skeletal muscles (quadriceps, tibialis anterior, and gastrocnemius) were harvested from the MCK-PGC-1β transgenics and littermate controls. Transverse sections were generated from the muscles and stained with antibodies against CD31 (PECAM), an endothelial-specific marker that highlights capillaries. As shown in Fig. 6A, transgenic expression of PGC-1β in skeletal myocytes leads to a dramatic induction of capillary density in skeletal muscle. There was an approximately fourfold increase in capillaries/mm2 (Fig. 6B) and a twofold increase in capillaries per muscle fiber (Fig. 6C). The cross-sections of fibers of MCK-PGC-1β animals are smaller, as reported previously (3), explaining why the induction of the capillary/fiber ratio is less than that of capillary/mm2. The MCK-CRE transgene is activated in the late embryogenesis/early postnatal period, long after basic muscle development is complete; it is thus unlikely that the observed dramatic increases in vascular density are a developmental phenomenon. PGC-1β in skeletal myocytes thus powerfully induces angiogenesis in vivo.
Fig. 6.
Angiogenesis in MCK-PGC-1β transgenic mice. A: transverse frozen sections from gastrocnemius (GAS), quadriceps (QUAD), and tibialis anterior (TA) from the muscle-specific PGC-1β transgenic animals (MCK-PGC-1β) were immunostained for CD31; n = 4/group. B: quantification of CD31-positive cells/mm2. C: quantification of CD31-positive cells per individual myofiber; n = 3 high-power fields from 4 animals/group. Error bars indicate SE; *P < 0.05 compared with control.
DISCUSSION
We show here that the coactivator PGC-1β can drive robust angiogenesis in skeletal muscle in vivo. We cannot exclude the possibility that PGC-1β might also be increasing vasculogenesis, although this process is not thought to occur in postnatal skeletal muscle. Although angiogenesis and vasculogenesis are fundamentally different processes, the net result would still be increased vascular density as observed. PGC-1β is well established as a powerful driver of mitochondrial biogenesis (3, 35, 38). Mitochondria require fuel and oxygen delivered via the vasculature. PGC-1β can thus coordinate the consumption of fuel and oxygen (mitochondria) with their delivery (blood vessels) in skeletal muscle.
PGC-1β likely induces angiogenesis in large part via the strong induction of VEGF, and this induction is independent of hypoxia and HIF activity. Angiogenesis has been thought to occur primarily in the context of hypoxia. Most common models of angiogenesis, including retinopathy of prematurity models and many tumor models, are triggered by hypoxia (10, 31), and most studies investigating how cells increase expression of VEGF have focused on HIF-1 activation. However, many instances of angiogenesis likely do not require hypoxia, including postnatal growth in numerous tissues, cold-induced angiogenesis in adipose tissue (44), and exercise-induced angiogenesis in skeletal muscle (9). Some of these differences may reflect different mechanisms for physiological and pathological angiogenesis. The PGC-1 coactivators are thus excellent candidates for mediating these instances of hypoxia-independent vessel growth. It will be interesting to see how well PGC-1 coactivators induce angiogenesis in tissues other than skeletal muscle.
Mice lacking PGC-1β have not been reported to have a gross vascular phenotype (18, 34), although this has not been studied in depth. Mice lacking PGC-1α in skeletal muscle do not have decreased vascular density at baseline (9). Therefore, the PGC1s may have redundant roles in baseline vessel density in muscle. Alternatively, the PGC-1s may be involved primarily in responses to physiological stimuli rather than in baseline vascular homeostasis. We have shown previously that PGC-1α is induced by adrenergic signaling and is required for at least some forms of exercise-induced angiogenesis (9, 11, 17). In contrast to PGC-1α, little is known of the physiological stimulus for regulating PGC-1β expression/function in skeletal muscle. PGC-1α expression is sensitive to multiple extracellular cues, whereas in general PGC-1β expression is less easily induced. Evaluation of vascular homeostasis in mice lacking both PGC-1α and PGC-1β in skeletal muscle will help address some of these questions.
Interestingly, the angiogenic genes induced by PGC-1β differ from those induced by PGC-1α. Despite this, PGC-1β induces angiogenesis in skeletal muscle seemingly as robustly as PGC-1α does. PGC-1α and -β share significant conserved homology (1, 20) as well as many transcriptional targets (19). However, it is clear that they also have nonredundant roles. In the liver, for example, PGC-1α induces gluconeogenesis, whereas PGC-1β regulates lipid trafficking (22, 42, 45). In the case of fiber type determination, muscle-specific overexpression of PGC-1α increases type I and IIA fibers, whereas PGC-1β does not despite the fact that both PGC-1α and PGC-1β transactivate mouse enhancer factor-2, a key transcriptional regulator of fiber type determination (3, 21, 26). Thus, despite their homology and target specificity, PGC-1α and -β can orchestrate different biological responses. It will be of interest to determine whether and how the new blood vessels induced by PGC-1α and -β differ.
Angiogenesis is a complex process, requiring the coordination of multiple cell types in space and time. The PGC-1 coactivators are known to coordinate large programs of gene expression in numerous contexts. They are thus also well suited to coordinate angiogenesis. Investigating how the PGC-1s coordinate this complex process is likely to lead to new insights into the mechanism of angiogenesis.
GRANTS
G. C. Rowe has received support from the National Heart, Lung, and Blood Institute (NHLBI; 5T32-HL-007374-31) and a UNCF-Merck Science Initiative (postdoctoral fellowship), and Z. Arany is supported by the NHLBI, the Smith Family Foundation, the Ellison Foundation, and the Harvard Stem Cell Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are delcared by the authors.
REFERENCES
- 1.Andersson U, Scarpulla RC. Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol Cell Biol 21: 3738–3749, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Arany ZP. High-throughput quantitative real-time PCR. Curr Protoc Hum Genet 11: Unit 11.10, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bloor CM. Angiogenesis during exercise and training. Angiogenesis 8: 263–271, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 19: 223–229, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Breier G. Angiogenesis in embryonic development—a review. Placenta 21, Suppl A: S11–S15, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. J Appl Physiol 104: 1304–1312, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA 106: 21401–21406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature 438: 960–966, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol 298: C572–C579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413: 179–183, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15: 259–266, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Jain RK. Molecular regulation of vessel maturation. Nat Med 9: 685–693, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1α mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab 297: E92–E103, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Strömstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4: e369, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277: 1645–1648, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 120: 261–273, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. HIF-1α in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 293: R2059–R2069, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10: 1173–1183, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101: 6570–6575, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11: 683–688, 1999 [DOI] [PubMed] [Google Scholar]
- 27.O'Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci USA 106: 2188–2193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125: 1275–1287, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res 107: 825–838, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54: 17–28, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8: S62–S67, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 19: 176–182, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Smith SK. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab 12: 147–151, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104: 5223–5228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 278: 26597–26603, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4: 13–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4: 453–464, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284: 21379–21385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11: 503–516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25: 10684–10694, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem 282: 36642–36651, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab 3: 99–110, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, Cannon B, Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9: 99–109, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001 [DOI] [PubMed] [Google Scholar]